Abstract
Tim (Timeless) and Tipin (Tim-interacting protein) form a stable heterodimeric complex that influences checkpoint responses and replication fork progression. We report that the Tim-Tipin complex interacts with essential replication fork proteins and affects their biochemical properties. The Tim-Tipin complex, reconstituted and purified using the baculovirus expression system, interacts directly with Mcm complexes and inhibits the single-stranded DNA-dependent ATPase activities of the Mcm2-7 and Mcm4/6/7 complexes, the DNA unwinding activity of the Mcm4/6/7 complex, and the DNA unwinding and ATPase activity of Cdc45-Mcm2-7-GINS complex, the presumed replicative DNA helicase in eukaryotes. Although stable interactions between Tim-Tipin and DNA polymerases (pols) were not observed in immunoprecipitation experiments with purified proteins, Tim was shown to interact with DNA pols α, δ, and ɛ in cells. Furthermore, the Tim-Tipin complex significantly stimulated the pol activities of DNA pols α, δ, and ɛ in vitro. The effects of Tim-Tipin on the catalytic activities of the Mcm complexes and DNA pols are mediated by the Tim protein alone, and distinct regions of the Tim protein are responsible for the inhibition of Mcm complex activities and stimulation of DNA pols. These results suggest that the Tim-Tipin complex might play a role in coupling DNA unwinding and DNA synthesis by directly affecting the catalytic activities of replication fork proteins.
Keywords: replication checkpoint, fork movement, genome integrity
Accurate and complete duplication of genetic information is essential for the maintenance of genome integrity. Cells are susceptible to many intrinsic and environmental threats during DNA replication, and inadequate responses to replication stress can lead to increased chromosome abnormalities and cancer (1, 2). As a part of surveillance systems monitoring replication stress, the replisome-associated proteins Tim (Timeless) and Tipin (Tim-interacting protein) play important roles in both DNA replication and genome maintenance (3).
Tim and Tipin are conserved in eukaryotes, and orthologs have been identified in several organisms, from yeasts to humans. The yeast orthologs of Tim/Tipin are Tof1/Csm3 in Saccharomyces cerevisiae and Swi1/Swi3 in Schizosaccharomyces pombe. Tim is named after the TIMELESS gene that was isolated as a circadian clock gene in Drosophila melanogaster (4), and Tipin was identified as a Timeless interacting protein using the yeast two-hybrid assay (5). Although some studies have shown the requirement of mammalian Tim for circadian rhythm (6), recent studies in yeast and mammals suggest that Tim plays significant roles in DNA replication and replication checkpoints (3).
In yeast, Tof1 and Csm3 have been shown to be components of the replisome progression complex (7). Tof1 interacts with Mrc1 during S phase and is required for intra-S phase checkpoint responses, such as Rad53 activation, arrest of replication fork movement, and recovery after replication fork stalling (8). In S. pombe, Swi1 and Swi3 are components of a replication fork protection complex that stabilizes stalled replication forks, and are required for activation of the replication checkpoint kinase Cds1 (9). Mutant strains of swi1 and swi3 exhibit increased genome instability, including formation of high levels of ssDNA, strand breaks, and recombination (10). Swi1 and Swi3 are involved in sister chromatid cohesion as well (11).
In mammalian cells, Tim and Tipin also interact with protein components of the replisome, including Mcm2 and DNA polymerase (pol) δ (12), and are involved in Chk1 and Chk2 activation in response to DNA damage or stalling of replication (13, 14). Depletion of Tim reduces the rate of replication fork progression even in the absence of DNA damage, suggesting that Tim is important not only for replication fork protection and checkpoint response, but also for replication (13). Consistent with this observation, deletion of Tof1 in yeast cells was found to slow the progression of replication forks (15).
Recent studies have suggested that Tim and Tipin play important roles in coordinating the DNA unwinding and DNA synthesis activities of the replisome. Significant levels of ssDNA were found to accumulate in swi1 and swi3 mutant yeast cells (10). Similar effects have been reported with the in vitro DNA replication system using Tipin-depleted Xenopus egg extracts (16) and in Tim-Tipin–depleted human cells (17). Dissociation of components of the DNA unwinding complex from the site of DNA synthesis was observed in Tof1-depleted yeast cells (8). Collectively, these observations along with the replisome association of Tim-Tipin suggest that the Tim-Tipin complex may help couple the DNA helicase and DNA pol activities of the eukaryotic replisome. The mechanism by which these activities are coupled is unclear, however.
In this study, we purified Tim, Tipin and Tim-Tipin proteins and examined whether they affect the biochemical properties of the replicative DNA helicase and DNA pols. We found that the Tim-Tipin complex interacts with the Cdc45-Mcm2-7-GINS (CMG) complex and DNA pols and significantly affects their catalytic activities. Our results suggest that the Tim-Tipin complex might couple DNA unwinding and DNA synthesis by affecting the biochemical properties of these replisome proteins.
Results
Tim Interacts Directly with Mcm Complexes.
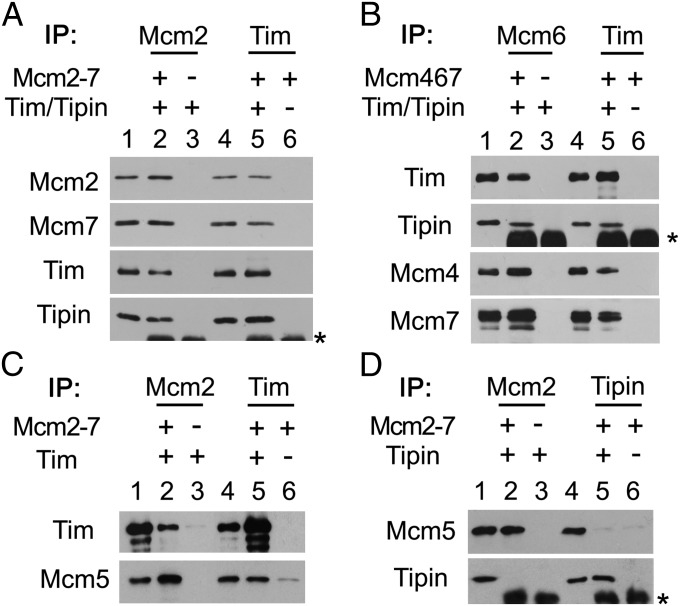
The Tim-Tipin complex was shown to associate with various replication fork proteins by immunoprecipitation experiments in mammalian cells (12). We examined whether the purified Tim and Tipin proteins interacted directly with purified Mcm complexes in vitro (Fig. 1). When equivalent amounts of Tim-Tipin and the Mcm2-7 (Fig. 1A) or Mcm4/6/7 complexes (Fig. 1B) were combined, immunoprecipitation of Mcm complexes with anti-Mcm2 or anti-Mcm6 antibodies resulted in the coprecipitation of the Tim-Tipin, and reciprocal immunoprecipitation with anti-Tim antibodies also precipitated the Mcm complexes. These results show that the Tim-Tipin interacted directly with the Mcm complexes presumably via one of the components of Mcm4, Mcm6, or Mcm7 proteins. Examining the interaction of Mcm complex with the Tim or Tipin protein alone, we found that immunoprecipitation of Tim precipitated the Mcm2-7 complex, and reciprocal immunoprecipitation of the Mcm2-7 complex also pulled down Tim (Fig. 1C). In contrast, Tipin showed no detectable interaction with the Mcm complexes (Fig. 1D). These results indicate that Tim is responsible for the interaction between the Tim-Tipin and Mcm complexes.
Fig. 1.
In vitro interactions of Tim-Tipin with Mcm complexes. Various combinations of purified proteins (5 pmol each), Mcm2-7 and Tim-Tipin (A), Mcm4/6/7 and Tim-Tipin (B), Mcm2-7 and Tim (C), or Mcm2-7 and Tipin (D), were combined and immunoprecipitated. Proteins precipitated by the indicated antibodies were resolved by SDS-9% PAGE, followed by Western blot analysis using the specified antibodies. Lanes 1 and 4 in each figure represent input materials (∼10%) for each immunoprecipitation. Asterisks indicate bands of Ig heavy chains.
Tim Directly Affects the Biochemical Activities of the Mcm Complexes.
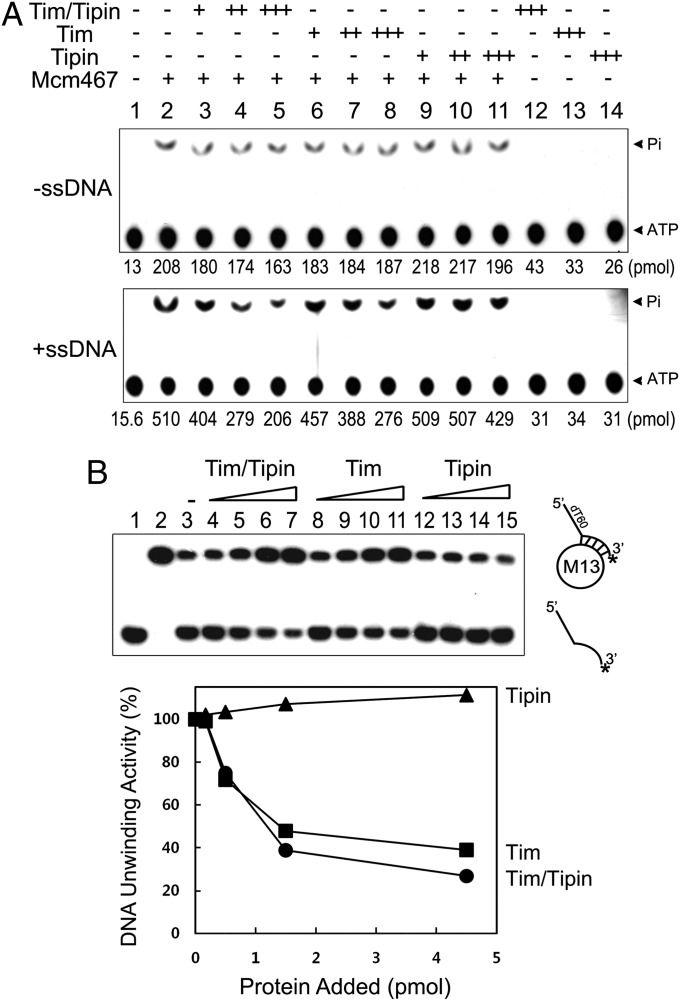
The Mcm complex is known to be a critical component of the replicative DNA helicase in eukaryotic cells (18, 19). We first examined whether the biochemical properties of Mcm4/6/7 complex were affected by the Tim-Tipin complex. As reported previously (20), the purified human Mcm4/6/7 complex has ATPase activity that is stimulated by ssDNA (Fig. 2A, lane 2) and DNA helicase activity (Fig. 2B, lane 3). The addition of the Tim-Tipin complex inhibited both ATPase (Fig. 2A) and DNA helicase (Fig. 2B) activities of the Mcm4/6/7 complex. Under the conditions used, the ATPase activity in the presence of ssDNA appeared to be affected more severely compared with that seen in the absence of ssDNA. The Tim protein alone also decreased the ATPase and DNA helicase activities of the Mcm 4/6/7 complex, whereas Tipin did not (Fig. 2). The purified human Mcm2-7 complex has weak ATPase activity (maximum rate of ATP hydrolysis ∼0.06 pmol/pmol/s), and this activity was also inhibited significantly by both the Tim-Tipin complex and the Tim protein alone (Fig. S1). Collectively, these results indicate that the Tim protein in the Tim-Tipin complex inhibits the enzymatic activities of Mcm complexes in vitro, and has the potential to inhibit the catalytic activity of the DNA unwinding complex that includes the Mcm complex in cells.
Fig. 2.
Influence of Tim-Tipin on biochemical properties of the Mcm4/6/7 complex. (A) The Mcm/4/6/7 complex (0.5 pmol) was preincubated with increasing amounts of Tim/Tipin, Tim, or Tipin (+, 0.33 pmol; ++, 1 pmol; +++, 3 pmol) at 4 °C for 2 h. The ATPase activity of each mixture was then measured. (B) (Upper) DNA helicase activity of Mcm4/6/7 complex (0.5 pmol) was measured in the presence of increasing levels of Tim-Tipin, Tim, or Tipin (0.25, 0.5, 1 and 2 pmol). Lane 1, boiled substrate; lane 2, no protein added; lane 3, Mcm4/6/7 alone; lanes 4–15, Mcm4/6/7 with Tim-Tipin, Tim, or Tipin proteins. (Lower) Quantitation of the duplex unwinding activity shown in the upper panel. Relative DNA unwinding activity was calculated as percent activity observed compared with the activity detected in the absence of additional proteins (shown in B, lane 3).
Tim Interacts Directly with CMG Complex and Inhibits Its Activity.
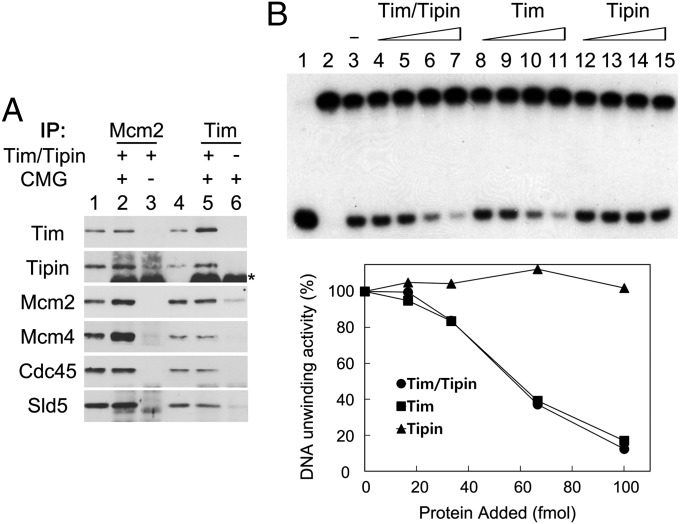
The Mcm complex is an essential component of the DNA unwinding complex during DNA replication, but the catalytic activity of the replicative DNA helicase requires formation of the CMG complex (19). The human CMG complex has been reconstituted using the baculovirus expression system and shown to have DNA helicase- and DNA-independent ATPase activities (21). Thus, we examined whether the Tim-Tipin complex interacts with and affects the biochemical properties of the CMG complex in vitro. When equivalent amounts of the purified Tim-Tipin and CMG complexes were mixed, these two complexes interacted stably in coimmunoprecipitation experiments, presumably through the interaction of Tim and Mcm proteins present in the CMG complex (Fig. 3A). The DNA helicase activity of CMG complex was significantly reduced by the addition of the Tim-Tipin complex or Tim protein, but not by the addition of Tipin (Fig. 3B), suggesting that Tim is responsible for the observed inhibited DNA helicase activity of the CMG complex. The inhibitory activity of Tim-Tipin complex appears to be specific for the CMG helicase activity, given that the Tim-Tipin complex did not affect the DNA helicase activity of SV-40 T-antigen (Fig. S2). The DNA-independent ATPase activity of CMG complex was also inhibited by the Tim-Tipin complex and by Tim (Fig. S3). These results suggest that the Tim-Tipin complex inhibits the catalytic activities of the CMG complex, and has a potential role during DNA replication by inhibiting the DNA unwinding activity of the CMG complex present at replication forks.
Fig. 3.
Interaction of Tim-Tipin with the CMG complex and its influence on the DNA helicase activity of the CMG complex. (A) Equivalent amounts of Tim-Tipin and CMG complexes (0.5 pmol) were combined and immunoprecipitated with antibodies against Mcm2 or Tim. Precipitated proteins were analyzed by Western blot analysis. Lanes 1 and 4 represent input materials (10%) for immunoprecipitation. Asterisks indicate bands of Ig heavy chains. (B) (Upper) DNA helicase activity of CMG complex (33 fmol) was measured after incubation in the absence or presence of increasing levels of Tim-Tipin, Tim, or Tipin (16.5, 33, 66, and 99 fmol) at 4 °C for 1 h. Lane 1, boiled substrate; lane 2, no protein added; lane 3, CMG alone; lanes 4–15, CMG with Tim-Tipin, Tim, or Tipin proteins. (Lower) Quantitation of the duplex unwinding activity shown in the upper panel. Relative DNA unwinding activity was calculated as percent activity observed compared with the activity detected with CMG complex alone (shown in B, lane 3).
Tim Interacts with Replicative DNA Pols and Stimulates Their Activities.
In previous studies, Tim-Tipin was shown to interact with replicative DNA pols in coimunoprecipitation experiments (12, 22). We examined whether Tim-Tipin interacts directly with replicative DNA pols. Initial coimmunoprecipitation experiments were carried out with purified proteins. Under the conditions used, however, no significant interactions between Tim-Tipin and DNA pol α, pol δ, or pol ɛ were detected. Interactions between the Tim-Tipin complex and the DNA pols might occur only transiently or perhaps may require other proteins for stabilization. Thus, we used bimolecular fluorescence complementation (BiFC) methods to analyze direct interactions in cells. In BiFC experiments, two proteins fused with the N-terminal or the C-terminal half of a fluorescence protein must associate with one another and remain in close proximity for the generation of a functional fluorescence signal (23). Our examination of interactions between Tim and the subunits of DNA pols (p68 subunit of pol α, p50 subunit of pol δ, and p17 subunit of pol ɛ) using BiFC revealed the production of fluorescence signals in each case (Fig. S4). When cells were treated with the CDK2 inhibitor NU6140 or with siRNA against Cdc7 kinase, no fluorescence signals were observed, although the expression levels of fusion proteins were similar to those detected in mock-treated cells (Fig. S4). These results indicate that the interactions observed in BiFC assays were specific and occurred only in S phase, which required both CDK and Cdc7 kinase activities. Taken together, these results suggest that Tim interacts directly with or is closely associated with the replicative DNA pols, presumably at replication forks.
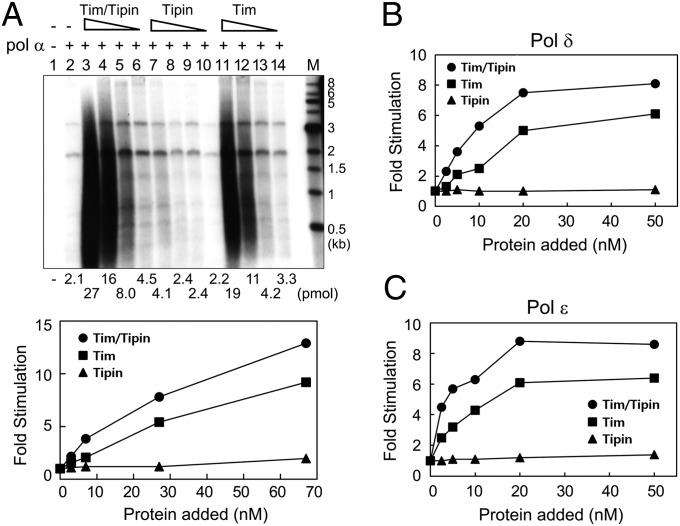
We next examined whether the catalytic activities of the DNA pols were affected by the Tim-Tipin complex. To evaluate the effect of Tim-Tipin on DNA pol α activity, we performed DNA pol activity assays using singly primed M13 ssDNA as the substrate. The addition of the Tim-Tipin complex significantly increased DNA synthesis catalyzed by DNA pol α, and the addition of the Tim protein alone also stimulated DNA pol α activity, albeit to a lower level than that seen with the Tim-Tipin complex (Fig. 4A). Under these conditions, the addition of 1 pmol of Tim-Tipin stimulated the incorporation of dNTPs by approximately 13-fold (Fig. 4A, lane 3); the addition of the same level of the Tim protein stimulated DNA synthesis by approximately ninefold (lane 11). Tipin alone had no significant effect on DNA pol α activity, however.
Fig. 4.
Influence of Tim-Tipin on the catalytic activities of DNA pols. (A) DNA synthesis catalyzed by the Pol α-primase was measured in the absence or presence of various concentrations of Tim-Tipin, Tim, or Tipin proteins (67, 27, 7, and 3 nM) using 5 fmol of singly primed M13 DNA as the substrate. After incubation at 37 °C for 30 min, aliquots were used to determine nucleotide incorporation (DEAE-cellulose paper adsorption) and size of products (1% alkaline-agarose gel electrophoresis). (B and C) DNA pol activities of pol δ (B) and pol ɛ (C) were examined in the absence or presence of Tim-Tipin, Tim or Tipin proteins (2.5, 5, 10, 30, and 50 nM) using the 89-mer TG annealed to 50-mer primer (5 pmol) as the substrate. Fold stimulation was calculated by dividing the activity observed in the presence of Tim- or Tipin-related proteins by the activity observed in the absence of these proteins.
The Tim-Tipin complex also stimulated the pol activities of pol δ (Fig. 4B) and pol ɛ (Fig. 4C). When a relatively short primed oligonucleotide template (90-mer TG annealed to 50-mer primer) was used as the substrate, both pol δ and pol ɛ activities were stimulated by the addition of the Tim-Tipin, and the maximum stimulation of pol δ and pol ɛ activities observed under our conditions was 8-fold and 8.5-fold, respectively. Tim alone also stimulated the pol activities of pol δ and pol ɛ approximately sixfold, whereas Tipin did not influence the activity of these enzymes.
Distinct Domains of Tim Are Responsible for Stimulation of DNA Pols and Inhibition of Mcm Complex.
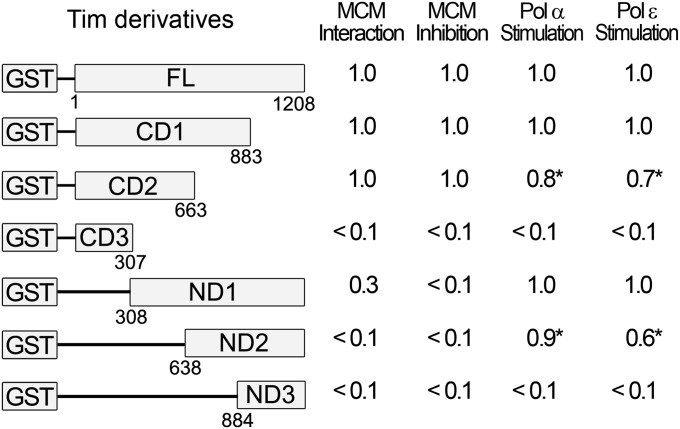
Regions in the Tim protein responsible for the stimulation of the DNA pols and the inhibition of the Mcm 4/6/7 helicase were examined. For this purpose, N-terminal as well as C-terminal truncated derivatives of Tim were prepared as GST-fusion proteins (Fig. S5), and their biochemical properties were examined. None of the N-terminal truncated proteins affected Mcm4/6/7 helicase activity, whereas the C-terminal truncated proteins CD1 and CD2 inhibited helicase activity (Fig. 5). The smallest truncated protein derivative that inhibited the Mcm helicase activity was CD2 (containing the N-terminal 663 amino acids). The fact that neither CD3 nor ND1 inhibited the activities associated with the Mcm complex indicates either that the complete CD2 region is required for Mcm inhibition or that the inhibitory domain resides near the border of the CD3 and ND1 proteins. The helicase inhibitory properties of the truncated Tim proteins were correlated with the strong interaction of these proteins with the Mcm4/6/7 complex (Fig. 5). When the individual GST-fused truncated Tim proteins were used as bait in pull-down assays with the Mcm4/6/7 complex, the Mcm complex was precipitated by the CD1 and CD2 proteins. These derivatives were effective inhibitors of the Mcm helicase activity. Although weak interactions between the Mcm complex and the ND1 protein were detected, these interactions did not lead to inhibited helicase activity of the Mcm complex.
Fig. 5.
C- and N-terminal truncations influence the biochemical properties of Tim. Tim and various truncated Tim derivatives were examined for their ability to interact with Mcm 4/6/7 (Mcm interaction), to inhibit DNA helicase activity of the Mcm 4/6/7 complex (Mcm inhibition), and to stimulate pol α and pol ɛ. These experiments were carried out as described in Figs. 1B, 2B, and 4 A and C, respectively. Each experiment was carried out using three concentrations of Tim or Tim derivatives and the data shown represent represent the average of three independent experiments. The activities detected with various Tim derivatives relative to that observed with full-length Tim are presented. The calculated SDs varied between ±0.02 and ±0.08, except for those values marked with an asterisk (SD ±0.2).
We also examined whether the truncated proteins stimulated the replicative DNA pols. The ND1, ND2, CD1, and CD2 derivatives stimulated the activity of pol α and pol ɛ, whereas the ND3 and CD3 derivatives were significantly less effective (Fig. 5). Given that both the ND2 and CD2 derivatives have stimulatory activity but ND3 or CD3 derivatives do not, the domains of the Tim protein responsible for pol stimulation appear to reside within the region of amino acids 308–883. Based on these analyses, we suggest that distinct regions of the Tim protein contribute to the inhibition of Mcm complex activities and the stimulation of DNA pols. Thus, Tim protein has the potential to interact simultaneously with both the CMG complex and DNA pols at replication forks and affect their catalytic properties.
Discussion
In many species, the Tim-Tipin complex plays important roles in S phase progression, stabilization of stalled replication forks, and checkpoint activation. Recent observations suggest that the Tim-Tipin complex is involved in coordinating both DNA unwinding and DNA synthesis activities of the replisome. These observations include the findings that ssDNA accumulates in Tim or Tipin-depleted cells from yeasts to human (10, 16, 17) and that components of the DNA unwinding complex dissociate from the site of DNA synthesis in Tof1-depleted yeast cells (8).
Here we report that the Tim-Tipin complex directly influences the biochemical properties of critical components of the replisome, consistent with the notion that the Tim-Tipin complex couples DNA unwinding and DNA synthesis. If these in vitro findings were to occur in vivo, Tim-Tipin could inhibit the DNA unwinding activity of the replisome as a negative regulator and prevent uncoordinated DNA unwinding during DNA replication. In addition, Tim-Tipin could interact with DNA pols and stimulate their pol activities. Although we could not examine the simultaneous interactions of the Tim-Tipin complex with both the Mcm complex and DNA pols, Tim-Tipin has the potential to influence both activities simultaneously, given that distinct domains are responsible for the inhibition and stimulation of the activities associated with the Mcm complex and DNA pols, respectively (Fig. 5).
Previous in vitro studies of the DNA helicase and DNA pol activities in the Escherichia coli replisome showed a slow rate of DNA unwinding catalyzed by DnaB alone (35 bp/s). However, interaction of DnaB with the Pol III holoenzyme (through the Tau subunit) increased the rate of DNA unwinding by approximately 20-fold (24). In contrast, replisome formation with the E. coli translesion DNA pols (pol II and pol IV) drastically reduced the unwinding rate of DnaB, suggesting that its helicase activity is regulated by the speed of DNA synthesis catalyzed by DNA pols (25). It is possible that the movement of the eukaryotic replisome is regulated as well. The coupling of the CMG helicase activity to the action of human pol ɛ led to the displacement of duplex regions greater than 10 kb, significantly longer than the duplex regions displaced by human CMG alone (up to 1 kb) (21).
In eukaryotes, the Tim-Tipin complex is a good candidate to functionally couple DNA unwinding and DNA synthesis activities. The reduced rate of replication fork progression in the absence of DNA damage observed in Tim-depleted cells (13) might be related to the lack of pol stimulation by Tim. If eukaryotic and prokaryotic replisomes behave in a similar manner, then the stimulation of DNA pol activity by Tim-Tipin could control the rate of translocation of the DNA unwinding complex. During normal replication fork progression, the inhibitory activity of Tim-Tipin on the CMG complex might not decrease the rate of fork progression under conditions in which the DNA pols stimulate the rate of DNA unwinding. However, when DNA synthesis is prevented by DNA damage or other stress insults, Tim-Tipin might stabilize stalled replication forks by inhibiting DNA unwinding activity. Consistent with this notion, we found that Tim-Tipin interacted stably with the CMG complex (Fig. 3), but not with the DNA pols in vitro.
In our experiements, Tim interacted with Mcm and CMG complexes, but Tipin did not. It has been suggested that both Tim and Tipin interact directly with Mcm proteins (26); however, that study examined interactions between Tim and Tipin with individual subunits of the Mcm2-7 complex in crude extracts, whereas we examined interactions of Tim and Tipin with Mcm and CMG complexes using purified proteins. Further studies are needed to determine whether interactions with individual subunits occur in the context of the Mcm2-7 or CMG complexes.
Previous studies have shown that Tipin also plays important roles in stabilizing stalled replication forks in yeast (10) and humans (16, 27). In the present study, Tim alone inhibited the Mcm complex and stimulated the DNA pols, whereas Tipin did neither. The role of Tipin in replication fork stabilization might be attributed in part to the finding that the stability of the Tim protein in cells is greatly decreased in Tipin depleted cells (27). Thus, Tipin depletion also may lead to instability of stalled replication forks by indirectly affecting the level of the Tim protein in human cells. However, Tim and Tipin also have been shown to be essential for Chk1 phosphorylation in response to DNA damage and replication stress, and this role cannot be attributed to the influence of Tim on the CMG complex or DNA pols. Tipin was shown to be required for the chromatin binding of Claspin (16), and its interaction with RPA appears to be required for Chk1 phosphorylation (28). Thus, Tim and Tipin clearly play additional roles in response to genotoxic stress that are independent of their influence on the CMG complex and DNA pols.
How the Tim-Tipin complex affects the biochemical properties of the Mcm complexes and DNA pols is not clear. One possibility is that the Tim-Tipin complex affects these activities by interacting with DNA. Indeed, the Tim-Tipin complex, as well as the Tim protein, exhibited weak DNA-binding activity under our experimental conditions (Fig. S6). However, our examination of the DNA-binding activities of truncated Tim derivatives revealed no correlation between DNA-binding activity and effects on DNA pols or Mcm4/6/7 helicase activity (Fig. S7). The effects of the Tim-Tipin complex on Mcm complexes appeared to be related solely to protein–protein interactions. In our experiments, interactions between Tim and replicative pols were observed only in cells, and depended on the presence of CDK and Cdc7 kinase activities (Fig. S4). Given that the Tim-Tipin complex is a component of the replication fork, and that formation of replication forks requires CDK and Cdc7, the dependence on these kinases might indicate that replication forks are essential for the observed interactions. However, further studies are needed to determine whether these kinases influence the properties of these proteins.
Materials and Methods
Cell Culture and Reagents, Preparation of Recombinant Proteins, Immunoprecipitation, and BiFC Assays.
Purification of recombinant proteins and other methods are described in SI Materials and Methods.
DNA Helicase and ATPase Activity Assays.
For the preparation of the substrate used in the helicase activity assay, an 80-mer oligonucleotide, 5′-T60GTTTTCCCAGTCACGACGTT-3′ was synthesized and annealed to M13mp18 ssDNA. After the 3′ end of the annealed 80-mer DNA was labeled with [α-32P] dGTP and the Klenow fragment, the labeled substrate was purified by spin-column chromatography with Sephacryl S-400 (GE Healthcare). DNA helicase and ATPase activities of Mcm and CMG complexes were measured as described previously (21).
DNA Replication Assays.
The activity of DNA pol α was measured using singly primed M13 mp18 as the substrate, and the activities of DNA pol δ and ɛ were measured using singly primed M13 or primed oligonucleotides (89-mer TG40 repeat) substrates, as described previously (29).
Supplementary Material
Acknowledgments
This research was supported by National Research Foundation of Korea Grants KRF-2007-C00522 and 2011-0015907 (funded by the Ministry of Education, Science, and Technology), and by National Institutes of Health Grant GMS R01 GM034559.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222494110/-/DCSupplemental.
References
- 1.Kolodner RD, Putnam CD, Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002;297(5581):552–557. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- 2.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane RJ, Mian S, Dalgaard JZ. The many facets of the Tim-Tipin protein families’ roles in chromosome biology. Cell Cycle. 2010;9(4):700–705. doi: 10.4161/cc.9.4.10676. [DOI] [PubMed] [Google Scholar]
- 4.Myers MP, Wager-Smith K, Wesley CS, Young MW, Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995;270(5237):805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 5.Gotter AL. Tipin, a novel timeless-interacting protein, is developmentally co-expressed with timeless and disrupts its self-association. J Mol Biol. 2003;331(1):167–176. doi: 10.1016/s0022-2836(03)00633-8. [DOI] [PubMed] [Google Scholar]
- 6.Barnes JW, et al. Requirement of mammalian Timeless for circadian rhythmicity. Science. 2003;302(5644):439–442. doi: 10.1126/science.1086593. [DOI] [PubMed] [Google Scholar]
- 7.Gambus A, et al. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol. 2006;8(4):358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 8.Katou Y, et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424(6952):1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi E, Noguchi C, McDonald WH, Yates JR, 3rd, Russell P. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol Cell Biol. 2004;24(19):8342–8355. doi: 10.1128/MCB.24.19.8342-8355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommariva E, et al. Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage. Mol Cell Biol. 2005;25(7):2770–2784. doi: 10.1128/MCB.25.7.2770-2784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansbach AB, et al. RFCCtf18 and the Swi1-Swi3 complex function in separate and redundant pathways required for the stabilization of replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe. Mol Biol Cell. 2008;19(2):595–607. doi: 10.1091/mbc.E07-06-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotter AL, Suppa C, Emanuel BS. Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork-associated factors. J Mol Biol. 2007;366(1):36–52. doi: 10.1016/j.jmb.2006.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unsal-Kaçmaz K, et al. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27(8):3131–3142. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Wood PA, Hrushesky WJ. Mammalian TIMELESS is required for ATM-dependent CHK2 activation and G2/M checkpoint control. J Biol Chem. 2010;285(5):3030–3034. doi: 10.1074/jbc.M109.050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tourrière H, Versini G, Cordón-Preciado V, Alabert C, Pasero P. Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol Cell. 2005;19(5):699–706. doi: 10.1016/j.molcel.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Errico A, Costanzo V, Hunt T. Tipin is required for stalled replication forks to resume DNA replication after removal of aphidicolin in Xenopus egg extracts. Proc Natl Acad Sci USA. 2007;104(38):14929–14934. doi: 10.1073/pnas.0706347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KD, Fu MA, Brown EJ. Tim-Tipin dysfunction creates an indispensible reliance on the ATR-Chk1 pathway for continued DNA synthesis. J Cell Biol. 2009;187(1):15–23. doi: 10.1083/jcb.200905006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labib K, Tercero JA, Diffley JF. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000;288(5471):1643–1647. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- 19.Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci USA. 2006;103(27):10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You Z, Masai H. DNA binding and helicase actions of mouse MCM4/6/7 helicase. Nucleic Acids Res. 2005;33(9):3033–3047. doi: 10.1093/nar/gki607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J. Properties of the human Cdc45/Mcm2-7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci USA. 2012;109(16):6042–6047. doi: 10.1073/pnas.1203734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Errico A, et al. Tipin/Tim1/And1 protein complex promotes Pol alpha chromatin binding and sister chromatid cohesion. EMBO J. 2009;28(23):3681–3692. doi: 10.1038/emboj.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerppola TK. Visualization of molecular interactions using bimolecular fluorescence complementation analysis: characteristics of protein fragment complementation. Chem Soc Rev. 2009;38(10):2876–2886. doi: 10.1039/b909638h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Dallmann HG, McHenry CS, Marians KJ. Coupling of a replicative polymerase and helicase: A tau-DnaB interaction mediates rapid replication fork movement. Cell. 1996;84(4):643–650. doi: 10.1016/s0092-8674(00)81039-9. [DOI] [PubMed] [Google Scholar]
- 25.Indiani C, Langston LD, Yurieva O, Goodman MF, O’Donnell M. Translesion DNA polymerases remodel the replisome and alter the speed of the replicative helicase. Proc Natl Acad Sci USA. 2009;106(15):6031–6038. doi: 10.1073/pnas.0901403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Numata Y, Ishihara S, Hasegawa N, Nozaki N, Ishimi Y. Interaction of human MCM2-7 proteins with TIM, TIPIN and Rb. J Biochem. 2010;147(6):917–927. doi: 10.1093/jb/mvq028. [DOI] [PubMed] [Google Scholar]
- 27.Yoshizawa-Sugata N, Masai H. Human Tim/Timeless-interacting protein, Tipin, is required for efficient progression of S phase and DNA replication checkpoint. J Biol Chem. 2007;282(4):2729–2740. doi: 10.1074/jbc.M605596200. [DOI] [PubMed] [Google Scholar]
- 28.Kemp MG, et al. Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress. J Biol Chem. 2010;285(22):16562–16571. doi: 10.1074/jbc.M110.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bermudez VP, Farina A, Tappin I, Hurwitz J. Influence of the human cohesion establishment factor Ctf4/AND-1 on DNA replication. J Biol Chem. 2010;285(13):9493–9505. doi: 10.1074/jbc.M109.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.