Abstract
Sympatric speciation has been controversial since it was first proposed as a mode of speciation. Subterranean blind mole rats (Spalacidae) are considered to speciate allopatrically or peripatrically. Here, we report a possible incipient sympatric adaptive ecological speciation in Spalax galili (2n = 52). The study microsite (0.04 km2) is sharply subdivided geologically, edaphically, and ecologically into abutting barrier-free ecologies divergent in rock, soil, and vegetation types. The Pleistocene Alma basalt abuts the Cretaceous Senonian Kerem Ben Zimra chalk. Only 28% of 112 plant species were shared between the soils. We examined mitochondrial DNA in the control region and ATP6 in 28 mole rats from basalt and in 14 from chalk habitats. We also sequenced the complete mtDNA (16,423 bp) of four animals, two from each soil type. Remarkably, the frequency of all major haplotype clusters (HC) was highly soil-biased. HCI and HCII are chalk biased. HC-III was abundant in basalt (36%) but absent in chalk; HC-IV was prevalent in basalt (46.5%) but was low (20%) in chalk. Up to 40% of the mtDNA diversity was edaphically dependent, suggesting constrained gene flow. We identified a homologous recombinant mtDNA in the basalt/chalk studied area. Phenotypically significant divergences differentiate the two populations, inhabiting different soils, in adaptive oxygen consumption and in the amount of outside-nest activity. This identification of a possible incipient sympatric adaptive ecological speciation caused by natural selection indirectly refutes the allopatric alternative. Sympatric ecological speciation may be more prevalent in nature because of abundant and sharply abutting divergent ecologies.
Keywords: adaptation, ecological stress, radio-tracking, metabolism, microscale
The origin and nature of species, the mystery of mysteries (1) and “the most important single event in Evolution” (2), have always been problematic in evolutionary studies (2–7). We adhere to the Biological Species Concept (2), recognizing its merits and demerits (2). The recent resurgence in speciation studies highlights many past obscurities (4), including sympatric speciation (7–18). Nevertheless, many basic questions related to adaptation and speciation, including sympatric speciation, still await resolution based primarily on the genomic sequence studies, such as in Drosophila (8), or even in species that presumably originated sympatrically, such as in the fly Rhagolites (9) or cichlid fishes in Africa and Neotropical America (10).
The mode of species origin is still a major focus of heated debate. Does speciation occur primarily in allopatry, i.e., requiring complete geographic isolation, or can it occur in parapatry and peripatry, where limited gene exchange operates, or even in sympatry, where free gene exchange occurs, as suggested by Darwin (1)? Darwin envisaged allopatric, parapatric, and sympatric modes of speciation, but neither he nor his followers estimated their proportions in nature, which remain enigmatic and limited (4). Moreover, no speciational mode aspect is as controversial as Darwin’s view that species also can speciate sympatrically (1). Theoretical studies (17) support the possible existence of sympatric speciation, but the evidence from nature is scant and awaits further investigation (4). Even the definition of sympatric speciation has been questioned, and the proposition has been advanced to estimate gene flow and selection rather than assigning cases to discrete categories such as sympatric and allopatric speciation (16, 18). Spalacidae have been studied extensively as an evolutionary model (3, 19–30). Can sympatric speciation occur in blind mole rats, Spalax, where allopatry and/or peripatry prevail (3, 22)? Blind subterranean mole rats, genus Spalax, provide a unique evolutionary model of adaptation linked to ecological speciation (a full reference list, is available at http://evolution.haifa.ac.il, Nevo list of publications). The ecological and chromosomal speciational trend of Spalax in Israel into increasingly arid environments occurred in the Pleistocene, during the last 2.00–2.35 million years (3, 22). It generated four species associated intimately with four climatic regimes with increasing aridity stress southwards and eastwards representing an ecologically adaptive speciational trend: Spalax golani, 2n = 54; Spalax galili, 2n = 52; Spalax carmeli, 2n = 58; Spalax judaei, 2n = 60 (22). Diploid chromosome numbers (2n) and allozyme genetic heterozygosity are positively correlated with aridity stress in Israeli Spalax, as also is true in Asia Minor, which is 30 times the size of Israel (24). Nonchromosomal genetic speciation with four species, 2n = 60, has been described in the Spalax ehrenbergi superspecies in Jordan (25, 26).
Earlier we identified microevolution in action in S. galili in northern Israel involving the edaphically adaptive genomic divergence of amplified fragment length polymorphisms (AFLP) (27). We compared 10 individuals from two abutting populations living in 5 square kilometers in two contrasting ecologies of chalk and basalt soils. Of 729 AFLP loci spread across the genome in both coding and noncoding regions, 433 (59.4%) were polymorphic, with 211 unique alleles related to soil type. Genetic polymorphism was significantly higher on the more xeric and ecologically stressful chalky soil than on the mesic basalt soil. In addition, swimming behavioral profiles were dramatically divergent between these populations (29): The chalk-dwelling animals drowned when thrown into the water, whereas basalt-dwelling animals swam, following regional patterns (30).
Recently, Tzur (28) identified mtDNA divergence between two S. galili populations living in two sharply contrasting ecologies of chalk and basalt soils (Fig. 1), separated by only a few meters to hundreds of meters without any topographic barrier. The population of Alma (ALM) lives in basalt soil, and the population of Kerem Ben Zimra (KBZ) lives in chalk. At KBZ, the geology consists of white Senonian chalk covered by rendzina soil with Sarcopterium spinosum and Majorana syriaca. In contrast, at ALM, the geology consists of dark, reddish-brown volcanic Pleistocene basalt soil with Carlina hispanica–Psorelea bituminosa plant formations. Soil fungi also displayed distinct chalk–basalt divergence (31) (Fig. 2). The genetic divergence found in the ALM–KBZ microsite is a remarkable pattern for a mammal that can disperse tens to hundreds of meters in each generation (3). Clearly, these results cannot be explained by migration (which causes homogenization) or by chance, which excludes sharp genetic inter-soil divergence. Edaphic natural selection was hypothesized to be the only evolutionary adaptive force that could have caused the sharp ecological contrasts in the genetic divergence seen in the mitochondrial genomes of S. galili (2n = 52). The stimulus of different geologies for plant speciation has been reviewed by Kruckeberg (32). The edaphic factor in the origin of plant species has been reviewed by Rajakaruna (33).
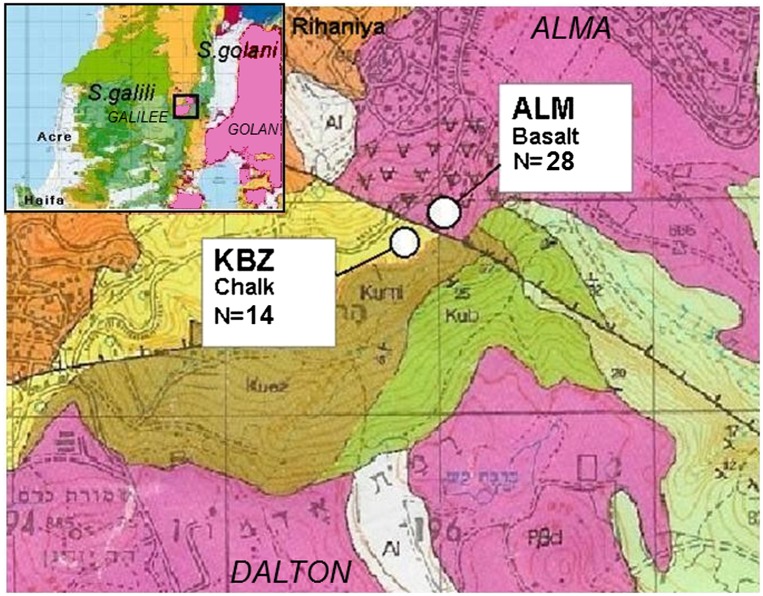
Fig. 1.
Geological map of the studied Dalton region in the Upper East Galilee, Israel. S. galili sampling sites, soil type, and number of captured animals are indicated. Colors indicate bedrock type: yellow, chalk; pink, basalt. A bold black line indicates a geological fault. The length of square edge in the map represents 1,000 meters. The image is based on Levitte (54). The smaller map in the upper-left shows the geology of Northern Israel. Note the sharp border between the adjacent examined populations of chalk and basalt.
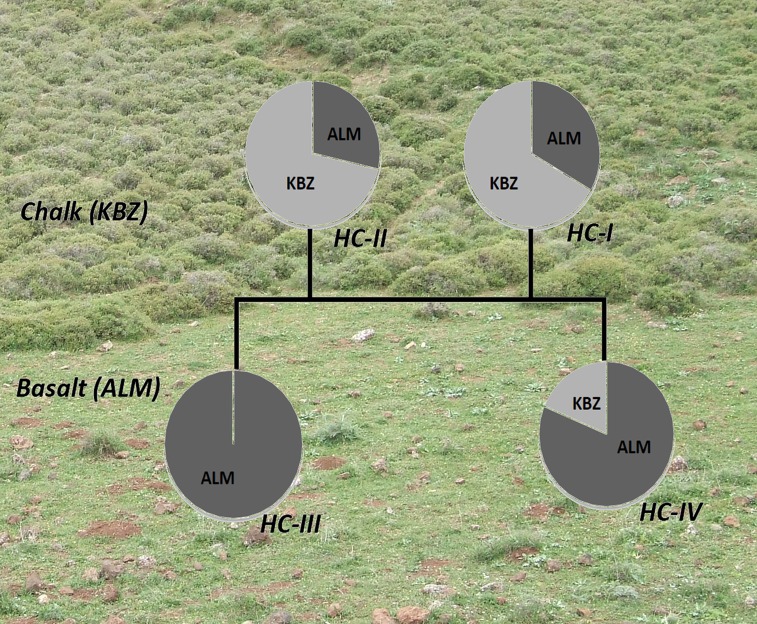
Fig. 2.
Picture of the site and pie graphs of the schematic phylogenetic relations of HC and their frequencies in basalt or chalk at the microsite. The background shows the sharp divergence of vegetation between chalk and basalt area. See Fig. 1 for geological map of the region and Fig. S1 for the schematic phylogenetic relations between HC. Please note that the pie graphs are not scaled to difference in the sample sizes.
Here we extended these studies, primarily through extensive analysis of complete mtDNA, physiology, and behavior, to investigate a possible incipient sympatric speciation in the blind subterranean mammal Spalax. Notably, Spalax usually speciates peripatrically (3, 22), but in the ALM–KBZ microsite we identified a possible nonchromosomal incipient sympatric speciation in blind mole rats.
Results
Analysis of Phylogenetic Trees.
Population statistics were conducted on mtDNA haplotype sequences in the concatenated DNA fragments (1,281 bp) of the control region sequence combined with the ATP6 sequence. To compare local and regional differences, we added 10 specimens of S. golani (2n = 54) from outside the ALM–KBZ microsite (Table S1). The most abundant haplotypes were H6, H2, H12, and H9. The haplotypes and their frequencies among different populations are presented in Tables S2 and S3.
The maximum likelihood-based phylogenetic trees of the identified haplotypes (Table S1), based on the HKY85 model, are presented in Figs. S1 and S2A. Fig. S1 describes haplotype distribution at the microsite. The tree identified well-separated haplotype clusters (HC), especially haplotypes HC-III and HC-IV, prevailing in the basalt (82%), and haplotypes HC-I and HC-II, prevailing in the chalk (78.5%). Fig. S2A shows the pattern with sequences of the Israeli mole rat species, S. golani (2n = 54) and S. galili at the microsite (Table S1). The S. galili subclade separates animals from ALM basalt and KBZ chalk very clearly. The phylogenetically nearest species to S. galili from the microsite is S. golani (2n = 54) from the Golan Heights and Mount Hermon. S. golani is regarded as the oldest of the four Israeli Spalax species (22). Interestingly, among S. galili samples, the HC that is well separated from the others is HC-III= (H9), which apparently represents a haplotype cluster adapted to basalt. Moreover, the HC-III amino acid sequence of ATP6 gene is identical to the sequence found in S. golani in the basalt plateau of the Golan heights.
Population Structure and Intersample Genetic Differences.
As indicated by the formalized choice criterion ∆K, the best-fitting set of haplotype clusters is K = 4 (Fig. S3) (abbreviated as HC-I, HC-II, HC-III, and HC-IV; Tables S2 and S3). The following facts indicate strongly that the pooled sample represents the real structure in the studied microsite: (i) individual samples are strongly assigned to one cluster or another (Tables S2 and S3 and Figs. S1 and S2A); (ii) the proportions of individuals assigned to each defined cluster are asymmetric (HC-I = 9, HC-II = 7, HC-III = 10, and HC-IV = 16; Fig. S2A), which is a criterion for deciding whether the observed structure represents reality (34); (iii) the test of the obtained best-fitting model (K = 4, Ɵ > 0) against the null model (K = 1, Ɵ = 0) indicates that the (K = 4, Ɵ > 0) is preferred over the (K = 1, Ɵ = 0) model. See SI Materials and Methods for details about calculating ∆K.
Genetic Differences Between Basalt- and Chalk-Dwelling Mole Rat Populations.
Significant differences in mtDNA genetic diversity were found between the mole rat populations in the two soil types of the microsite (Fig. 2). In the ALM basalt population (n = 28), only five haplotypes were identified: HC-I: H2 (n = 3); HC-II: H8 (n = 2), HC-III: H9 (n = 10), HC-IV: H6 (n = 12); and H11 (n = 1). These five haplotypes show low genetic (haplotype) diversity (Hd) of 0.678. Two of these haplotypes, H9 and H6, comprised 73% of the total ALM population. In contrast, in the adjacent KBZ population (n = 14), we found eight haplotypes: HC-I: H2 (n = 3), H19 (n = 1), H20 (n = 2); HC-II: H8 (n = 3), H12 (n = 2); HC-IV: H6 (n = 1), H7 (n = 1), and H11 (n = 1). These haplotypes show a higher level of diversity, 0.924. The difference in Hd in the ALM and KBZ populations is significant by the test of the difference between two means (P < 0.001, binomial test; eight haplotypes among 14 animals in the chalk population versus five haplotypes among 28 animals in the basalt population) (for details on statistics, see SI Materials and Methods).
Significant differences between the ALM and KBZ populations were obtained by several tests {means of haplotype-based differentiation statistics [χ2 = 25.3, P = 0.003, degree of freedom (df) = 9] and sequence-based differentiation statistics [nearest-neighbor statistic (Snn): 0.74, Permutation test, PM, P < 0.0001]} and were supported by information criterion r (mean value of r = 0.75 from 20 runs). The maximum possible concordance between the two populations is 66.22% (the maximum possible K = 0.60), indicating that up to 40% of the variability could be attributed to the differing soil types. The haplotypes H6 and H9, with significantly different frequencies in the populations on the two soil types (Tables S2 and S3), are candidates for diversifying selection.
Basalt-Specific Haplotype and mtDNA Recombination.
Haplotype H9 differs most from the other studied haplotypes (Fig. 2 and Figs. S1 and S2A). The ATP6 haplotype H9 sequence raises a point of particular interest. This haplotype was found exclusively in the ALM basalt-dwelling population and was absent in the adjacent chalk-dwelling KBZ population. Moreover, in complete mtDNA sequences we identified a significant recombination between H9 and H12 in the studied area (Fig. S2B). The recombination is highly significant, as indicated by five detection methods. The ATP6 amino acid sequence in this haplotype revealed a gene product identical to that found in the species S. golani (rechecked in S. golani controls). The ATP6 H9 sequence contains asparagine in position 185 (the codon AAC) in the ALM basalt population, similar to S. golani on the basalt plateau of the Golan Heights. In contrast, all S. galili ATP6 sequences from the other samples (28) contain serine in that position (the codon AGC). The identified mutation in the codon AAC seems to be under positive selection pressure, because the frequency of the H9 haplotype is significantly higher in populations living on basalt soil. Future functional studies should investigate the direct phenotypic effect of this variant.
Activity Patterns Outside the Nest Revealed by Radio-Tracking.
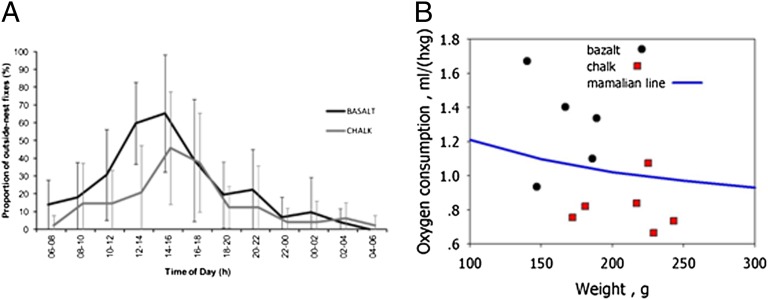
The outside-nest activity of mole rats from both microsites was concentrated into one single daily peak between 10:00 AM and 4:00 PM (Fig. 3A). However, there was a significant difference in the overall amount of the outside-nest activity between animals from the two different soils. The animals living on basalt soils were located outside the nest significantly more often than those living on chalk [F1, 17 = 8.9, P < 0.01; analysis of covariance (ANCOVA); Fig. 3A]. The effects of sex and the interaction of sex and soil type were not significant (P = 0.28 and 0.82, respectively).
Fig. 3.
(A) Daily activity patterns of mole rats in basalt and chalk habitats. Data are shown as means ± SD. (B) Oxygen consumption is significantly higher in mole rats from the basalt region (black circles) than in mole rats from the chalk region (red squares).
Oxygen Consumption: Resting Metabolic Rate.
Mean oxygen consumption was 1.33 ± 0.27 mL O2⋅h−1⋅g−1 in the basalt mole rats and was 0.89 ± 0.14 mL O2⋅h−1⋅g−1 in the chalk mole rats, indicating significant differences in resting metabolic rates between the mole rat populations on the abutting soil types (P < 0.05; Mann–Whitney U test) (Table S4 and Fig. 3B).
Discussion
Genome and Phenome Evolution of Subterranean Mammals at Global, Regional, and Local Scales.
One of nature’s best long-term global evolutionary experiments, which occurred about 45 Mya, is mammals adapting to life underground. This remarkable evolution followed stepwise climatic cooling and drought stresses during the transition from the Middle Eocene to the Early Oligocene (3). The evolutionary history of spalacids during the Neogene (35) and Pleistocene (3, 22, 23) also was channeled by climatic stresses and oscillations. Thus, ecological stresses influenced the evolution of spalacids in both adaptation and speciation. The evolution of the four species of Spalax in Israel is climatically determined through coupled underground stresses (3, 22, 23) and aboveground gradients of increasing aridity southward and eastward. The speciation mode of the four species of the S. ehrenbergi superspecies in Israel was allopatric or peripatric, which are regarded as the major modes of speciation in the Spalacidae (3, 22). Our present study highlights the possibility of incipient sympatric speciation.
Sharp Ecological Divergence as the Basis for Biological Divergence.
The two examined populations of S. galili (2n = 52) (27, 28) in the eastern Upper Galilee Mountains in northern Israel abut one another, separated by a geological fault and alternative lithologies and soils (Figs. 1 and 2). The studied area covers 0.04 km2. The ALM and KBZ mole rats live in sharply divergent ecologies: dark Early Pleistocene basalt faulted against white Senonian chalk (Figs. 1 and 2). Plant formations (Table S5) and soil fungi (31) demonstrate sharp intersoil divergence. No geographical barriers separate the two mole rat populations except for the divergent rock, soil, and plant ecologies. The chalk-dwelling mole rat population is much sparser than the basalt-dwelling population (around 1:5), probably because the chalk-dwelling population is subjected to much higher ecological stress. The chalk habitat is drier, with a much lower density of geophytes (the major food resource of mole rats). Notably, the resting metabolic rate or oxygen consumption was higher in basalt-dwelling animals (Fig. 3B). Mammals of low body mass have high specific oxygen consumption, as found in our study. However, judging from the allometric equation (36), this difference in mass could be responsible for a difference of 0.02 in oxygen consumption, whereas our study found a difference in oxygen consumption of 0.44. A low resting metabolic rate is typical of mammals in arid soils with low productivity. Hypothetically, different mtDNA haplotypes and variations in mitochondrial genes may play functional roles in the oxygen consumption during aerobic respiration that is being done by the mitochondria. Radio-tracking also revealed significant intersoil divergence in the activity levels of the ALM and KBZ mole rat populations (Fig. 3A). Moreover, laboratory experiments in aquaria revealed that animals originated from basalt soils swam significantly longer than animals from chalk soils, which drowned quickly (29). This local pattern reflects the regional pattern across Israel, in which northern mole rats swim better than southern ones (30). Superior swimming ability in Spalax may be associated with the extent and level of flooding and free-standing water, which is higher regionally in north Israel and is higher locally on basalt than on chalk (30). Preliminary biological laboratory comparisons, requiring additional critical verification, revealed that the ALM and KBZ populations demonstrate divergent habitat and food selection, as well as mate choice, which is the basis for prezygotic reproductive-isolating mechanisms in mole rats (37).
Nuclear and Mitochondrial Genomes Display Similar Genetic Divergence Between Chalk- and Basalt-Dwelling Populations with Higher Diversity in Chalk-Dwelling Populations.
Adaptive edaphic nuclear coding and noncoding genomic AFLP divergence previously showed higher genomic diversity on the more stressful chalk habitat (27). Mitochondrial results by Tzur (28), elaborated here, on a microscale transect of basalt and chalk across the geological fault display parallel patterns. Genetic polymorphism in mtDNA, like that of AFLP in the nuclear genome, is higher in the more stressful chalk habitat than in the milder basalt habitat. This parallelism in both nuclear and mtDNA genomic diversity corroborates the environmental theory of genetic diversity (38). Genetic polymorphism increases southward in Israel in 13 genera involving 21 species, 142 populations, and 5,474 individuals tested for 27 enzymatic loci (39). This regional pattern has been substantiated at global, regional, and local scales (38). A dramatic demonstration of genetic divergence on the local scale is caused by interslope differences at the level of microclimatic stress in the “Evolution Canyon” microsite model. Sixty-four percent of the model organisms studied for genetic diversity displayed higher levels of polymorphism on the drier and hotter south-facing slope (40). The organization and evolution of molecular genetics and genomic diversities in nature at global, regional, and local scales are nonrandom and structured, displaying regularities across life forms, and are positively correlated with, and partly predictable by, abiotic and biotic environmental heterogeneity and stress (38), as was found in the ALM and KBZ populations. Evolution towards biodiversity, even in small populations such as those studied here, is driven primarily by natural selection as an adaptive trait coping with ecological diversity, stress, and change. Natural selection includes diversifying, balancing, as well as cyclical and purifying, selective regimes, interacting with but ultimately overriding the effects of mutation, migration, and stochasticity (38). Natural selection appears as the last editor determining fitness and survival.
Mitochondrial Genome in Adaptation and Incipient Ecological Speciation.
The genetic divergence that was demonstrated by the significantly different mtDNA frequencies in the two populations suggests that natural selection operates directly on the mtDNA genome or indirectly on unknown genomic loci. Here we minimize the potential isolation by distance, without topographic barriers, and present additional critical evidence supporting the hypothesis of possible (or even probable) incipient sympatric speciation in S. galili. We found genetic and phenotypic differences between the contiguous ALM and KBZ S. galili populations (Fig. 2). The two populations reside in the same Mediterranean climatic regime, with about 600 mm annual rainfall (41), and at the same altitude (760 m above sea level), but their habitats differ drastically in geology (Fig. 1), lithology, and vegetation (Fig. 2). The single most important factor dividing the area into contrasting ecologies is the geological and lithological background. In the Upper Galilee Mountains there are only two basalt flows, the Alma and Dalton basalt islands, which are isolated and surrounded by chalk soil (Fig. 1). The basalt and chalk soils vary in chemistry, structure, texture, and vegetation (42, 43). The basalt represents a cooler and more humid habitat than the dry and warm chalk, which is more steppic in nature, as documented by a comparison of soil fungi (31). Significant mtDNA divergence was found between the mole rat populations occupying the two soil types. The distance separating the two populations (a minimum of a few meters to a maximum of 200 m) is smaller than the species’ dispersal range in one generation. Up to 40% of the mtDNA genetic diversity is attributable to the contrasting soil types. Significant differences in the distribution and structure of haplotype clusters in the ALM and KBZ populations (Fig. 2) negate stochasticity and clearly indicate that diversifying natural selection overrules gene flow, as is true in the Evolution Canyon (44–46). Strong divergent selection may be an important evolutionary force driving not only adaptation but, most importantly, the possible incipient sympatric ecological speciation in S. galili at the ALM–KBZ microsite.
An interesting case is haplotype H9 (28), which is known exclusively from basalt-dwelling mole rats (Fig. S2A), and it is under higher selection pressure than other identified haplotypes in both soil types. It may be a relict haplotype that does not hybridize with other haplotypes, as indicated by its restriction to the ALM basalt. Interestingly, this haplotype is present only in a small area of the studied basalt island and may have originated as a recombinant between H12 and unknown haplotypes (Fig. S2B). More investigation is needed to determine if H9 is a hybrid haplotype. It is remarkable that H9 including ATP6 gene amino acid sequence also occurs in the basalt plateau of the Golan Heights, suggesting that H9 is adapted to the basalt niche. It is noteworthy that incipient ecological speciation also occurs in Evolution Canyon (dubbed the “Israeli Galapagos”) across life forms, in bacteria, wild barley, Drosophila melanogaster, and Acomys cahirinus (46). Adaptive ecologically divergent polymorphisms keep evolving into incipient sympatric speciation as envisaged by Darwin (1). Importantly, blind mole rats exhibit mate choice, with individuals choosing mates whose odors are similar to their own genotypic odor (47). Prezygotic reproductive-isolating mechanisms play a major role in mole rat speciation across Israel (37). Our preliminary results (awaiting critical verification) suggest the operation of soil-based mate choice and habitat selection in the investigated populations. In future studies it will be important to substantiate prezygotic reproductive-isolating mechanisms that may operate through olfaction or through vocal and seismic communication systems (3). Currently, the reproductive isolation is hypothesized indirectly by the drastically decreased gene flow between the two mole rats populations.
Sympatric Speciation.
The prevalence of sympatric speciation in nature is still unknown and is hotly debated (4), despite much up-dated information and theoretical support since it was advocated by Darwin (1) as an important mode of species formation in nature. Theoretically, sympatric speciation must involve resource differentiation (4), as is clearly the case in our study, which is based on ecological speciation. The central premise of ecological speciation is that reproductive isolation is a consequence of divergent selection pressures imposed by different niches (48–50), in our case the chalk and basalt. Divergent selection on resource use, niche, food, and mate choices may drive positive assortative mating and sympatric speciation. Recent studies of widespread genomic divergence in the fly Rhagoletis pomonella, a classical model of sympatric speciation via host plant (48), reveal widespread genomic divergence of “genomic islands of speciation” during sympatric speciation (9, 49). Sequential sympatric speciation across trophic levels can cascade: The parasitic wasp has formed new incipient species as a result of specializing on diversifying fly hosts, including the recently derived apple-infesting race of Rhagoletis pomonella (50). Genomics of adaptation and speciation in the famous adaptive radiation of cichlid fishes in Africa and the Neotropics has been overviewed recently (10) and studied recently in Cameron Crater Lake Cichlids (14).
The supporting evidence for a possible (or even probable) incipient sympatric speciation in our study primarily involves historical geological evidence. Most of the Upper Galilee Mountains consist of Upper Cretaceous carbonates, including the Senonian chalk of Kerem Ben Zimra. The basalt flow of Alma is Pleistocene and represents a new geological island of volcanic basalt flow over the old carbonaceous Upper Cretaceous rocks and, most importantly, forming an island, together with the nearby second basaltic island of Dalton. The closest extensive basaltic regions are in the Golan Heights, across the rift valley, and in the eastern Lower Galilee. The geographic distance of the ALM–KBZ region from the Golan Heights and eastern Lower Galilee is in tens of kilometers. The ALM–KBZ region is small, a few square kilometers (and our investigated area is 0.04 square kilometers) within the much larger range of S. galili distributed over hundreds of square kilometers of carbonates throughout the entire Upper Galilee. The ALM–KBZ site is effectively a stasipatric island that emerged from a volcanic eruption in the Pleistocene. The rate of DNA evolution in the S. ehrenbergi superspecies indicates an approximate divergence time of 2.0–2.35 Mya to the branch leading to the oldest species in the Israeli superspecies, S. golani (2n = 54), found in the Golan Heights and Mount Hermon (51). The geological history of the ALM basalt plateau suggests a Pleistocene origin for the two basalt islands. The incipient emergence of the new species on the basalt most probably is the result of the sympatric evolution of species in the abutting chalk and basalt ecologies. Usually, discussions of allopatric speciation do not involve sharp ecological divergence dependent on specific geology (but see later). Both the novel lithology (the volcanic basalt) and the sharp geological fault separating the new basalt from the older chalk may be enough to generate incipient in situ speciation in the two sharply divergent geological and biological ecologies. This system is interesting because it does permit sympatric speciation without the need to rule out speciation via allopatry or even secondary contact. The contact is primary and was dictated by the volcanic eruption and the geological fault line. Once the basalt flowed over the chalk, the alternative selective pressure started to separate the mole rat populations ecologically in the very same homeland (patria) and without the need to evoke a secondary contact. It is important to note that our discussion deals with incipient sympatric speciation. Because we now have the full nuclear genome of the basalt-dwelling mole rat, it will be interesting to resequence the entire genome of the chalk-dwelling mole rats to reject allopatry speciation fully. We now witness the process of ongoing speciation of a new basalt-dwelling population that emerged from the older one living on the chalk. This process of incipient sympatric speciation generates a strong selection against ongoing gene flow between basalt- and chalk-dwelling mole rat populations. Thus, the adaptation of mole rats across the geological fault between basalt and chalk is not superficial. It is an in-depth ecological incipient sympatric speciation, like that unveiled in diverse taxa across phylogeny, from bacteria to mammals, in the Evolution Canyon (46). Evolving prezygotic and postzygotic reproductive-isolating mechanisms emerge in association with the different basalt and chalk ecologies. We already have witnessed mate, habitat, and food choices associated with the soil origin of the animals; these observations now need to be verified statistically. Our hypothesis of a possible incipient sympatric speciation also can be tested in the second, nearby basalt island of Dalton within the extended carbonaceous region in the Upper Galilee. Remarkably, several plant species, e.g., genus Crocus, also speciate between basalt (Crocus aleppicus) and chalk (Crocus hyemalis) in the same region. The dramatically divergent basalt and chalk ecologies are fruitful sites for future studies of sympatric speciation in diverse taxa across life forms, from viruses and bacteria to algae, fungi, plants, and animals, as is true in Evolution Canyon (45).
Importantly, although the biota commonly is determined by climate, geology enriches discontinuity and habitat diversity. When slope, rock, and vegetation are discontinuous, the probability for speciation increases, as is evident in microevolutionary responses to heavy metals and serpentine endemism (32). The edaphic factor in the origin of plant species, including serpentine outcrops, mine tailings, guano deposits, and salt flats, are important in plant speciation (33). Hybrid sterility over tens of meters between ecotypes adapted to serpentine and nonserpentine soil was described by Moyle et al. (11). Among his extensive studies on divergence in local plant populations, Antonovics (12) described the long-term persistence of prereproductive isolation at a mine boundary.
Although the frequency of sympatric speciation in nature is still unknown, sharply divergent ecologies caused by geological, lithological, edaphic, and vegetational divergence are common. Moreover, our studies at Evolution Canyon (40, 46) suggest that incipient sympatric speciation is common in nature, resulting not from secondary contact of allopatric populations but from sharp ecological divergences associated with geology, soil, or climate. Although many more such cases need to be examined and evaluated critically, sympatric speciation is theoretically possible (17) and is demonstrated clearly by polyploidy in plants (mentioned in ref. 5). Our study suggests that it will be fruitful to explore the possibility of sympatric speciation in sharply divergent ecologies in subterranean mammals, where the rule is allopatric/peripatric speciation (3). Our case suggests that nonchromosomal speciation also may occur in spalacids, as we have already shown earlier (25, 26). Our present study suggests that sympatric speciation, recognized as theoretically possible, does occur in nature. The current scant evidence for sympatric speciation (4) may derive from investigators exploring complex ecologies [e.g., in cichlid studies in Africa and the Neotropics (9, 15)] rather than clear-cut settings of sharp ecological divergences and using the powerful tool of the new genomics. Skepticism about the high frequency of sympatric speciation (4) may be premature. Darwin’s insight regarding the prevalence of sympatric speciation in nature (1) may prove correct, not only in clear cases such as polyploidy but also in the sharp, local ecological contrasts prevailing in nature.
Conclusions and Prospects.
Our results demonstrate a genetic and phenotypic divergence in Spalax at a microsite. It encompasses sharply contrasting ecologies and suggest that this process of possible, or even probable, incipient ecological speciation may occur generally in small mammals. Strong edaphic selection is the only known evolutionary driving factor that can explain the divergence in mtDNA, as well as in nuclear AFLP discovered earlier, in Spalax populations living on different but adjacent soil types (27). Strong divergent natural selection can overrule gene flow caused by sharp ecological heterogeneity, as evidenced by interslope microclimatic divergence in Evolution Canyon (45), or by geological edaphic selection, as in the ALM–KBZ mole rats described here. Determining how frequent sympatric speciation actually is in nature will require future studies in both nuclear and mitochondrial population genomics associated with sharp ecological heterogeneity and geological or climatic divergence. The Evolution Canyon (40, 46) and ALM–KBZ models described here suggest that, as initially claimed by Darwin (1), sympatric speciation may be much more frequent in nature than generally believed. When the genomic revolution (52) permitted the analysis of whole genomes, genome-wide patterns of divergence ecological speciation became a focus of interest (7, 53). A major question is how individual speciation genes are arrayed within the genome and how this array affects speciation. An approach involving more integrative and theoretical work is warranted. An understanding of the evolutionary forces driving speciation will disentangle the roles of different processes such as natural selection, gene flow, and the recombination rate in speciation. More experimental work and sequencing plus theoretical studies are required in the future to enable a shift from descriptive to predictive studies that will highlight the causes and consequences of genome-wide patterns of speciation (7). We have just completed sequencing the Spalax genome from the basalt-dwelling populations; resequencing the genome from the Spalax chalk-dwelling population may highlight both adaptive and speciational genes selected by the rock, soil, and sharp vegetation divergences (43) within the same macroclimatic regime.
Materials and Methods
Contrasting Basalt and Chalk Habitats, Animals Examined, and Genetic Parameters Studied.
The blind mole rats were collected from a defined region in the Upper Eastern Galilee Mountains (Table S2), near the village of Rihaniya, on an Alma Pleistocene basalt plateau and Kerem Ben Zimra Senonian chalk (33°2.5′ N, 35°29.2' E, altitude 760 m) (54). The sample for genetic analysis contained 42 adult mole rats of the S. galili (2n = 52), collected in the edaphically divergent ALM–KBZ microsite and named accordingly (Figs. 1 and 2). The animals were captured live in the field during the wet seasons of 2000–2006 and were kept in the animal facilities at the Institute of Evolution, University of Haifa, Israel. All animals were treated in accordance with the Ethics committee, University of Haifa guidelines. Animals were grouped by population, which was determined a priori by the location and soil type of the capture site. The following segments of mtDNA were sequenced: (a) the mtDNA control region in all samples; (b) the mtDNA gene ATP6 sequenced in each of the mtDNA haplotypes found in the initial sequencing phase; (c) the complete mtDNA molecule (16,423 bp) in four of the most abundant haplotypes (GenBank accession numbers, JN571129–JN571132) (Tables S2 and S3). For details of DNA extractions, sequencing, bioinformatics software, and statistical analysis, see SI Materials and Methods.
Daily Activity Patterns.
Twenty mole rats (eight from KBZ and 12 from ALM) were radio-tracked in their natural burrow systems in January, 2012 to reveal differences between the inhabitants of the two soil types in the daily amount and patterns of outside-nest activity (see SI Materials and Methods for details) (55, 56).
Oxygen Consumption.
To measure oxygen consumption, the mole rat was weighed and then placed in a thermoregulated 29.5 °C (mole rat thermoneutrality) chamber. The top cover of the chamber was sealed, and the chamber was supplied with airflow at 680 mL/min (Table S4). At 5-min intervals the oxygen concentration and the activity of the animal were recorded. Animals were active initially; the resting level was recorded after the animal had been at rest for at least 20 min. Oxygen consumption was calculated as a mean after 20 min of rest and also after 5 min of minimal oxygen consumption (57).
Supplementary Material
Acknowledgments
We thank Dr. Josephine Todrank, Prof. Giora Heth, Prof. Dan Mishmar, and Dr. Imad Shams for comments and suggestions for improving the manuscript and Ema Hrouzková, Lucie Pleštilová, Miloš Vitámvás, Stephan Koeppen, Veronika Dvořáková, and Neta Massad for assistance with radio-tracking. This study was supported by the Grant Agency of Czech Republic, project P506/11/1512. Y.H. received scholarships from the Ministry of Science and Technology and the Council for Higher Education in Israel. E.N. received support from the Ancell Teicher Foundation for Genetics and Molecular Evolution.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. JN571129–JN571132]. Additional accession numbers are listed in Table S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222588110/-/DCSupplemental.
References
- 1.Darwin C. The Origin of Species by Means of Natural Selection, or the Preservation of Favored Races in the Struggle for Life (John Murray; London), reprinted (1977) New York: Modern Library; 1859. [Google Scholar]
- 2.Mayr E. Animal Species and Evolution. Cambridge, MA: Belknap; 1963. [Google Scholar]
- 3.Nevo E. Mosaic Evolution of Subterranean Mammals: Regression, Progression and Global Convergence. Oxford, UK: Oxford Univ Press; 1999. [Google Scholar]
- 4.Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- 5.Ayala FJ, Fitch WM. Genetics and the origin of species: An introduction. Proc Natl Acad Sci USA. 1997;94(15):7691–7697. doi: 10.1073/pnas.94.15.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton AR. (1998) Species and speciation: Geography, population structure, ecology, and gene trees. Endless Forms: Species and Speciation, eds Howard DJ, Berlocher SH (Oxford Univ Press, Oxford, UK), pp 32–43. [Google Scholar]
- 7.Nosil P, Feder JL. Genomic divergence during speciation: Causes and consequences. Philos Trans R Soc Lond B Biol Sci. 2012;367(1587):332–342. doi: 10.1098/rstb.2011.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulathinal R, Stevison L, Noor M. The genomics of speciation in Drosophila, and introgression estimated using low coverage genome sequencing. PLoS Genet. 2009;5:e1000550. doi: 10.1371/journal.pgen.1000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel AP, et al. Widespread genomic divergence during sympatric speciation. Proc Natl Acad Sci USA. 2010;107(21):9724–9729. doi: 10.1073/pnas.1000939107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan S, Elmer KR, Meyer A. Genomics of adaptation and speciation in cichlid fishes: Recent advances and analyses in African and Neotropical lineages. Philos Trans R Soc Lond B Biol Sci. 2012;367(1587):385–394. doi: 10.1098/rstb.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyle LC, Levine M, Stanton ML, Wright JW. Hybrid sterility over tens of meters between ecotypes adapted to serpentine and non-serpentine soils. Evol Biol. 2012;39(2):207–218. [Google Scholar]
- 12.Antonovics J. Evolution in closely adjacent plant populations X: Long-term persistence of prereproductive isolation at a mine boundary. Heredity (Edinb) 2006;97(1):33–37. doi: 10.1038/sj.hdy.6800835. [DOI] [PubMed] [Google Scholar]
- 13.Turner TL, Bourne EC, Von Wettberg EJ, Hu TT, Nuzhdin SV. Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat Genet. 2010;42(3):260–263. doi: 10.1038/ng.515. [DOI] [PubMed] [Google Scholar]
- 14.Martin CH. Weak disruptive selection and incomplete phenotypic divergence in two classic examples of sympatric speciation: Cameroon Crater Lake cichlids. Am Nat. 2012;180(4):E90–E109. doi: 10.1086/667586. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer J, Misof B, Schliewen UK. Speciation within genomic networks: A case study based on Steatocranus cichlids of the lower Congo rapids. J Evol Biol. 2012;25(1):138–148. doi: 10.1111/j.1420-9101.2011.02409.x. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick BM, Fordyce JA, Gavrilets S. What, if anything, is sympatric speciation? J Evol Biol. 2008;21(6):1452–1459. doi: 10.1111/j.1420-9101.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilets S. Perspective: Models of speciation: What have we learned in 40 years? Evolution. 2003;57(10):2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 18.Bird CE, Fernandez-Silva I, Skillings DJ, Toonen RJ. Sympatric speciation in the post “modern synthesis” era of evolutionary biology. Evol Biol. 2012;39(2):158–180. [Google Scholar]
- 19.Nevo E. Evolutionary theory and processes of active speciation and adaptive radiation in subterranean mole rats, Spalax ehrenbergi superspecies in Israel. Evol Biol. 1991;25:1–125. [Google Scholar]
- 20.Nevo E, Honeycutt RL, Yonekawa H, Nelson K, Hanzawa N. Mitochondrial DNA polymorphisms in subterranean mole-rats of the Spalax ehrenbergi superspecies in Israel, and its peripheral isolates. Mol Biol Evol. 1993;10(3):590–604. doi: 10.1093/oxfordjournals.molbev.a040026. [DOI] [PubMed] [Google Scholar]
- 21.Nevo E, Beiles A, Spradling T. Molecular evolution of cytochrome b of subterranean mole rats, Spalax ehrenbergi superspecies, in Israel. J Mol Evol. 1999;49(2):215–226. doi: 10.1007/pl00006544. [DOI] [PubMed] [Google Scholar]
- 22.Nevo E, Ivanitskaya E, Beles A. Adaptive Radiation of Blind Subterranean Mole Rats: Naming and Revisiting the Four Sibling Species of the spalax ehrenbergi superspecies in Israel: Spalax galili (2n=52), S. golani (2n=54), S. carmeli (2n=58) and S. judaei (2n=60) Leiden, The Netherlands: Bachkhuys Publishers; 2001. [Google Scholar]
- 23.Nevo E. Stress, adaptation, and speciation in the evolution of the blind mole rat, Spalax, in Israel. Mol Phylogenet Evol. 2012 doi: 10.1016/j.ympev.2012.09.008. Available at http://dx.doi.org/10.1016/j.ympev.2012.09.008. Accessed December 2012. [DOI] [PubMed] [Google Scholar]
- 24.Nevo E, Filippucci MG, Redi C, Korol AB, Beiles A. Chromosomal speciation and adaptive radiation of mole rats in Asia Minor correlated with increased ecological stress. Proc Natl Acad Sci USA. 1994;91(17):8160–8164. doi: 10.1073/pnas.91.17.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanitskaya E, Nevo E. Cytogenetics of mole rats of the Spalax ehrenbergi superspecies from Jordan (Spalacidae, Rodentia) Z Saugetierkd. 1998;63:336–346. [Google Scholar]
- 26.Nevo E, Ivanitskaya E, Filippucci MG, Beiles A. Speciation and adaptive radiation of subterranean mole rats, Spalax ehrenbergi superspecies, in Jordan. Biol J Linn Soc Lond. 2000;69:263–281. [Google Scholar]
- 27.Polyakov A, Beharav A, Avivi A, Nevo E. Mammalian microevolution in action: Adaptive edaphic genomic divergence in blind subterranean mole-rats. Proc Biol Sci. 2004;271(Suppl 4):S156–S159. doi: 10.1098/rsbl.2003.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzur S. 2007. Edaphic selection at a microsite on mtDNA polymorphism in blind mole rat, Spalax galili, Dalton, Israel. MSc Thesis, University of Haifa, Haifa, Israel.
- 29.Freund S. 2008. Swimming as an adaptive parameter (regionally and locally) in the life of Spalax ehrenbergi. MSc Thesis, University of Haifa, Haifa, Israel.
- 30.Hickman G, Nevo E, Heth G. Geographic variation in the swimming ability of Spalax ehrenbergi (Rodentia, Spalacidae) in Israel. J Biogeogr. 1983;10:29–36. [Google Scholar]
- 31.Grishkan I, Tsatskin A, Nevo E. Diversity of cultured microfungal communities in surface horizons of soils on different lithologies in Upper Galilee, Israel. Eur J Soil Biol. 2008;44(42):180–190. [Google Scholar]
- 32.Kruckeberg A. An essay: The stimulus of unusual geologies for plant speciation. Syst Bot. 1986;11(3):455–463. [Google Scholar]
- 33.Rajakaruna N. The edaphic factor in the origin of plant species. Int Geol Rev. 2004;46(5):471–478. [Google Scholar]
- 34.Pritchard JK, Wen X, Falush D. 2010. Documentation for Structure Software: Version 2.3 (Univ of Chicago Press, Chicago)
- 35.Hadid Y, et al. Is evolution of blind mole rats determined by climate oscillations? PLoS ONE. 2012;7(1):e30043. doi: 10.1371/journal.pone.0030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22(3):453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- 37.Heth G, Nevo E. Origin and evolution of ethological isolation in subterranean mole rats. Evolution. 1981;35:259–274. doi: 10.1111/j.1558-5646.1981.tb04885.x. [DOI] [PubMed] [Google Scholar]
- 38.Nevo E. Molecular evolution and ecological stress at global, regional and local scales: The Israeli perspective. J Exp Zool. 1998;282:95–119. [Google Scholar]
- 39.Nevo E, Beiles A. Genetic parallelism of protein polymorphism in nature: Ecological test of the neutral theory of molecular evolution. Biol J Linn Soc Lond. 1988;25:229–245. [Google Scholar]
- 40.Nevo E. 2009. Evolution in action across life at “Evolution Canyons,” Israel. Trends in Evolutionary Biology 1:e3.
- 41. The Atlas of Israel: Cartography, Physical and Human Geography (1985) (Survey of Israel, Tel Aviv)
- 42.Arieh S. The Soil of Israel. Berlin: Springer-Verlag; 2007. [Google Scholar]
- 43.Rabinovitch A. Parent Rock, Soil, and Vegetation in Galilee. Israel: Nature Reserve Authority, Kibuts Hameuhad; 1986. [Google Scholar]
- 44.Nevo E. Evolution of genome-phenome diversity under environmental stress. Proc Natl Acad Sci USA. 2001;98(11):6233–6240. doi: 10.1073/pnas.101109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nevo E. Selection overrules gene flow at “Evolution Canyons,” Israel. Advances in Genetics Research. 2011;5(3):67–89. [Google Scholar]
- 46.Nevo E. “Evolution Canyon”: A microcosm of life’s evolution focusing on adaptation and speciation. Israel Journal of Ecology & Evolution. 2006;52:485–506. [Google Scholar]
- 47.Tzur S, Todrank J, Juergens A, Nevo E, Heth G. Odour–genes covariance within a natural population of subterranean, Spalax galili blind mole rats. Bot J Linn Soc. 2009;96(3):483–490. [Google Scholar]
- 48.Bush G. The taxonomy, cytology and evolution of the genus Rhagoletis in North America (Diptera Tephritidae) Bulletin of The Museum of Comparative Zoology. 1996;134:431–562. [Google Scholar]
- 49.Nosil P. 2012. Degree of sympatry affects reinforcement in Drosophila. International Journal of Organic Evolution, 10.1111/j.1558-5646.2012.01817.x.
- 50.Forbes A, Powell THQ, Stelinski L, Smith J, Feder JL. 2009. Sequential sympatric speciation across trophic levels. Science 323:776–779. [DOI] [PubMed]
- 51.Catzeflis FM, Nevo E, Ahlquist JE, Sibley CG. Relationships of the chromosomal species in the Eurasian mole rats of the Spalax ehrenbergi group as determined by DNA-DNA hybridization, and an estimate of the spalacid-murid divergence time. J Mol Evol. 1989;29(3):223–232. doi: 10.1007/BF02100206. [DOI] [PubMed] [Google Scholar]
- 52.Schuster SC. Next-generation sequencing transforms today's biology. Nat Methods. 2008;5(1):16–18. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- 53.Schluter D. The Ecology of Adaptive Radiation. Oxford, UK: Oxford Univ Press; 2000. [Google Scholar]
- 54.Levitte D. 2001. Geological Map of Zefat 1:50,000 (Geological Survey of Israel, Jerusalem)
- 55.Šklíba J, Šumbera R, Chitaukali WN, Burda H. Determinants of daily activity patterns in a free-living afrotropical solitary subterranean rodent. J Mammal. 2007;88:1009–1016. [Google Scholar]
- 56.Šklíba J, Šumbera R, Chitaukali WN, Burda H. Home-range dynamics in a solitary subterranean rodent. Ethology. 2009;115:217–226. [Google Scholar]
- 57.Arieli R, AR A, Shkolnik A. Metabolic responses of a fossorial rodent (Spalax ehrenbergi) to simulated burrow conditions. Physiol Zool. 1977;50:61–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.