Abstract
Leaf-wax n-alkanes 2H/1H ratios are widely used as a proxy in climate reconstruction. Although the broad nature of the relationship between n-alkanes δ2H values and climate is appreciated, the quantitative details of the proxy remain elusive. To examine these details under natural environmental conditions, we studied a riparian broadleaf angiosperm species, Populus angustifolia, growing on water with a constant δ2H value and monitored the δ2H values of leaf-wax n-alkanes and of stem, leaf, stream, and atmospheric waters throughout the entire growing season. Here we found the δ2H values of leaf-wax n-alkanes recorded only a 2-wk period during leaf flush and did not vary for the 19 weeks thereafter when leaves remained active. We found δ2H values of leaf-wax n-alkanes of P. angustifolia record conditions earlier in the season rather than fully integrating the entire growing season. Using these data, we modeled precipitation δ2H values during the time of wax synthesis. We observed that the isotope ratios of this precipitation generally were 2H-enriched compared with mean annual precipitation. This model provides a mechanistic basis of the often-observed 2H-enrichment from the expected fractionation values in studies of broadleaf angiosperm leaf-wax δ2H. In addition, these findings may have implications for the spatial and temporal uses of n-alkane δ2H values in paleoapplications; when both plant community and growth form are known, this study allows the isolation of the precipitation dynamics of individual periods of the growing season.
Keywords: biomarkers, compound-specific isotope analysis, geographic information system, isoscape, stable isotopes
Stable isotopes serve as tracers and integrators of both environmental and physiological signals within plant materials. Terrestrial plant leaf waxes (i.e., n-alkanes, n-acids, and others) and their isotope ratios have attracted much interest as a higher plant-specific biomarker for paleoclimate reconstruction (1), because these compounds are easily identified and isolated from a variety of geologic materials and are relatively robust to geologic alteration (2). The environmental information recorded in hydrogen isotope ratios of leaf waxes have become a prevalent proxy to reconstruct ancient climates (3–5), mountain building events (6, 7), and floral transitions (8). However, critical questions related to the interpretation of ancient higher plant biomarkers remain unanswered, especially with respect to the extent of the leaf life cycle recorded by this proxy.
Environmental and climate transect studies have shown that δ2H values of higher plant n-alkanes and environmental waters are correlated (9, 10), but temporal observations of δ2H values of n-alkanes have produced conflicting data on the nature of this relationship (11–15) that require clarification to elucidate how δ2H values of ancient n-alkanes can be interpreted. To reconcile these issues and to provide the critical constraints for climate reconstructions using δ2H values of n-alkanes, carefully designed and controlled biologic experiments are needed that consider plant physiology and phenology (1). In addition, biological experiments also must take into account how leaf-wax n-alkanes are mixed and transported into geologic materials and become climate archives. The issue then becomes how to make highly controlled greenhouse experiments comparable to natural ecosystem experiments, where environmental parameters are less defined, and then how to expand these results to geologic settings where very little information about a plant’s environment is known.
To combine the control of a greenhouse experiment with the natural variability of ecosystem trials, we studied a common, riparian broadleaf angiosperm species, Populus angustifolia, in its native environment (Big Cottonwood Canyon, UT) exposed to soil water with a naturally constant isotope ratio. The use of a native riparian species under a constant soil water δ2H value provides an ideal test to separate the effects of source and leaf water 2H/1H variation on δ2H values of n-alkanes in natural, real-world conditions. Here, we measured the hydrogen isotope ratios of leaf-wax n-alkanes (δ2Hn-alkane) and stem (δ2Hxylem), leaf (δ2Hleaf), stream (δ2Hstream), and atmospheric (δ2Hatmo) waters throughout the growing season from the bud phase to leaf senescence. In this study, we addressed when leaf waxes of broadleaf angiosperms are formed and if and how δ2H values of modern deciduous tree leaf-wax n-alkanes vary after the time of wax synthesis. We then expanded our findings to larger regional scales to provide a framework to interpret other modern ecosystem δ2Hn-alkane data. This experiment on plants living in an environment with a naturally constant water source but with dynamic humidity through the growing season informs the interpretation of modern and ancient n-alkane δ2H datasets.
Results
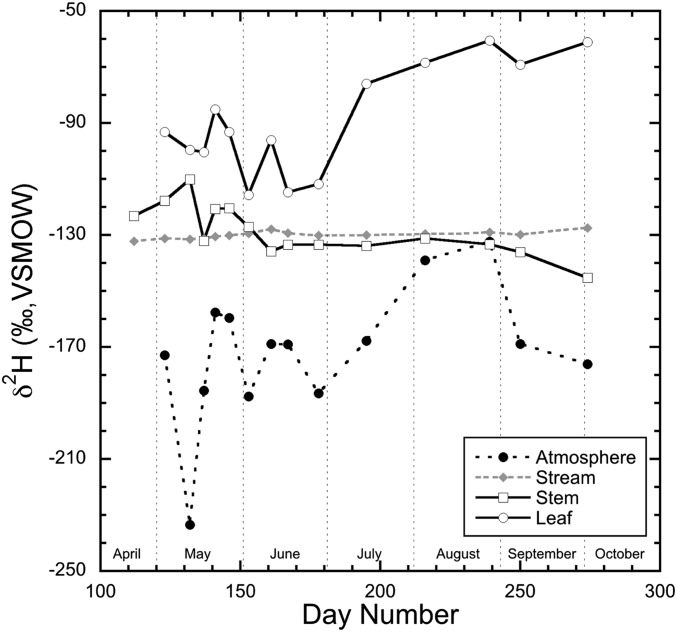
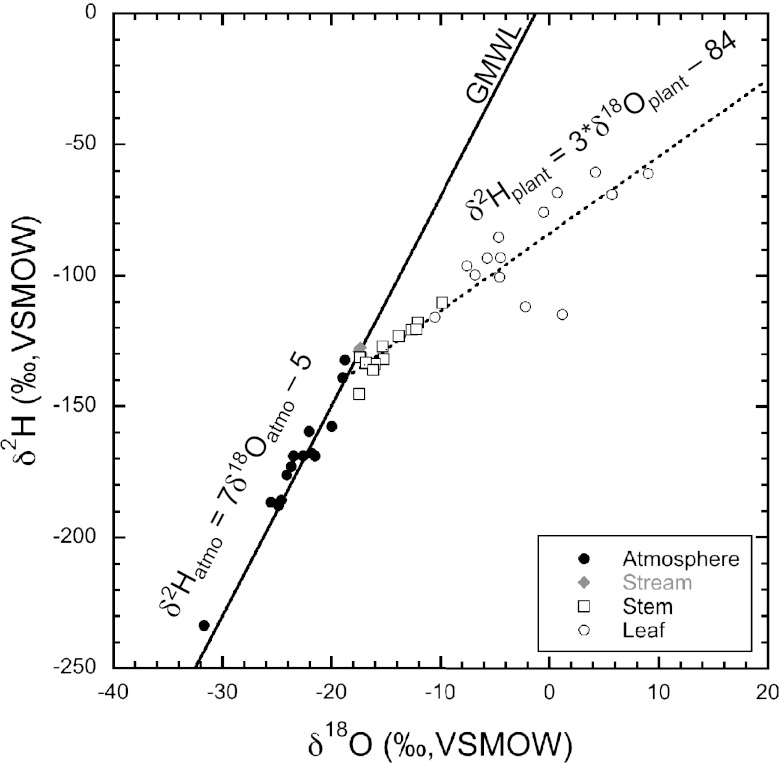
We found δ2H values of stream water did not change (−130 ± 1‰; all SDs are reported at 1σ) throughout the study interval (Fig. 1). Earlier Roden and Ehleringer (16) established that stream δ2H values within Big Cottonwood Creek remained relatively static because melting winter snow pack contributed the majority of the creek water. Throughout the study interval, the δ2H values of atmospheric water vapor varied from −233 to −132‰, with more 2H-enriched values later in the growing season after leaf expansion. Atmospheric water shows nearly 100‰ variation in δ2H value throughout the season (Fig. 1). This variation likely reflected different air masses and atmospheric conditions in the region, because all atmospheric water samples fall on the Global Meteoric Water Line (Fig. 2).
Fig. 1.
Hydrogen isotope variations of atmospheric water vapor, stream water, stem water, and leaf water during the study interval.
Fig. 2.
Cross-plot of hydrogen and oxygen isotope values of atmospheric water vapor, stream water, stem water, and leaf water. The Global Meteoric Water Line (GMWL) is shown by the solid black line (δ2H = 8*δ18O − 10). Regressions between the hydrogen and oxygen isotope values of atmospheric water vapor (δ2Hatmo) and plant waters (i.e., stem and leaf waters, δ2Hplant) are shown by the dotted and dashed lines, respectively, and are described as δ2Hatmo = 7*δ18Oatmo − 5 (R2 = 0.95) and δ2Hplant = 3*δ18Oplant − 84 (R2 = 0.85), respectively.
To reduce sun/shade variations and to focus on whole plant–water–wax interactions throughout the 2010 season, we homogenized leaves into a single sample from the four cardinal points around the tree, taking particular care to sample only mature leaves from the initial leaf flush. Bud break occurred shortly after day 146, with leaf expansion occurring between days 153 and 161 (Fig. 3). Leaf-water δ2H values ranged from −61 to −116‰. Bud water showed little difference before leaf flush (±7 ‰), followed by large variations in the δ2H value of leaf waters that indicated a strong positive correlation with atmospheric water vapor (R2 = 0.77) until September (Fig. 1).
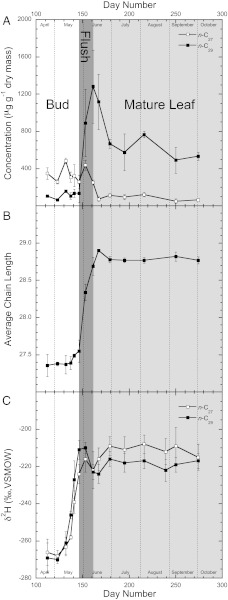
Fig. 3.
(A) Concentration of n-C27 and n-C29 normal alkanes on buds and leaves of the narrowleaf cottonwood (P. angustifolia). White, dark gray, and light gray colors represent the bud, leaf-flush, and mature-leaf phases, respectively. Error bars represent SDs around the average of the three individual trees used in this study. (B) Average n-alkane chain length of bud and leaf waxes across the studied interval. Error bars represent SDs around the average of the three individual trees used in this study. (C) Hydrogen isotope values of bud and leaf-wax n-alkanes from P. angustifolia. Error bars represent the SDs of all analyses of an individual compound from the sampled day.
Stem-water δ2H values ranged from −110 to −145‰ and showed more 2H-enriched values in the initial growing season before leaf expansion (before day 153). After leaf expansion, δ2H values of stem and stream waters were similar (−135 ± 5‰), suggesting that stream water was the dominant source water for the plants. During leaf expansion the δ2H value of stem waters became more 2H-depleted, and after expansion δ2H values remained steady through the remainder of the study interval (Fig. 1). This pattern in stem-water δ2H values has been observed previously in species common to the Intermountain West of the United States (17) and has been explained as increased transpirational flux during bud swelling and leaf flush as residual xylem water from the previous growing season is incorporated. Throughout the previous winter this residual xylem water can be subjected to evaporation that can enrich the xylem water significantly in 2H (17). Although this pattern of 2H enrichment of xylem water in winter is common in the arid western United States (17), regions with milder and more humid winters may not be affected as significantly. Nonetheless, this phenomenon may influence the δ2H values of stem water during the period of leaf flush and thus may influence leaf-wax δ2H values during this interval.
Bud and leaf-wax n-alkanes were extracted from three mature P. angustifolia individuals located on the stream edge and were found to be composed predominately of n-C27 and n-C29 (Fig. 3A). In all individuals, the average chain length changed systematically from 27.4 ± 0.2 to 28.8 ± 0.1, corresponding with changes from bud to leaf-flush and finally to leaf-expansion phases (Fig. 3B). After the initial development of leaf wax, the concentrations of n-C27 and n-C29 n-alkanes decreased and then stabilized for the remainder of the growing season. From bud to leaf, the δ2H values of n-C27 and n-C29 from the individual trees changed from −268 ± 4‰ and −268 ± 8‰, respectively, to −216 ± 8‰ and −215 ± 8‰, respectively, (Fig. 3C).
Discussion
Wax Distributions and Isotope Ratios Reflect Leaf-Expansion Phase.
We found distinct patterns in the n-alkane distributions and δ2H values of field-grown P. angustifolia leaf waxes during specific phenologic intervals. In particular, we found a distinct n-alkane distribution and δ2H value during the bud and leaf-expansion phases and a molecular and isotopic transition between these phases during the leaf-flush period. Although we observed a general coherence between n-alkane distribution and δ2H variations, we noted subtle differences in timing of the initiation of these variations (Fig. 3). Specifically, we found the concentration of n-C29 and the average chain length of P. angustifolia leaf-wax n-alkanes began to increase after day 146, during the leaf-flush interval (Fig. 3 A and B). In comparison, Fig. 2C shows that δ2H values of these same n-alkanes become more 2H-enriched after day 123, more than 3 wk before the beginning of the leaf-flush interval and the wax-development period. Furthermore, the δ2H values of the n-alkanes reached their most 2H-enriched and steady δ2H value before the increase in the concentration of n-C29 and in the average chain length. These differences in timing indicate that before wax development on the leaf surface, new waxes are synthesized in or on the bud before bud break and leaf flush and that these new bud waxes have an isotope ratio very similar to the waxes that are deposited on the leaf surface at leaf flush. These data suggest that the bud waxes are produced near the end of the previous growing season with stem water and are carried over the winter. Here in P. angustifolia, new leaf-wax n-alkanes are produced during the bud-swelling phase of leaf development, preceding bud break. During bud swelling, new waxes are produced in limited amounts with the influx of water and stored sugars.
Nonetheless, we found that, after the leaf expanded, leaf-wax n-alkane distributions and δ2H values of field-grown P. angustifolia trees did not vary further, even though changes in leaf water occurred in response to changes in the environment. Specifically, n-C27 and n-C29 δ2H values remained constant from day 146–274 and 161–274, respectively [(F1, 14 = 131.6 and F1, 14 = 134.6 for n-C27 and n-C29 δ2H values, respectively; Tukey–Kramer honestly significant difference (HSD) α = 0.001]. There are several possible explanations for this observed pattern: (i) leaf waxes are continually produced or reworked from an H-source that does not vary isotopically during the growing season; or (ii) leaf waxes are produced from an H-pool only at leaf flush and remain unaltered for the remainder of the growing season. Here we found δ2H values of stem water remained nearly constant during the growing season, although large variations in the δ2H values of leaf water were observed (Fig. 1). Although the δ2H values of stem water remain constant, it can be ruled out as the direct H-source, because isotope-tracer experiments have shown that the hydrogen isotope ratios of leaf waxes derive from leaf-water pools (15). Furthermore, the hydrogen isotope ratios of leaf water vary throughout the growing season (Fig. 1), and thus we would have expected leaf-wax δ2H values to vary if waxes were produced continually from leaf-water H-sources after leaf expansion. We did not observe this pattern, and these data suggest that P. angustifolia leaf waxes are not reworked once they are developed, or, if they are reworked, that the isotope ratios are not altered significantly (Fig. 3C). These data indicate that the interval during which the leaf flushes and waxes develop is the only period throughout the growing season when environmental conditions can affect leaf-wax δ2H values (Fig. 3). During this interval, leaf water and resulting leaf waxes would be subject to the effects of humidity and atmospheric water vapor, as suggested by previous workers (11, 13), and should be related to these parameters. Nonetheless, the net biosynthetic fractionation (ɛ) between leaf water and n-C27 and n-C29 n-alkanes was −123 ± 14‰, consistent with previous estimates (18). Also, we found the calculated apparent fractionation between the δ2H values to leaf waxes and leaf water varied throughout the growing season, not because of changes in leaf-wax δ2H values but rather because of variations in leaf-water δ2H values, in contrast to previous suggestions (13). Together these data suggest that the δ2H values of P. angustifolia n-alkane leaf waxes record only a finite period during the early growing season and do not record variations in local climate either before or after the development of leaf wax.
In Deciduous Species δ2H Values Reflect Plant Environment only at the Time of Wax Synthesis.
At the ecosystem level, plant communities can be composed of species with various growth habits (i.e., herb, grass, shrub, tree) or dominated largely by a single growth form. Although different growth forms may have distinct phenological characteristics, leaf development and wax formation can be characterized into two categories: continuous and finite growth. Grasses have intercalary meristems that allow continuous leaf-blade expansion and wax development throughout the day and lifetime of the leaf. Both controlled greenhouse experiments (14) and field experiments (19, 20) support that the idea that the δ2Hn-alkane values of grass leaf-wax n-alkanes reflect a continuous growth process. These studies show that the δ2Hn-alkane values of grasses vary with the δ2H values of source and leaf water (14, 19, 20). This pattern supports the hypothesis that the δ2Hn-alkane values of grass leaf waxes reflect the leaf-water environment during leaf expansion.
In contrast to grasses, broadleaf deciduous species form leaf cuticle during the brief period of leaf expansion, which may last 5–20 d, with the majority of leaf-wax formation subsiding after leaf expansion (21–25). Botanical information predicts and multiple empirical studies support the notion that isotope ratios of leaf waxes in trees, shrubs, and herbs remain relatively constant throughout the lifetime of a leaf. Both greenhouse (15, 26) and field (12) experiments support the hypothesis that δ2Hn-alkane values of tree leaf waxes develop by a determinate process. Our findings support these previous studies, because we find that the interval during which the leaf flushes and waxes develop is the only period when the external environment affects the δ2H values of leaf waxes. Nonetheless, studies indicate that cuticular waxes of broadleaf angiosperms (27) and their δ2Hn-alkane values may vary during a growing season (13, 28, 29). In these latter cases, the variation often is explained as reflecting short-term changes in the isotopic composition of leaf water and soil water. Although it is clear that some angiosperm and gymnosperm species may rework leaf waxes under specific environmental stresses (26, 30), the isotopic variations observed in previous naturally grown deciduous angiosperm datasets also could have been caused by mixed sampling of mature and secondary lammas leaves. Also discrepancies between previous datasets and this work may be a function of sampling a combination of sun and shade leaves as well as sampling a variety of trees tapping different soil water sources.
Although this study focused on a single deciduous species, P. angustifolia shares the leaf phenologic characteristics of other woody temperate species throughout North America; that is, leaf flush occurs primarily early in the growing season (31, 32). When scaled to an ecosystem level, these data suggest that the environmental signal in δ2H values from deciduous species leaf waxes would be weighted toward the early growing season and would record environmental conditions only during that time. For deciduous species in different biomes, the growing season may be quite different. Consider, for example, vegetation in tropical monsoonal climates, which flush as precipitation arrives once or twice a year, or in a seasonal tropical rainforest that receives precipitation throughout the year (31). Thus, we surmise that δ2H records of deciduous leaf waxes from contrasting biomes may record the leaf-water environment in very different seasonal phases of the annual water cycle.
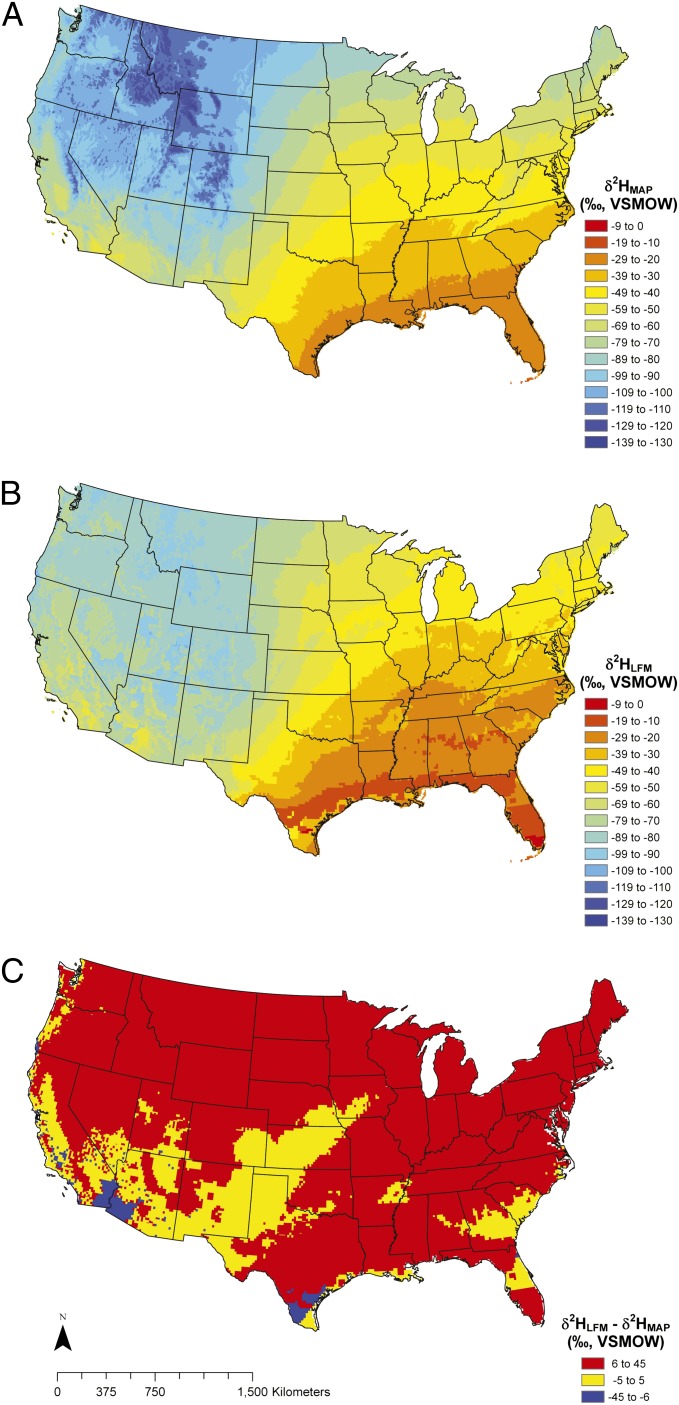
Nonetheless, in this case, as with most midlatitude broadleaf angiosperms, leaf-wax formation occurs in the early spring and thus reflects the leaf-water environment of that period. Here we construct a δ2H isotopic landscape model (an isoscape) that expresses the δ2H value of precipitation that falls during the month following the last frost (Fig. 4B). Because bud break and leaf-wax development of broadleaf angiosperms typically occurs only after the last frost, this biologically defined model provides a base layer for comparing leaf-wax δ2H records extracted from modern deciduous plants. Although we predict that δ2H values of precipitation will display a geospatial pattern similar to the mean annual δ2H values, we find the precipitation that falls during the month of the last freeze, when we determined that plant synthesize leaf waxes, is more 2H-enriched than the mean annual δ2H value throughout the majority of the United States (red regions in Fig. 4C).
Fig. 4.
(A) Interpolated δ2H values of mean annual precipitation (MAP). (B) Interpolated δ2H values of precipitation in the last freeze month (LFM). Individual LFM precipitation δ2H values were extracted from www.waterisotopes.org. The month of the last freeze was defined as the median date of the last 0 °C. (C) The difference between the δ2H values of LFM precipitation and MAP. Red and blue regions show positive and negative anomalies, respectively; yellow regions have similar LFM and MAP precipitation δ2H values. Both LFM and MAP precipitation δ2H values have a 0.083° grid cell resolution with a 95% confidence interval of 4‰ (46).
Differences in modeled precipitation δ2H values are not unexpected; however, mean annual precipitation δ2H values are used commonly to model leaf-wax δ2H values from plants (19, 33–35) and sediments (9, 36). Often when mean annual precipitation δ2H values are used to calculate the apparent fractionation between the δ2H values of leaf wax and source waters, the resulting fractionation is smaller than expected (i.e., leaf waxes are more enriched in 2H than predicted). These studies place significant interpretation on variations in plant form and seasonal relative humidity that require the changes in the δ2H value of leaf water to explain these fractionations. Although certainly it is true that in some cases humidity affects leaf-water (37–41) and leaf-wax δ2H values at the time of synthesis (11, 13), the disconnect between precipitation δ2H values and botanical studies of leaf-wax δ2H values also may be explained, in part, by the timing of wax synthesis. These precipitation isoscape models indicate that variations in humidity and leaf-water δ2H values may not be needed in all cases to reconcile these apparent fractionations. For example, Hou et al. (42) found leaf-wax δ2H values varied by nearly 70‰ at a single locality in Massachusetts, with C3 grasses more depleted in 2H than other functional types (i.e., trees, herbs, and shrubs). In this case, differences in physiology between C3 dicots and monocots were suggested to be the controlling factor of apparent fractionation. In a recent review, Sachse et al. (1) found similar relationships across a much larger dataset and suggested that rooting depth, leaf architecture, plant form, and biochemistry control leaf-water and leaf-wax δ2H values. Although these studies and others (19, 33–35, 42, 43) show that plant physiology, biochemistry, rooting depth, and growth habit clearly affect leaf-water and leaf-wax δ2H values at the time of synthesis, it also may be important to consider the interval of time at which wax synthesis takes place when comparing n-alkane δ2H values from different plant functional groups. Given that different deciduous growth forms have either continuous or finite leaf development and wax formation, it is anticipated that different biomes and plant forms have different ɛ values between leaf wax and source water (i.e., mean annual precipitation in most cases). Grasses have continuous leaf-blade and leaf-wax development throughout the growing season, whereas broadleaf species produce leaf waxes during the early growing season; therefore the differences in apparent fractionation between leaf waxes and mean annual precipitation should be expected, given the differences in leaf phenology and timing of wax synthesis. Our model (Fig. 4) demonstrates that broadleaf species leafing out in the early growing season likely have source water with a different δ2H value during the time of wax synthesis than grasses that continually produce waxes, even though the grasses and broadleaf species might grow in the same precipitation and soil environments. In these examples, the leaf-wax δ2H values in broadleaf species should be more positive than in grasses.
Here, we have shown that the δ2H values of field-grown deciduous leaf waxes reflect only the leaf-water environment in the early phases of the growing season. This study system is ideal for studying the temporal trends in leaf-wax δ2H values and for teasing apart the conflicting controls of soil and leaf-water 2H-enrichment. Nonetheless, it is important to consider that these riparian plants were grown on a stable natural water source, which may not be the case for other species, growth forms, and/or environments. In these other cases, ground and soil water is likely the most important water source for a plant. Ground and soil water may or may not be sensitive to variations in early growing season precipitation, so this isoscape may not be appropriate for all plant systems. Nonetheless, we provide a precipitation isoscape specific to deciduous angiosperm species that share phenologic characteristics with P. angustifolia and a conceptual model that should be widely applicable to many other plants and biomes. This isoscape does not incorporate any information about early growing season humidity, and future modeling efforts can build and expand on this contribution by including information about humidity and growth forms and extrapolating the data to larger spatial scales.
Knowledge of Leaf Phenology Can Inform Paleoclimate Reconstructions.
Geologic studies frequently use leaf-wax δ2H values to reconstruct ancient climate signals, but few consider how leaf-wax δ2H values in different plant biomes are related to the interval of wax development. As we have shown here, the δ2H values of deciduous angiosperms leaf waxes reflect a leaf-water environment only in the early phases of the growing season. Although sedimentary n-alkane δ2H records represent a long-term, catchment-integrated average of environmental signals, we predict that in biomes and catchments dominated by deciduous broadleaf angiosperms with a single leaf flush, climate signals within sedimentary leaf-wax records will be weighted toward the water environment at the time of leaf flush. However, it is likely that sedimentary leaf-wax δ2H records derived from vegetation in some biomes (e.g., grasslands, rainforests) would not be subject to these constraints and would record seasonal climatic signals. Thus, these data suggest that, if nature of the plant community is constrained in sedimentary records, then leaf-wax δ2H signals can be isolated to individual periods of the growth seasons.
Materials and Methods
Three narrowleaf cottonwood (P. angustifolia) individuals were sampled from a riparian environment within Big Cottonwood Canyon, UT (40.633° N, 111.723° W; 1,892 m above sea level). Throughout the 2010 season, stem and leaf samples were collected from the same individual plants. Two leaf aliquots, one sample for water extraction and the other for lipid extraction, were collected from the four cardinal points of the tree. Four or five leaves were collected for leaf-water analysis and were stored in 4-mL baked-glass vials. Stem samples were collected from the first branch point from the tree trunk and were stored in 4-mL baked-glass vials. All leaf- and stem-water sample vials were sealed with Parafilm (Pechiney Plastic Packaging Company) and stored in the freezer until the time of processing. Four to eight leaves were collected for leaf-lipid analysis and were stored in paper coin envelopes. Leaf-lipid samples were dried immediately for 48 h in a 50 °C oven upon return to the laboratory. We collected atmospheric water vapor using a custom cryo-trap. Atmospheric vapor from 3 m above ground level was pumped into a glass cold trap submerged in dry ice/ethanol slurry. After 1 h, the frozen vapor was thawed and transferred to a 4-mL baked-glass vial, sealed with Parafilm, and stored in the freezer until the time of processing.
Stem and leaf waters were extracted using a cryogenic vacuum water-extraction method, followed by the addition of activated charcoal to remove water-soluble organic compounds following West et al. (44). The stable isotope abundances of water samples were analyzed on a Picarro model L1102-i water analyzer. Each sample was analyzed four times (four consecutive replicate injections) alongside a set of three laboratory reference materials [Sirfer Zero (0.1‰), EV (−72.5‰), DI (−123.0‰)] that previously had been calibrated to the Vienna Standard Mean Ocean Water (VSMOW) scale. Using delta notation, stable isotope ratios are calculated as δ = [(Rsamp/Rstd) −1], where R represents the 2H/1H abundance ratio, and Rsamp and Rstd are the ratios in the sample and standard, respectively. δ2H values are expressed relative to the standard VSMOW. The measurement precision for water H analysis was 0.5‰.
Leaf-wax lipids were extracted from leaves, and hydrocarbons were isolated following Pedentchouk et al. (12). Compounds were identified and abundance was quantified using a GC-MS with a fused silica, DB-5 phase column with helium as the carrier, at a flow of 1.5 mL/min. The GC oven temperature program used was 60–320 °C at 15 °C/min with an isothermal for 30 min. Compounds were identified by comparing elution times with n-alkane standards. Compound concentrations were quantified using a four-point calibration curve generated from authentic reference materials. Average chain length is defined as the average area-weighted chain length of higher plant n-alkanes from n-C23 through n-C33.
Compound-specific isotope analyses were performed using a Thermo Trace 2000 GC (Thermo Scientific) coupled to a Finnigan Delta V isotope ratio mass spectrometer interfaced with a high-temperature conversion system. The H3+ factor, determined daily during these analyses, before standard calibration and sample analysis was 2.42 ± 0.25 (1σ, n = 43). All compound-specific δ2H values are expressed relative to the VSMOW scale. Individual n-alkane isotope ratios were corrected to primary in-house n-alkane reference materials that previously had been calibrated to the VSMOW scale and were analyzed daily at several concentrations [n-C18 (−70‰), n-C20 (−54‰), n-C22 (−39‰), n-C24 (−36‰), n-C28 (−250‰), and n-C32 (−236‰)]. The accuracy for compound-specific measurements was ± 3‰ (1σ, n = 165) as determined from a secondary quality-control n-alkane reference material [n-C36 (−240 ‰)]. Apparent fractionation (ɛ) is defined as [Rlipid/Rwater − 1], where R represents the 2H/1H abundance ratio, and Rlipid and Rwater are the ratios in the n-alkane and water, respectively.
Statistical analysis was completed using Prism 5.0c for Mac OS X. The isotope data for all measurements (n-C27, n = 116; n-C29, n = 160) from a given sampling day (n = 15) were compared using one-way ANOVA and a Tukey–Kramer HSD post hoc test to identify differences at α = 0.001. Basic descriptive statistics for all compounds and sampling days are provided in Tables S1 and S2.
Monthly precipitation δ2H values were estimated using a raster dataset downloaded from www.waterisotopes.org (45). Raster datasets of the last freeze month were downloaded from the National Climatic Data Center’s Climate Maps of the United States (http://hurricane.ncdc.noaa.gov/cgi-bin/climaps/climaps.pl). The monthly precipitation and temperature data layers were imported into ArcGIS 9.3 and intersected. Individual precipitation δ2H values for the last freeze month then were merged into a single raster dataset.
Supplementary Material
Acknowledgments
This research was supported by National Science Foundation Grant IOS-1052551 (to J.R.E. and B.J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213875110/-/DCSupplemental.
References
- 1.Sachse D, et al. Molecular paleohydrology: Interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu Rev Earth Planet Sci. 2012;40(1):221–249. [Google Scholar]
- 2.Schimmelmann A, Sessions AL, Mastalerz M. Hydrogen isotopic (D/H) composition of organic matter during diagenesis and thermal maturation. Annu Rev Earth Planet Sci. 2006;34(1):501–533. [Google Scholar]
- 3.Pagani M, et al. Expedition 302 Scientists Arctic hydrology during global warming at the Palaeocene/Eocene thermal maximum. Nature. 2006;442(7103):671–675. doi: 10.1038/nature05043. [DOI] [PubMed] [Google Scholar]
- 4.Smith FA, Wing SL, Freeman KH. Magnitude of the carbon isotope excursion at the Paleocene-Eocene thermal maximum: The role of plant community change. Earth Planet Sci Lett. 2007;262(1):50–65. [Google Scholar]
- 5.Tipple BJ, et al. Coupled high-resolution marine and terrestrial records of carbon and hydrologic cycles variations during the Paleocene–Eocene Thermal Maximum (PETM) Earth Planet Sci Lett. 2011;311(1–2):82–92. [Google Scholar]
- 6.Hren MT, Pagani M, Erwin DM, Brandon M. Biomarker reconstruction of the early Eocene paleotopography and paleoclimate of the northern Sierra Nevada. Geology. 2010;38(1):7–10. [Google Scholar]
- 7.Polissar PJ, et al. Paleoaltimetry of the Tibetan Plateau from D/H ratios of lipid biomarkers. Earth Planet Sci Lett. 2009;287(1):64–76. [Google Scholar]
- 8.Tipple BJ, Pagani M. A 35 Myr North American leaf-wax compound-specific carbon and hydrogen isotope record: Implications for C4 grasslands and hydrologic cycle dynamics. Earth Planet Sci Lett. 2010;299(1–2):250–262. [Google Scholar]
- 9.Hou J, D'Andrea WJ, Huang Y. Can sedimentary leaf waxes record D/H ratios of continental precipitation? Field, model, and experimental assessments. Geochim Cosmochim Acta. 2008;72(14):3503–3517. [Google Scholar]
- 10.Sachse D, Radke J, Gleixner G. δD values of individual n-alkanes from terrestrial plants along a climatic gradient – Implications for the sedimentary biomarker record. Org Geochem. 2006;37(4):469–483. [Google Scholar]
- 11.Feakins SJ, Sessions AL. Controls on the D/H ratios of plant leaf waxes from an arid ecosystem. Geochim Cosmochim Acta. 2010;74(7):2128–2141. [Google Scholar]
- 12.Pedentchouk N, Sumner W, Tipple BJ, Pagani M. delta C-13 and delta D compositions of n-alkanes from modern angiosperms and conifers: An experimental set up in central Washington State, USA. Org Geochem. 2008;39(8):1066–1071. [Google Scholar]
- 13.Sachse D, Kahmen A, Gleixner G. Significant seasonal variation in the hydrogen isotopic composition of leaf-wax lipids for two deciduous tree ecosystems (Fagus sylvativa and Acer pseudoplatanus) Org Geochem. 2009;40(6):732–742. [Google Scholar]
- 14.Gao L, Burnier A, Huang Y. Quantifying instantaneous regeneration rates of plant leaf waxes using stable hydrogen isotope labeling. Rapid Commun Mass Spectrom. 2012;26(1):115–122. doi: 10.1002/rcm.5313. [DOI] [PubMed] [Google Scholar]
- 15.Kahmen A, Dawson TE, Vieth A, Sachse D. Leaf wax n-alkane δD values are determined early in the ontogeny of Populus trichocarpa leaves when grown under controlled environmental conditions. Plant Cell Environ. 2011;34(10):1639–1651. doi: 10.1111/j.1365-3040.2011.02360.x. [DOI] [PubMed] [Google Scholar]
- 16.Roden JS, Ehleringer JR. There is no temperature dependence of net biochemical fractionation of hydrogen and oxygen isotopes in tree-ring cellulose. Isotopes Environ Health Stud. 2000;36(3):303–317. doi: 10.1080/10256010008036389. [DOI] [PubMed] [Google Scholar]
- 17.Phillips SL, Ehleringer JR. Limited uptake of summer precipitation by bigtooth maple (Acer gradidentatum Nutt) and Gambel's oak (Quercus gambelii Nutt) Trees (Berl) 1995;9(2):214–219. [Google Scholar]
- 18.Chikaraishi Y, Naraoka H, Poulson SR. Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants. Phytochemistry. 2004;65(10):1369–1381. doi: 10.1016/j.phytochem.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Smith FA, Freeman KH. Influence of physiology and climate on δD of leaf wax n-alkanes from C3 and C4 grasses. Geochim Cosmochim Acta. 2006;70(5):1172–1187. [Google Scholar]
- 20.Sachse D, Gleixner G, Wilkes H, Kahmen A. Leaf wax n-alkane δD values of field-grown barley reflect leaf water δD values at the time of leaf formation. Geochim Cosmochim Acta. 2010;74(23):6741–6750. [Google Scholar]
- 21.Kolattukudy PE. Cutin biosynthesis in Vicia faba leaves. Plant Physiol. 1970;46(5):759–760. doi: 10.1104/pp.46.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riederer M, Markstadter C. In: Plant Cuticles: An Integrated Functional Approach. Kersteins G, editor. Oxford, UK: BIOS Scientific Publishers; 1996. pp. 189–200. [Google Scholar]
- 23.Jetter R, Kunst L, Samuels AL. In: Biology of the Plant Cuticle. Rieder M, Muller C, editors. Oxford, UK: Blackwell Publishing; 2006. pp. 145–181. [Google Scholar]
- 24.Jetter R, Schäffer S. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 2001;126(4):1725–1737. doi: 10.1104/pp.126.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauke V, Schreiber L. Ontogenetic and seasonal development of wax composition and cuticular transpiration of ivy (Hedera helix L.) sun and shade leaves. Planta. 1998;207(1):67–75. [Google Scholar]
- 26.Mortazavi B, et al. Does the 13C of foliage-respired CO2 and biochemical pools reflect the 13C of recently assimilated carbon? Plant Cell Environ. 2009;32(10):1310–1323. doi: 10.1111/j.1365-3040.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 27.Maffei M, Mucciarelli M, Scannerini S. Environmental factors affecting the lipid metabolism in Rosmarinus officinalis L. Biochem Syst Ecol. 1993;21(8):765–784. [Google Scholar]
- 28.Lockheart MJ, Van Bergen PF, Evershed RP. Variation in the stable carbon isotope composition of individual lipids from the leaves of modern angiosperms: Implications for the study of higher land plant-derived sedimentary organic matter. Org Geochem. 1997;26(1–2):137–153. [Google Scholar]
- 29.Lockheart MJ, Poole I, Van Bergen PF, Evershed RP. Leaf carbon isotope compositions and stomatal characters: Important considerations for palaeoclimate reconstructions. Org Geochem. 1998;29(4):1003–1008. [Google Scholar]
- 30.Bacic T, Krstin L, Rosa J, Popovic Z. Epicuticular wax on stromata of damaged silver fir trees (Abies Alba Mill.) Acta Societatis Botanicorum Poloniae. 2005;74(2):159–166. [Google Scholar]
- 31.Barthélémy D, Caraglio Y. Plant architecture: A dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Ann Bot (Lond) 2007;99(3):375–407. doi: 10.1093/aob/mcl260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cline MG, Harrington CA. Apical dominance and apical control in multiple flushing of temperate woody species. Can J For Res. 2007;37(1):74–83. [Google Scholar]
- 33.Liu W, Yang H. Multiple controls for the variability of hydrogen isotope compositions in higher plant n-alkanes from modern ecosystems. Glob Change Biol. 2008;14(9):2166–2177. [Google Scholar]
- 34.Liu W, Yang H, Li L. Hydrogen isotopic compositions of n-alkanes from terrestrial plants correlate with their ecological life forms. Oecologia. 2006;150(2):330–338. doi: 10.1007/s00442-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 35.McInerney FA, Helliker BR, Freeman KH. Hydrogen isotope ratios of leaf wax n-alkanes in grasses are insensitive to transpiration. Geochim Cosmochim Acta. 2011;75(2):541–554. [Google Scholar]
- 36.Polissar PJ, Freeman KH. Effects of aridity and vegetation on plant-wax dD in modern lake sediments. Geochim Cosmochim Acta. 2010;74(20):5785–5797. [Google Scholar]
- 37.Flanagan LB, Ehleringer JR. Stable isotope composition of stem and leaf water: Applications to the study of plant water use. Funct Ecol. 1991;5(2):270–277. [Google Scholar]
- 38.Kahmen A, et al. Effects of environmental parameters, leaf physiological properties and leaf water relations on leaf water δ18O enrichment in different Eucalyptus species. Plant Cell Environ. 2008;31(6):738–751. doi: 10.1111/j.1365-3040.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 39.Roden JS, Ehleringer JR. Observations of hydrogen and oxygen isotopes in leaf water confirm the craig-gordon model under wide-ranging environmental conditions. Plant Physiol. 1999;120(4):1165–1174. doi: 10.1104/pp.120.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson TE, Ehleringer JR. Isotopic enrichment of water in the “woody” tissue of plants: Implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochim Cosmochim Acta. 1993;57(14):3487–3492. [Google Scholar]
- 41.Dawson TE, et al. Stable isotopes in plant ecology. Annu Rev Ecol Syst. 2002;33(1):507–559. [Google Scholar]
- 42.Hou J, D'Andrea WJ, MacDonald D, Huang Y. Hydrogen isotopic variability in leaf waxes among terrestrial and aquatic plants around Blood Pond, Massachusetts (USA) Org Geochem. 2007;38(6):977–984. [Google Scholar]
- 43.Bi X, et al. Molecular and carbon and hydrogen isotopic composition of n-alkanes in plant leaf waxes. Org Geochem. 2005;36(10):1405–1417. [Google Scholar]
- 44.West AG, Patrickson SJ, Ehleringer JR. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun Mass Spectrom. 2006;20(8):1317–1321. doi: 10.1002/rcm.2456. [DOI] [PubMed] [Google Scholar]
- 45.Bowen GJ, Revenaugh J. Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res. 2003;39(10):1299. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.