Abstract
Inability to recruit new adipose cells following weight gain leads to inappropriate enlargement of existing cells (hypertrophic obesity) associated with inflammation and a dysfunctional adipose tissue. We found increased expression of WNT1 inducible signaling pathway protein 2 (WISP2) and other markers of WNT activation in human abdominal s.c. adipose tissue characterized by hypertrophic obesity combined with increased visceral fat accumulation and insulin resistance. WISP2 activation in the s.c. adipose tissue, but not in visceral fat, identified the metabolic syndrome in equally obese individuals. WISP2 is a novel adipokine, highly expressed and secreted by adipose precursor cells. Knocking down WISP2 induced spontaneous differentiation of 3T3-L1 and human preadipocytes and allowed NIH 3T3 fibroblasts to become committed to the adipose lineage by bone morphogenetic protein 4 (BMP4). WISP2 forms a cytosolic complex with the peroxisome proliferator-activated receptor γ (PPARγ) transcriptional activator zinc finger protein 423 (Zfp423), and this complex is dissociated by BMP4 in a SMAD-dependent manner, thereby allowing Zfp423 to enter the nucleus, activate PPARγ, and commit the cells to the adipose lineage. The importance of intracellular Wisp2 protein for BMP4-induced adipogenic commitment and PPARγ activation was verified by expressing a mutant Wisp2 protein lacking the endoplasmic reticulum signal and secretion sequence. Secreted Wnt/Wisp2 also inhibits differentiation and PPARγ activation, albeit not through Zfp423 nuclear translocation. Thus adipogenic commitment and differentiation is regulated by the cross-talk between BMP4 and canonical WNT signaling and where WISP2 plays a key role. Furthermore, they link WISP2 with hypertrophic obesity and the metabolic syndrome.
Keywords: mesenchymal stem cells, adipogenesis
The global epidemic of obesity is the major cause of the dramatic increase in type-2 diabetes (1). However, not all obese phenotypes are equally detrimental, and obesity characterized by an abdominal and intraabdominal fat accumulation is particularly harmful (2, 3). Around 20–30% of obese individuals exhibit a normal insulin sensitivity and metabolic profile and, similarly, around 30% of nonobese individuals can be characterized as inappropriately insulin-resistant (2–4). Thus, the fat mass per se is not the direct cause, but rather the associated dysregulated and inflamed adipose tissue accompanying enlargement of the adipose cells (hypertrophic obesity) (4–9). Hypertrophic obesity is a consequence of a reduced ability to recruit and differentiate new preadipocytes in the abdominal s.c. adipose tissue as shown in both in vivo and in vitro studies in humans (7, 10).
Several animal models have provided evidence of the metabolic consequences of an impaired ability to store excess fat in the s.c. adipose tissue and to recruit new adipose cells. Lipoatrophy leads to insulin resistance and lipid storage in ectopic sites, and this is reversed by adipose tissue transplantation (11). Overexpressing adiponectin in the adipose tissue in mice leads to morbid obesity but with hypercellular, rather than hypertrophic, adipose tissue and with maintained insulin sensitivity and metabolism (12).
Abdominal obesity in humans is a marker of the metabolic syndrome (13) and is also associated with ectopic lipid accumulation in other sites, including visceral/intraabdominal fat and the liver, as a consequence of an inability to store excessive lipids in the s.c. depot (13, 14). This inability seems to be even more pronounced in Asian Indian and Japanese ethnic groups; they become insulin-resistant and preferentially accumulate intraabdominal and liver fat following a modest increase in body mass index (BMI) (15, 16).
Canonical WNT regulates cell fate and differentiation and must be terminated to allow precursor cells to enter the adipose lineage (17–21), and bone morphogenetic protein 4 (BMP4) has been shown to promote commitment of the early precursor cells (22, 23). However, the molecular mechanisms regulating commitment of stem cells/precursor cells into the adipose lineage are currently unknown. We here show that canonical WNT and BMP4 signaling converge at the level of WNT1 inducible signaling pathway protein 2 (WISP2) and that this cytosolic and secreted molecule is a key regulator of adipogenic commitment and peroxisome proliferator-activated receptor γ (PPARγ) activation by both inhibiting the nuclear targeting of the transcriptional activator zinc finger protein 423 (Zfp423) (24) and by inhibiting PPARγ activation through extracellular signals.
Results
WNT/WISP2 Is Activated in Hypertrophic Obesity.
We first examined the expression of several WNT-regulated genes in the abdominal s.c. adipose tissue in relation to adipose cell size and abdominal waist circumference (i.e., hypertrophic obesity). WISP2 mRNA levels, a well-established marker of canonical WNT activation (23, 25), correlated positively with adipose cell size (Fig. 1A) as well as waist circumference in 36 nondiabetic individuals. Insulin sensitivity, measured with the euglycemic clamp, was also negatively correlated with adipose cell size in these individuals, independent of obesity, supporting the idea that cell size is a better marker of insulin sensitivity than BMI (4).
Fig. 1.
Canonical WNT is activated in hypertrophic obesity. (A) WISP2 expression in the adipose tissue correlates with adipose cell size (r = 0.42, P = 0.012) and waist circumference (r = 0.35, P = 0.038) in 36 healthy individuals. (B) The expression of WISP2 is positively correlated with other WNT-activated genes including Cyclin D1 (r = 0.58, P = 0.011), Fibronectin (r = 0.47, P = 0.032), BMP4 (r = 0.45, P = 0.035), and PPARδ (r = 0.65, P = 0.003). (C) WISP2 protein is highly expressed in human adipose tissue, but not in isolated mature adipocytes. (D) WISP2 gene expression is markedly decreased during adipogenesis of both 3T3-L1 mouse preadipocytes and (E) human primary preadipocytes. (F) The reduction in WISP2 gene expression during adipocyte differentiation of CD133+ precursor cells is negatively correlated with BMI and fat cell size (r = −0.68, P = 0.016).
Also other WNT-regulated genes, such as cyclin D1, correlated with fat cell size as well as with WISP2 mRNA levels. Significant correlations were also found for several other known WNT target genes (Fig. 1B). Together, these results provide evidence for enhanced canonical WNT activation in the s.c. adipose cells in hypertrophic obesity.
Canonical WNT is mainly active in undifferentiated cells, and we also found WISP2 to be highly expressed in human mesenchymal stem cells, CD133+ precursor cells, and preadipocytes (Table S1), and WISP2 protein was only found in the intact adipose tissue containing stromal precursor cells but not in isolated mature adipose cells (Fig. 1C).
WISP2 mRNA levels markedly decreased when we differentiated 3T3-L1 and human preadipocytes to adipocytes (Fig. 1 D and E). However, the down-regulation of WISP2 following differentiation of both abdominal s.c. preadipocytes and CD133+ cells was markedly less in cells from individuals with hypertrophic obesity (Fig. 1F), further supporting enhanced WNT/WISP2 activation in the stromal cells and inability to adequately suppress this pathway.
WISP2 Identifies the Metabolic Syndrome.
We compared WISP2 expression in the s.c. and visceral adipose tissue in lean, overweight, and obese individuals with or without the metabolic syndrome. WISP2 expression was considerably (three- to fourfold, P < 0.001) higher in the abdominal s.c. than in the visceral adipose tissue and was further increased in obesity (Fig. 2A). Importantly, it was significantly higher in the abdominal s.c. adipose tissue in equally obese individuals fulfilling the criteria of the metabolic syndrome (P < 0.001) (Fig. 2A). In contrast, there was no significant difference in WISP2 expression in the visceral adipose tissue between these two groups (Fig. 2A). Consistent with this, WISP2 expression in the abdominal adipose tissue correlated significantly and positively with waist circumference and amount of intraabdominal fat and negatively with degree of insulin sensitivity measured with the euglycemic clamp (Fig. 2B).
Fig. 2.
WISP2 expression predicts the metabolic syndrome. (A) The expression of WISP2 is higher in abdominal s.c. compared with visceral adipose tissue from the same individuals and is also higher in equally obese individuals fulfilling the criteria for the metabolic syndrome (P < 0.001) in the s.c. but not the visceral adipose tissue. (B) The expression of WISP2 is positively correlated with intraabdominal fat mass (r = 0.744, P < 0.001) and negatively related to insulin sensitivity measured with the euglycemic clamp (r = −0.531, P = 0.002). (C) WISP2 expression in the adipose tissue is positively correlated with waist circumference (r = 0.83, P = 0.003) and intraabdominal fat mass (r = 0.68, P = 0.03) in newly detected type-2 diabetic patients.
WISP2 mRNA levels in s.c. adipose tissue also correlated positively with waist circumference and intraabdominal visceral fat accumulation in newly detected and untreated subjects with type-2 diabetes (Fig. 2C). Together, these data show that hypertrophic obesity with expanded abdominal adipose cells is associated with inappropriate WNT/WISP2 activation in s.c. fat together with abdominal obesity, accumulation of ectopic intraabdominal fat, and insulin resistance.
WISP2 Is a Secreted Adipokine Inhibiting Adipogenesis.
We verified that Wisp2 is induced by β-catenin following the addition of the canonical Wnt3a ligand or a GSK3β inhibitor (SB 216763) (Fig. S1A). Wisp2 is highly expressed in undifferentiated cells and shows a predominant intracellular cytosolic distribution. However, it is also a secreted protein, as shown by expressing a myc-tagged Wisp2 in 3T3-L1 preadipocytes (Fig. S1B).
We then characterized the effect of WISP2 as a secreted protein by adipose precursor cells by adding recombinant murine or human WISP2 to the medium of undifferentiated 3T3-L1 or human preadipocytes. Like Wnt3a (7), WISP2 increased the proliferation of both 3T3-L1 and human preadipocytes and also prevented their differentiation (Fig. S1 C–E).
To examine its role in adipose cell differentiation, we stably transfected 3T3-L1 preadipocytes with a doxycycline-inducible shRNA because cell growth was reduced by adding siRNA to the medium. Knockdown (∼90%) of endogenous Wisp2 with doxycycline induced a marked and spontaneous (i.e., no differentiation mixture required) differentiation of the committed 3T3-L1 cells with activation of the c/ebpδ,, Pparγ, c/ebpα, aP2, and Glut4 genes (Fig. 3 A and B).
Fig. 3.
Inhibition of WISP2 induces spontaneous adipogenesis. (A) Knockdown of WISP2 by doxycycline-activated shRNA induced adipogenic genes and (B) spontaneous differentiation of 3T3-L1 cells. (C) Oil Red O staining of 3T3-L1 cells 72 h after indicated treatments in control cells, after differentiation mixture (diff) has been added and with or without the addition of 1.5 ng/mL TNFα, 10% Wnt3a, or 200 ng/mL rmWISP2 as shown. No differentiation mixture was added to the shWISP2 cells. (D) The presence of rhWISP2 (250 ng/mL) or Wnt3a (10%) prevented adipocyte commitment and differentiation of hMSC shown by Oil Red O staining and (E) expression of adipogenic markers during differentiation. Black bars, two cycles of induction/maintenance, 12 d; white bars, three cycles of induction/maintenance, 21 d. (F) Wnt3a and rmWisp2 increase Lrp6 phosphorylation (Ser1490) in 3T3-L1 cells. All results shown are representive of two to four experiments.
To further validate the role of Wisp2 as a mediator of canonical Wnt and its inhibitory effect on adipogenesis, we added Wnt3a or Wisp2 to control and Wisp2 shRNA 3T3-L1 cells. As expected, Wnt3a completely prevented differentiation of control cells but not in cells lacking Wisp2, whereas adding rmWisp2 to these cells markedly reduced their differentiation (Fig. 3C). Similarly, the well-established ability of TNFα to inhibit adipogenesis was prevented by down-regulating Wisp2 with shRNA (Fig. 3C), clearly documenting that this cytokine can inhibit adipogenesis in preadipocytes through Wnt activation (19, 20).
We then examined the effect of WISP2 on adipogenic differentiation of human mesenchymal stem cells. Addition of rhWISP2, like Wnt3a, markedly reduced the ability of human mesenchymal stem cells (hMSCs) to enter the adipogenic pathway (Fig. 3D) and induction of adipose genes (Fig. 3E). Thus, WISP2, like Wnt3a, can prevent the differentiation of both stem cells and committed preadipocytes to mature adipose cells. This inhibitory effect of WISP2 and Wnt3a on PPARγ activation was not due to an increase in the recently described inhibitory serine 273 phosphorylation of PPARγ (26).
The specific receptor for WISP2 is currently unknown but, like canonical WNT ligands, recombinant WISP2 induced phosphorylation of the WNT coreceptor LRP5/6 (Fig. 3F). Thus, it is possible that WISP2 signaling also involves the Frizzled receptors/LRP 5/6 pathways.
WISP2 Inhibits Adipogenic Commitment by BMP4.
To understand the mechanisms whereby WISP2 inhibits adipogenic differentiation, we characterized the effect in both the committed 3T3L1 preadipocytes as well as in the uncommitted NIH 3T3 fibroblasts. NIH 3T3 fibroblasts lack responsiveness to PPARγ ligands and have a poor adipogenic potential and are, therefore, a good model to examine effects on adipogenic commitment.
We first explored the possibility that WISP2 is able to cross-talk with BMP4, a well-recognized inducer of precursor cell commitment to the adipose lineage and of PPARγ activation (22, 23).
Knocking down Wisp2 by itself induced Pparγ activation in both 3T3-L1 preadipocytes and NIH 3T3 fibroblasts (Fig. 4A). This effect was equal to or greater than the effect of adding BMP4 to wild-type cells (Fig. 4A). Furthermore, the effect of BMP4 was dramatically amplified in both cells when Wisp2 also was reduced (Fig. 4A). A similar effect was seen for the Pparγ-regulated genes aP2, Glut4, and adiponectin (Fig. 4A). Adding the PPARγ ligand rosiglitazone alone had no effect in NIH 3T3 wild-type cells but dramatically increased the activation of the Pparγ-regulated genes in both NIH 3T3 and 3T3-L1 Wisp2 siRNA cells, further documenting the increased Pparγ activation and responsiveness to the ligand (Fig. 4A). Taken together, these results show that Wisp2 cross-talks with BMP4 and its ability to induce adipogenic commitment and Pparγ activation in uncommitted NIH 3T3 fibroblasts as well as in committed 3T3-L1 preadipocytes.
Fig. 4.
Wisp2 forms a complex with Zfp423 and inhibits BMP4-induced commitment and adipogenic differentiation of NIH 3T3 fibroblasts and 3T3-L1 preadipocytes. (A) Pparγ activation and adipogenic differentiation of NIH 3T3 fibroblasts (Upper) and 3T3-L1 preadipocytes (Lower) by BMP4 (40 ng/mL) and rosiglitazone (Rosi; 1.0 µM) in wild-type and Wisp2 siRNA cells. (B) The effect of BMP4 and rosiglitazone on adipogenic differentiation is inhibited by Zfp423 siRNA in NIH 3T3 fibroblasts (Left) and 3T3-L1 preadipocytes (Right). (C) Coimmunoprecipitaion of Flag-tagged Zfp423 and myc-tagged Wisp2. The Wisp2/Zfp423 complex is dissociated by BMP4 and this is inhibited by Noggin (100 ng/mL). The Zfp423 SMAD mutant (ΔSBD) also bound to Wisp2 but this complex was not dissociated by BMP4. (D) BMP4-induced activation of Pparγ is completely prevented in cells expressing the SMAD mutant (ΔSBD) Zfp423 compared with full-length Zfp423 in Wisp2-expressing cells. However, the SMAD mutant was able to activate Pparγ in response to BMP4 when Wisp2 was reduced with siRNA. The results shown are representative of two to four experiments.
WISP2 Inhibits Zfp423 Nuclear Translocation by BMP4.
Zfp423 was recently shown to be a transcriptional activator of Pparγ and inducer of adipogenic determination (24). To characterize the possibility that Wisp2 cross-talks with Zfp423, we knocked down this transcriptional activator together with Wisp2. Interestingly, this completely prevented the induction of Pparγ and associated adipogenic genes (Fig. 4B) following Wisp2 siRNA in both NIH 3T3 cells and in 3T3-L1 cells (Fig. 4B). Furthermore, the ability of BMP4 to activate Pparγ expression was completely inhibited in NIH 3T3 cells and also markedly reduced in 3T3-L1 cells, as was the effect of rosiglitazone on aP2 induction (Fig. 4B). These results clearly confirm that Zfp423 is critical for Pparγ activation and show that Wisp2 interacts with this molecule as well as with the ability of BMP4 to induce Pparγ activation.
We then explored the possibility that Wisp2 is associated with Zfp423 in a BMP4-regulated manner by performing coimmunoprecipitation experiments following expression of myc-tagged Wisp2 and Flag-tagged Zfp423. The use of a tagged protein was necessary because our currently available antibodies do not allow good immunoprecipitations of Wisp2. As shown in Fig. 4C, Wisp2 was coimmunoprecipitated with Zfp423 under nonstimulated conditions whereas this was dissociated by adding BMP4. Furthermore, adding the BMP4 inhibitor, Noggin, prevented the ability of BMP4 to dissociate the Wisp2/Zfp423 complex. To further verify this concept, we examined the adipogenic differentiation of NIH 3T3 cells under these same conditions. As shown in Fig. 5A, BMP4 had a small effect in wild-type NIH 3T3 cells, whereas knocking down Wisp2 alone induced a clear adipogenic effect on differentiation consistent with the Pparγ activation (Fig. 4 A and B). However, adding BMP4 had a dramatic adipogenic effect in Wisp2 siRNA cells, and this effect was completely inhibited by also knocking down Zfp423 (Fig. 5A). These results are consistent with the gene expression patterns (Fig. 4 A and B).
Fig. 5.
Adipogenic differentiation of NIH 3T3 fibroblasts by BMP4 is regulated by Wisp2 and Zfp423. (A) Oil Red O staining of NIH 3T3 cells, transfected with scrambled or Wisp2 siRNA with or without Zfp423 siRNA, followed by BMP4 treatment (72 h) as shown and incubated with differentiation mixture (8 d). (B) BMP4-induced nuclear translocation of Zfp423 is regulated by intracellular Wisp2. Zfp423 immunostaining of NIH 3T3 cells, transfected with either scrambled siRNA or Wisp2 siRNA and following addition of BMP4 (48 h). Note the speckled accumulation of nuclear Zfp423 protein (Upper) merged with nuclear DAPI staining (Lower). (C) Pparγ activation by BMP4 in NIH 3T3 cells transfected with scrambled or mutant Myc-tagged Wisp2 lacking the ER sequence and in the presence or absence of Wisp2 siRNA and (D) cellular expression of the mutant Myc-Wisp2 protein. The results shown are representative of experiments repeated at least twice.
Taken together, these results show that BMP4 dissociates the Wisp2/Zfp423 cytosolic complex and that either knocking down Wisp2 alone (Fig. 4 A and B) or overexpressing Zfp423 (24) (Fig. 4D) induces Pparγ and adipogenesis similar to the effect of adding BMP4, whereas the effect of BMP4 was dramatically amplified when Wisp2 also was reduced (Fig. 4 A and B).
We then examined the potential role of the SMAD sequences in Zfp423 for BMP4 activation of PPARγ, as reported by Gupta et al. (24), by overexpressing a SMAD mutant protein in NIH 3T3 fibroblasts. Overexpressing the SMAD mutant of Zfp423 in Wisp2-expressing cells completely inhibited the effect of BMP4 on Pparγ induction (Fig. 4D). In contrast, BMP4 markedly increased PPARγ activation when the SMAD mutant was expressed in cells with wisp2 siRNA (Fig. 4D). Furthermore, overexpressing this mutant alone increased Pparγ activation to a similar extent as wild-type Zfp423, showing that it was indeed capable of activating Pparγ. Thus, the SMAD sites in Zfp423 are critical for the dissociation of the Wisp2/Zfp423 complex by BMP4 but not for the ability to increase Pparγ activation.
This concept was further corroborated by the findings that the SMAD mutant also bound to Wisp2 but it was not dissociated by BMP4 in Wisp2-expressing cells (Fig. 4C), consistent with the inhibited effect of BMP4 to activate PPARγ in these cells (Fig. 4D).
Because Zfp423 is a transcriptional activator of Pparγ (24)), we analyzed the possibility that BMP4-induced dissociation of the Wisp2/Zfp423 complex also allowed the nuclear translocation of Zfp423 in NIH 3T3 cells. As shown in Fig. 5B, Zfp423 nuclear targeting was increased by BMP4 in wild-type cells but this effect was dramatically amplified by knocking down Wisp2. These results are consistent with the effects seen on Pparγ activation (Fig. 4 A and B) and ability of the cells to undergo adipogenesis (Fig. 5A).
Because Wisp2 is both a cytosolic and a secreted protein, we next examined the effect of BMP4 in scrambled and Wisp2 siRNA NIH 3T3 fibroblasts overexpressing a mutant Myc-tagged Wisp2 protein lacking the endoplasmic reticulum (ER) signal sequence, thus preventing its secretion. As shown in Fig. 5C, the effect of BMP4 on PPARγ activation was almost completely inhibited by expressing the mutant Wisp2 protein in Wisp2 siRNA cells, supporting the key role of intracellular Wisp2 in regulating BMP4-induced adipogenic commitment. Fig. 5D documents the cellular expression of the mutant Myc-Wisp2 protein, and we also verified that this protein was not recovered in the incubation medium, in contrast to full-length Myc-Wisp2 (Fig. S1B).
Adipogenic Differentiation, but Not Commitment, also Requires Inhibition of Extracellular Wnt/Wisp2.
We further characterized the cross-talk between BMP4 and Wnt/Wisp2 to ascertain that Wisp2 or Wnt3a did not have an inhibitory effect on BMP4 signaling measured as pSMAD 1/5/8 activation in NIH 3T3 cells, but no such interaction was seen (Fig. S2A). We also examined whether BMP4-induced commitment with Zfp423 nuclear translocation inhibited Wisp2 expression as a marker of inhibition of canonical Wnt activation. However, there was no effect of BMP4 on Wisp2 mRNA levels (Fig. S2B). Thus, BMP4 induces commitment by dissociating the Zfp423/Wisp2 complex but does not induce full adipogenic differentiation under concurrent Wnt/Wisp2 activation, as suggested by the small effect of BMP4 on PPARγ activation (Fig. 4A) and on differentiation of wild-type NIH 3T3 cells (Fig. 5A).
To further examine this, we exposed the NIH 3T3 Wisp2 siRNA cells to BMP4 in the presence or absence of added Wnt3a/Wisp2 ligands to examine whether commitment, as defined by the targeting of Zfp423 to the nucleus, was still possible in these cells. As shown in Fig. S2C, BMP4 still targeted Zfp423 to the nucleus in the presence of Wnt3a or recombinant Wisp2 in the medium, although differentiation (Fig. 3C), like the Pparγ and aP2 genes (Fig. 4A), remained inhibited. These results indicate that extracellular Wnt/Wisp2 also exert a direct inhibitory effect on Pparγ activation that is not overcome by only targeting Zfp423 to the nucleus with BMP4. This concept is further supported by our finding of a direct inhibitory effect of both Wisp2 and Wnt3 on PPARγ activation in reporter assays (Fig. S2D).
BMP4 Activates the ZFP423 Gene and Nuclear Translocation in Human Cells.
We first verified that WISP2 knockdown in human preadipocytes with siRNA promotes their differentiation. This was also the case because FABP4 was markedly increased but human preadipocytes did not undergo spontaneous differentiation like the 3T3-L1 cells because these cells require an exogenous PPARγ ligand, indicating that they, unlike 3T3-L1 adipocytes, cannot produce an endogenous ligand.
We then examined the effect of WISP2 siRNA and BMP4 on nuclear translocation of ZFP423. As shown in Fig. S3A, both WISP2 siRNA and BMP4 increased nuclear ZFP423, as in NIH 3T3 fibroblasts. However, the interindividual differences of this effect of BMP4 in cells from six subjects were quite large, probably a consequence of the presence of both committed and uncommitted cells in the human stromal vascular cell pool. Importantly, we also noted that BMP4 in the culture medium increased ZFP423 mRNA levels two- to fourfold (Fig. S3B), which is similar to what was seen in NIH 3T3 cells (two- to fivefold after 48–72 h in Wisp2 siRNA cells).
Taken together, these data show that canonical WNT activation, through WISP2, is a supreme regulator of PPARγ in both murine and human preadipocytes and that this is exerted at two different levels, involving both commitment and differentiation, as schematically illustrated in Fig. 6.
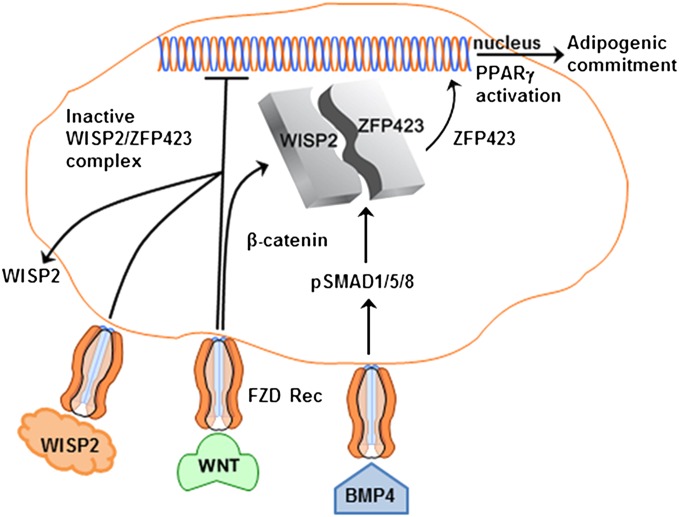
Fig. 6.
Schematic illustration of the interactions between BMP4 and WNT signaling. WISP2 is highly expressed in mesenchymal precursor cells and prevents PPARγ activation by binding ZFP423 in the cytosol. BMP4 induces SMAD1/5/8 phosphorylation, which interacts with the SMAD binding sites in ZFP423, dissociates the WISP2/ZFP423 complex, and allows ZFP423 nuclear entry for PPARγ activation. This allows cellular commitment but overall differentiation is also regulated by extracellular WNT/WISP2, which prevents PPARγ activation through still unclear mechanisms.
Discussion
These results clearly show that canonical WNT remains inappropriately activated in the stromal cells in hypertrophic obesity and this is also associated with inhibited normal precursor cell recruitment and differentiation to new preadipocytes. Intriguingly, we have recently shown that hypertrophic obesity is associated with a genetic predisposition for type-2 diabetes, but not for obesity itself (9, 27, 28), thus providing a mechanism for the well-established association between insulin resistance and heredity for type-2 diabetes. Furthermore, these results suggest an important role of genetic factors for canonical WNT activation in hypertrophic obesity. To what extent this is associated with the TCF7L2 or other risk genotypes (29) is under investigation.
We show here that WISP2 is both a cytosolic and a secreted protein that prevents adipogenic commitment and PPARγ-induced differentiation through at least two major mechanisms; one is by retaining Zfp423, a key transcriptional activator of Pparγ (24), in the cytosol, and this complex becomes dissociated by BMP4, allowing nuclear import of Zfp423 and cell commitment to the adipose lineage. This is the key mechanism whereby BMP4 induces adipogenic commitment, as shown in the studies in which a nonsecreted WISP2 protein was expressed. Interestingly, BMP4 was also able to increase ZFP423 gene activation, adding another layer of cross-talk in promoting adipogenic differentiation. The other mechanism is through WISP2 secretion and extracellular canonical WNT/WISP2-related signals whereby PPARγ activation is reduced. Clearly, commitment through BMP4 signaling is still possible because neither WISP2 nor WNT3a inhibited BMP4-mediated pSMAD1/5/8 activation or the nuclear targeting of ZFP423. Inhibition of secreted WNT/WISP2 is likely to occur through the induction of the secreted inhibitors of WNT including WIF, SFRP 1/2, or DICKKOPF 1/2 (30). DICKKOPF 1 (DKK1) is particularly interesting because we have recently shown that it is both a marker and a mediator of excellent adipogenic differentiation of human precursor cells (31). Furthermore, the marked impairment of precursor cell differentiation in hypertrophic obesity is overcome by adding both DKK1 and BMP4 (31).
It is currently unclear how these secreted inhibitors of canonical WNT/WISP2 are regulated and activated. We have found that PPARγ ligands can increase Dkk1 secretion in 3T3-L1 cells (32). However, this occurs quite late and when Pparγ already is induced and cannot account for terminating the concurrent Wnt activation, which would be required. This is obviously an area of great interest for ongoing research. However, once expressed and active, Pparγ activation by endogenous or exogenous ligands is able to further cross-talk with the canonical Wnt pathway because it enhances beta-catenin degradation and, thus, plays an important role in maintaining Wnt inhibition and the adipocyte differentiated state (21, 33).
Interestingly, canonical Wnt activation is also able to cross-talk with insulin signaling and action. We have previously reported that Wnt activation reduces glucose transport in response to insulin as well as impairs IRS1 phosphorylation and downstream Akt phoshorylation by insulin in adipose cells (21). Furthermore, it was recently shown that the liver became insulin-resistant and that gluconeogenesis was enhanced by Wnt activation (34). Thus, Wnt activation not only regulates adipogenic commitment and cell differentiation but can also induce insulin resistance, another key property of hypertrophic obesity and the metabolic syndrome.
Only one previous study has been performed with Wisp2 and its effect on adipogenesis. In that study, overexpressed Wisp2 in 3T3-L1 cells only induced a stimulating effect on proliferation but no inhibition of differentiation (35). The reason for this negative finding is unclear, not least because stimulation of proliferation usually impairs adipogenic differentiation. However, it is possible that the expressed Wisp2 was not secreted, which would preclude the extracellular inhibitory signals, or that the expressed protein did not bind cytosolic zfp423, as shown here. We have validated our results in several cell lines, including hMSC, and also shown the opposite effects by inhibiting endogenous Wisp2 expression in the cells.
A recent proteomics study also found WISP2 to be secreted by human adipose tissue (36), confirming our present results and supporting WISP2 as a novel adipokine. To what extent WISP2 can be involved in other effects and signaling of BMP/TGFβ ligands in adipogenic and other cells is currently under investigation. Interestingly, it was recently reported that WISP2 reduced epithelial-mesenchymal transformation in MCF7 breast cancer cells (37), suggesting that the TGFβ superfamily may be a target for WISP2 actions.
Taken together, our results show that canonical WNT signaling inhibits adipogenic commitment and differentiation through WISP2, which retains ZFP423 in the cytosol and, as a secreted molecule, also elicits an extracellular signaling pathway that can inhibit PPARγ activation. Furthermore, as a secreted molecule, WISP2 can probably exert both autocrine and paracrine (and possibly endocrine) regulation and be an important adipokine mediating cross-talk between the adipose tissue and other cells.
These findings identify WISP2 as a potential target for drug development. Specific inhibition of WISP2 would allow recruitment and differentiation of new preadipocytes following weight gain and, thereby, adequate storage of excess lipids in the s.c. tissue rather than the accumulation of ectopic fat and the development of insulin resistance and the metabolic syndrome.
Materials and Methods
Biopsies of human adipose tissue were obtained from the s.c. and visceral depots from 89 carefully phenotyped individuals for qRT-PCR mRNA analyses of WISP2 and other genes and for protein analyses. The study protocols were approved by the Ethics Committees of the University of Gothenburg, University of Kuopio, and Charles University and were in accordance with the Declaration of Helsinki. Cellular proteins were extracted and subjected to PAGE separation and immunoblotting. Immunoprecipitations of tagged proteins were performed following expression of myc-Wisp2 and Flag-Zfp423 and after incubating the cells with 40 ng/mL BMP4 and 100 ng/mL Noggin as shown.
Wisp2 was stably knocked down with doxycycline-inducible shRNA in 3T3-L1 preadipocytes or transiently with siRNA in NIH 3T3 fibroblasts and 3T3-L1 preadipocytes. Immunofluorescence of Zfp423 was examined in a Leica SP5 confocal microscope following permeabilization and incubation with specific antibodies following the indicated preincubations. Lipid accumulation was visualized with Oil Red O staining.
More complete information is given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Bruce M. Spiegelman for advice and reagents. The study was supported by Swedish Research Council Grant K2010-54X-03506-39-3, the Swedish Foundation for Strategic Research, the Swedish Diabetes Association, the Novo Nordisk Foundation, the IngaBritt and Arne Lundberg Foundation, and the Torsten and Ragnar Söderberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211255110/-/DCSupplemental.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 3.Marini MA, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30(8):2145–2147. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 4.Smith U, Hammarstedt A. Antagonistic effects of thiazolidinediones and cytokines in lipotoxicity. Biochim Biophys Acta. 2010;1801(3):377–380. doi: 10.1016/j.bbalip.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 7.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arner E, et al. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansson PA, et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003;17(11):1434–1440. doi: 10.1096/fj.02-1132com. [DOI] [PubMed] [Google Scholar]
- 10.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 11.Gavrilova O, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105(3):271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 14.Vidal-Puig A, Unger RH. Special issue on lipotoxicity. Biochim Biophys Acta. 2010;1801(3):207–208. doi: 10.1016/j.bbalip.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Chandalia M, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS ONE. 2007;2(8):e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand SS, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE) PLoS ONE. 2011;6(7):e22112. doi: 10.1371/journal.pone.0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20(1):16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. 2006;281(14):9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 20.Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14(7):1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson B, Smith U. Activation of canonical wingless-type MMTV integration site family (Wnt) signaling in mature adipocytes increases beta-catenin levels and leads to cell dedifferentiation and insulin resistance. J Biol Chem. 2010;285(18):14031–14041. doi: 10.1074/jbc.M110.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101(26):9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6(4):385–389. doi: 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- 24.Gupta RK, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waki H, et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5(5):357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Choi JH, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001;15(6):1101–1103. [PubMed] [Google Scholar]
- 28.Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE. 2011;6(4):e18284. doi: 10.1371/journal.pone.0018284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafson B, Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61(5):1217–1224. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafson B, Eliasson B, Smith U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: A link with osteogenesis. Diabetologia. 2010;53(3):536–540. doi: 10.1007/s00125-009-1615-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26(15):5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4(158):ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inadera H, Shimomura A, Tachibana S. Effect of Wnt-1 inducible signaling pathway protein-2 (WISP-2/CCN5), a downstream protein of Wnt signaling, on adipocyte differentiation. Biochem Biophys Res Commun. 2009;379(4):969–974. doi: 10.1016/j.bbrc.2008.12.185. [DOI] [PubMed] [Google Scholar]
- 36.Lehr S, et al. 2012. Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics 11(1):M111.010504.
- 37.Sabbah M, et al. CCN5, a novel transcriptional repressor of the transforming growth factor β signaling pathway. Mol Cell Biol. 2011;31(7):1459–1469. doi: 10.1128/MCB.01316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.