Abstract
Contemporary economic models hold that instrumental and impulsive behaviors underlie human social decision making. The amygdala is assumed to be involved in social-economic behavior, but its role in human behavior is poorly understood. Rodent research suggests that the basolateral amygdala (BLA) subserves instrumental behaviors and regulates the central-medial amygdala, which subserves impulsive behaviors. The human amygdala, however, typically is investigated as a single unit. If these rodent data could be translated to humans, selective dysfunction of the human BLA might constrain instrumental social-economic decisions and result in more impulsive social-economic choice behavior. Here we show that humans with selective BLA damage and a functional central-medial amygdala invest nearly 100% more money in unfamiliar others in a trust game than do healthy controls. We furthermore show that this generosity is not caused by risk-taking deviations in nonsocial contexts. Moreover, these BLA-damaged subjects do not expect higher returns or perceive people as more trustworthy, implying that their generous investments are not instrumental in nature. These findings suggest that the human BLA is essential for instrumental behaviors in social-economic interactions.
Keywords: economic trust, altruism
Classical economic models portray humans as rational selfish beings, whose decisions are based upon cost–benefit analyses and instrumental choice (1, 2). Contemporary views propose that economic decision making is not only rational and instrumental, but can also be affective-impulsive in nature and, in the classical sense, economically irrational (3, 4). This dichotomy is nicely illustrated in trust interactions between an investor and a trustee in the trust game (5). When an investor perceives a potential partner as trustworthy, high investment can be entirely instrumental because higher returns are anticipated. However, if the decision to trust is affective in nature, higher investments may occur for noninstrumental reasons in the absence of expectations for high profits.
Human neuroimaging data implicate the amygdala in modulating economic trust, but the findings are inconsistent and the specifics are poorly understood. Several studies have reported negative relationships between amygdala activity and economic trust (6–8), but other studies suggest no amygdala involvement in economic trust behaviors (6, 9, 10). A potential reason for this inconsistency is that in human research, the amygdala is typically investigated and discussed as a single unit (11, 12). Animal research, however, has clearly demonstrated that the amygdala has structurally and functionally separate subdivisions (13–15).
Research in rodents highlights two amygdala subregions, which might be relevant to human trust: the basolateral amygdala (BLA) and the central-medial amygdala (CMA) (16–18). These subregions play a pivotal role in economic behavior and decision making as part of the cortico-mesolimbic circuit involving the orbitofrontal cortex (OFC) and the nucleus accumbens (NAc) (14, 19–21). In rodents, the BLA has been proposed to subserve calculated instrumental behavior, whereas the CMA subserves affective-impulsive behaviors (14, 19).
To date, there is scarce information about the respective roles of these amygdala subregions in human behavior. Nonetheless, in the domain of fear processing, we recently showed that the distinction between instrumental and impulsive behaviors for the rodent BLA and CMA may also exist in humans. Humans with developmental focal bilateral damage limited to the BLA are hypervigilant to innate—i.e., unconditioned—fear cues (15). These data correspond to rodent research, which shows that the BLA instrumentally down-regulates unwarranted fear hypervigilance by inhibiting the impulsive-affective response pathway from CMA to brainstem (17, 22). Furthermore, these BLA-damaged humans also show impairments in fear conditioning; they are less able to learn contingencies between stimuli and their predictive cues.* Fear learning is an instrumental process vital for coping with the environment and, according to animal research, is subserved by the BLA (22). Accordingly, it is conceivable that subjects with BLA damage are also impaired in behaving instrumentally in social situations, because they lack impulse inhibition and may not acquire the behavioral repertoires to behave instrumentally.
It is intuitively understandable that an individual’s propensity to trust other people is not written in stone, but that these baseline trust levels are also determined by experiences in life—i.e., that “people’s baseline levels of suspicion will change over time based on their experiences in the world” (ref. 8, p. 8728). Betrayals of trust are highly aversive and may decrease baseline levels of trust (23). Taking such learning into account, one might predict that individuals with BLA damage show increased trust in situations in which others, who have learned through aversive experiences, show distrust. Thus, in many ways, developmental BLA damage should cause a relatively decreased instrumentality in social-economic interactions, which would also be consistent with observations of altruistic behaviors in children (24–27), as well as with recent evidence showing that giving is spontaneous and greed is calculated (28).
Here we investigate whether selective BLA damage in humans impairs instrumental social-economic choice behaviors. We compared the social interaction of 3 subjects with focal bilateral damage to the BLA, but a functional CMA (Fig. 1), and 12 controls in a one-shot trust game (29). Controls were matched for age, intelligence, economic income, and region (SI Text). Furthermore, we used additional paradigms for assessing risk-taking behaviors, expectations about back-transfers of the trustees, and trustworthiness ratings of unfamiliar faces to better understand the motivations behind their investment decisions in the trust game measure.
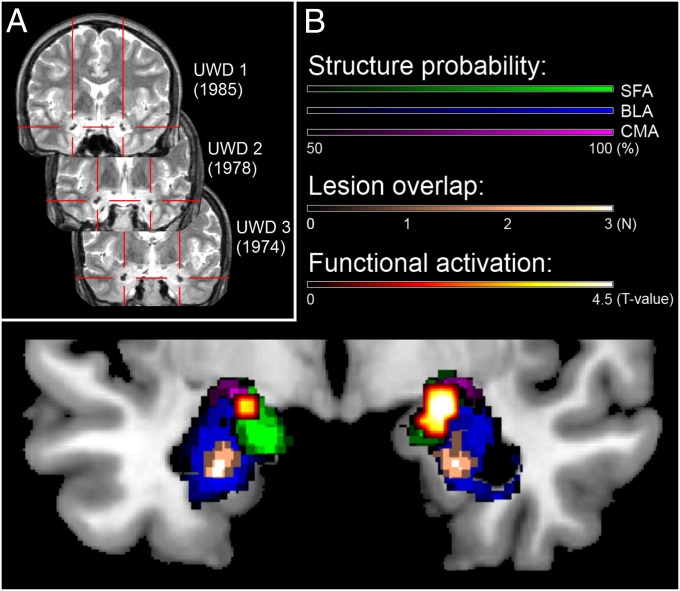
Fig. 1.
(A) T2-weighted MR images (coronal view) of the three subjects with Urbach–Wiethe disease (UWD), with their year of birth and red crosshairs indicating the calcified brain damage. (B) Structural and functional assessment of the bilateral amygdala in our group of three UWD subjects. Plotted are the cytoarchitectonic probability maps of the amygdala thresholded at 50% (47), structural lesion overlap, and functional activation during the emotion-matching task (48), all normalized to the Montreal Neurological Institute template brain. The structural method indicates that the lesions of the three patients are located in the BLA, whereas the functional method shows activation during emotion matching in the superficial amygdala (SFA) and CMA, but not in the BLA. This figure is adapted from Morgan et al. (38), wherein detailed structural MRI and functional MRI methods used are described.
Results
Trust Game.
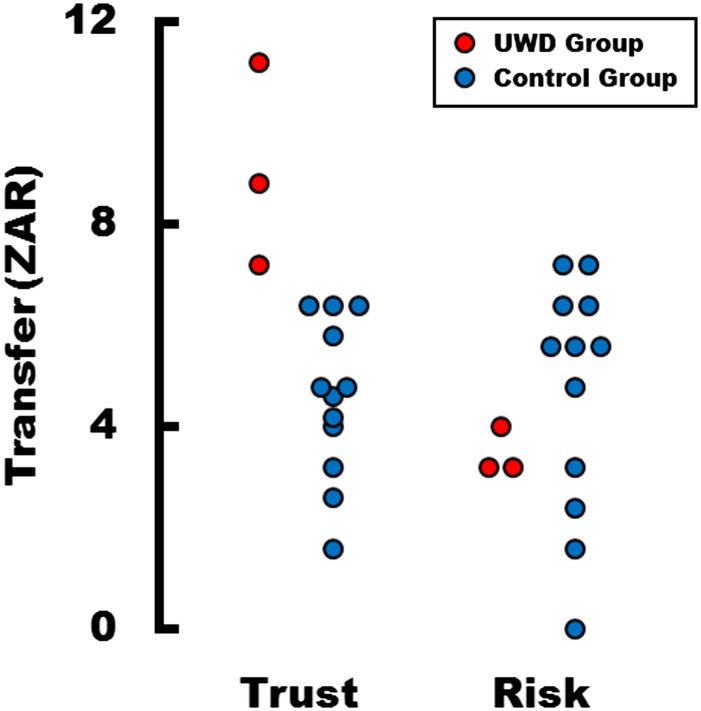
In the trust game, two players, an investor and a trustee, receive an initial endowment. The investor has to decide how much of the endowment to send to the trustee. The amount sent is then multiplied by three, and the trustee decides how much of the total money to return to the investor. Thus, if the investor gives money to the trustee and the latter shares the earnings of the transfer, both players end up with a higher monetary payoff (Fig. 2A). However, the trustee also has the option of betraying the investor's trust by keeping all of the money. Because sharing the earnings is costly for the trustee, a selfish trustee will not honor, but instead exploit the investor's trust. Thus, from the perspective of the investor, choosing a trusting action—that is, investing a large amount of money—is a risky decision, because there is no information about the trustee’s trustworthiness until the end of the experiment. Results showed that our BLA-damaged subjects transferred significantly more money to the anonymous trustees than control subjects did (Mann-Whitney test, P < 0.01, n = 15, two-tailed), with control-subjects transferring on average 4.56 South African Rand (ZAR) (range: 1.6–6.4 ZAR) and BLA-damaged subjects transferring on average 9.06 ZAR (range: 7.2–11.2 ZAR; Fig. 3). Strikingly, there is no overlap between Urbach–Wiethe disease (UWD) subjects and control subjects in trusting behavior, and the mean difference between the groups is near 100%. In sum, each BLA-damaged subject placed higher trust in the unknown opponent than any of the control subjects did.
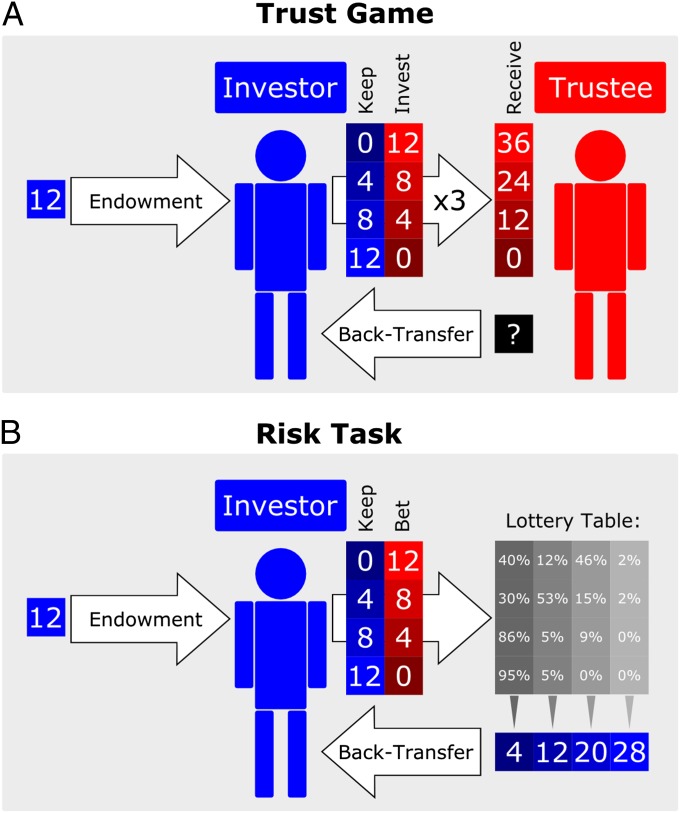
Fig. 2.
(A) Trust game. The investor and the trustee both received an endowment of 12 ZAR, and the investor decided how much of this endowment to send to the trustee. The amount sent was then multiplied by the factor of three, and the trustee decided how much of the money received to send back to the investor. Subjects played five single rounds in which the investor was only informed about the back-transfer at the end of the game. (B) Risk task. The trustee was replaced by a random mechanism in the form of four distinct lotteries. All four lotteries were wheel-of-fortune–type lotteries whose winning probabilities and outcomes approximated the risk associated with investor risk in the trust game. Subjects had to buy a total of five lottery tickets with different prices for the tickets corresponding to four different types of lotteries. Ticket prices corresponded directly to the transfer the investor could make in the trust game.
Fig. 3.
BLA-damaged subjects in the trust game transferred significantly more money to the anonymous trustees than control subjects (Mann-Whitney test, P < 0.01, n = 15, two-tailed), with control subjects transferring on average 4.56 ZAR and BLA-damaged subjects transferring 9.06 ZAR. Strikingly, there was no overlap between UWD and control subjects in trusting behavior, and the mean difference between the groups nears 100%. In the risk task, the average investment of the control group was 4.67 ZAR (range: 0.0–7.2 ZAR), which is even slightly above the average investment of the BLA-damaged subjects of 3.47 ZAR (range: 3.2–4.0 ZAR), but this difference clearly is not significant (Mann-Whitney test, P = 0.31, n = 15, two-tailed).
Risk Task.
Investors take risks when transferring amounts of money to trustees in the trust game because they do not know beforehand whether the trustee will reciprocate or exploit their trust. There is substantial evidence showing that humans are averse to such risks (30), but in the context of our results from BLA-damaged individuals, it is important to know whether the social nature of the risk played a role in the relative absence of risk aversion in our subjects or whether this observation is rather a general domain effect—that is, behavior in the trust game of the BLA-damaged subjects might be caused by a general lack of risk aversion due to relatively low non-domain-specific risk aversion.
Accordingly, we tested whether the BLA-damaged subjects would display a lack of risk aversion outside the social context of the trust game. We used a nonsocial risk-taking paradigm isomorphic to the trust game wherein the trustee is replaced by a random mechanism. The random mechanism in the risk experiment approximated trustees' decisions taken from another study (29). Therefore, the investors faced the identical monetary risks as in the trust game, but the transfer decisions were not based upon social interactions (Fig. 2B). In this risk paradigm, the average investment of the control group was 4.67 ZAR (range: 0.0–7.2 ZAR), which was not significantly different from, and even slightly above, the average investment of the UWD subjects of 3.47 ZAR (range: 3.2–4.0 ZAR) (Mann-Whitney test, P = 0.31, n = 15, two-tailed; Fig. 3B).
Trustworthiness Beliefs Task.
Another potential mechanism underlying the observed effect on social-economic behavior in our BLA-damaged subjects might be that they have socially naïve expectations of fairness of monetary return from anonymous trustees. In effect, our UWD-subjects might have highly optimistic expectations about the trustees’ monetary return. To examine this issue, we measured subjects’ expectations about the trustees’ back-transfer in another experiment wherein each subject’s expectations about the trustees’ back-transfers were assessed for each possible investor transfer of 0, 4, 8, and 12 ZAR. Subjects’ beliefs were paid according to trustees’ actual decisions derived from a previous study (29)—i.e., we paid more money if they made more accurate guesses, which incentivized them to really think about the trustees’ decisions. We found no significant difference between BLA-damaged and control subjects in the amount they expected to get back from the trustee (Mann-Whitney test, P > 0.80, n = 15, two-tailed). Although the control subjects on average believed to receive back 6.50 ZAR (4.4–8.8), BLA-damaged subjects expected a return of 6.71 ZAR (4.8–6.0). Thus, both UWD and control subjects expected that trustees would pay them back more or less the same amount, but notably, both groups did not expect fair sharing of the profits of the economic interaction. It should furthermore be noted that the expected return in both groups was rather low, which may be in keeping with the fact that all our subjects belong to the so-called “coloured” Namaqualand population, who endured long-term social and economic abuse during Apartheid (SI Text). In sum, both BLA-damaged and control subjects hold similar beliefs of return of their economic investments, and the sizeable group differences shown in the trust game therefore cannot be explained in terms of BLA-damaged subjects having socially naïve (optimistic) beliefs in the trustworthiness of others.
Fairness Expectations Interview.
To further inspect this critical issue, we performed a short structured interview wherein we questioned BLA-damaged and control subjects on expectations of the trustee’s returns and their actual investments in the trust game. Overall, BLA-damaged and control subjects expected the trustees to return their own initial investment, but not more than that, which concurs with their objective behavior in the trustworthiness beliefs task. Control subjects suggested that they for that reason invested a relatively low proportion of their total endowments in the trust game (i.e., group-mean < 40%), indicating that voluntary-instrumental decisions were involved. BLA-damaged subjects invested nearly twice that proportion (group-mean > 75%) but could not provide a rationale for their striking generosity in the interview, suggesting that their economic decisions were impulsive choices.
In the risk task, we showed that risk-taking preferences could not explain the behavior of our BLA-damaged subjects in the trust game. As humans tend to be less willing to take risks when other humans are the agents of uncertainty (31, 32), it might be suggested that subjects with BLA damage lack such social-risk aversion. However, we also show that BLA-damaged subjects’ expectations of the trustees’ back-transfers (i.e., their expectations of fairness of the trustees) are similar to expectations of control subjects and very low. However, whereas control subjects acted instrumentally on these expectations in investing only a low proportion of their endowments, subjects with BLA damage invested generously, despite strong expectations of unfair return.
This confidence about trustees' unfair returns as reflected on two measures suggest that the BLA-damaged subjects’ generous investments might be altruistic in nature and that they were not taking excessive social risks. Importantly, although trusting unfamiliar others involves social uncertainty, our BLA-damaged subjects were not uncertain, but even confident, in their expectations about the trustees’ unfair behavior. The expectations of unfairness are not surprising because our patients and controls derive from a group that has suffered extreme social and economic abuse in the past several decades, and their invariably low expectation of monetary return in the trust game is consistent with this history.
Facial Trustworthiness Task.
Expectations of unfairness may seem remarkable in BLA-damaged subjects in the light of findings with subjects with extensive amygdala damage (33) who show heightened trustworthiness ratings of unfamiliar faces. However, rodent amygdala models indicate that bilateral selective damage to the BLA produces very different effects than damage to other, or all, amygdala regions, also because of excitatory and inhibitory interactions between the amygdala subregions (14, 17, 19, 34). Heightened trustworthiness ratings in SM, a patient with full amygdala damage (33), may thus not be informative with respect to the effects of selective BLA damage. This conclusion is substantiated by our BLA-damaged subjects showing hypervigilance for innate fear cues (15), whereas SM is hypovigilant to innate fear cues (35). Indeed, we tested our BLA-damaged and matched control subjects with an adapted and validated version of the Adolphs et al. (33) facial trustworthiness task (36) and found no group differences (Mann-Whitney test, P = 0.47, n = 15, two-tailed). Furthermore, in agreement with the above findings of low expectations of monetary return in the trust game, the ratings of trustworthiness of BLA-damaged and control subjects were very low compared with other studies using this task (36, 37).
Discussion
We show that, compared with control subjects, humans with focal bilateral damage to the BLA invest generously in unfamiliar others in the trust game. These large economic investments were not due to general abnormalities in risk taking in BLA-damaged subjects. Furthermore, the investments of BLA-damaged subjects in trustees also cannot be attributed to differences in intelligence or to socially naïve expectations of fair return, because IQ levels, expectations about back-transfers of the trustees, and face-to-face trustworthiness ratings were similar for BLA-damaged and control subjects. Crucially, the objectively and subjectively assessed expectations of back-transfers and facial-trustworthiness ratings indicate that BLA-damaged subjects did not expect more fairness than the control subjects. BLA-damaged subjects, similar to control subjects, seem to have accurate conscious knowledge about the state of the social world, but in their social-economic behavior, BLA-damaged subjects do not behave in accordance with that knowledge.
Overall, our data suggest that the generosity displayed by the BLA-damaged subjects results from impairments in instrumental choice behaviors, which, according to rodent research, are subserved by the BLA (14, 19). This interpretation is further underscored when we consider that the large investments of BLA-damaged subjects arguably are affective-impulsive behaviors, which are subserved by the CMA, and in the absence of BLA regulation the CMA should come into prominence in social decision making (14, 15, 17, 19). One might argue that the economic choices of our BLA-damaged subjects appear irrational, and deficits in intelligence and especially working memory could underlie such irrational decisions. However, this explanation seems not to be the case because experimental groups were matched on intelligence (SI Text), and working memory performance in these BLA-damaged subjects is even above average (38). Furthermore, our data also agree with findings on fear behaviors in these same BLA-damaged subjects, who show acute unconditioned fear hypervigilance (an affective-impulsive behavior), whereas fear conditioning (an instrumental fear learning behavior) is impaired (15).* From a learning perspective, the generosity in the trust game of our BLA-damaged subjects might be considered pathological altruism (39), in the sense that inborn altruistic behaviors have not, due to BLA damage, been unlearned through negative social experience (24–27).
Our findings correspond to parallel amygdala subregion models of economic choice behavior in rodents, which suggest that the BLA subserves instrumental choice behavior by exerting direct and indirect actions via the OFC on the NAc. Furthermore, the BLA may, via the intercalated cell masses of the amygdala, inhibit the pathway for impulsive choice behavior from CMA to NAc (14, 15, 19). The BLA, in the corticomesolimbic system, is arguably the regulating hub underlying instrumental economic choice behaviors. Although both BLA and OFC are involved in these instrumental behaviors via their actions on the NAc, crucially, the OFC is in these and other behaviors dependent on, or regulated by the BLA (15, 40, 41). In sum, we argue that damage to the BLA in our subjects may have impaired instrumental choice behaviors both directly (BLA–NAc pathway) and indirectly (OFC–NAc pathway). Furthermore damage to the BLA may additionally have caused the increase in impulsive choice behavior by disinhibiting the CMA (BLA–CMA pathway) (14, 15, 19).
Importantly, BLA calcification in our subjects is a slowly progressive, developmental lesion, potentially allowing for compensatory neuroplasticity (42). Possible evidence of compensatory plasticity in emotion processing has been observed in a single case with the same genetic disease as our subjects, but with full amygdala damage (43). Our consistent findings in three cases with highly selective and homogenous BLA lesions nevertheless suggest that the human BLA is an indispensable part of the neural circuit underlying calculative instrumental choice behaviors. A remaining question is whether these subjects might alter their social-economic decisions when directly confronted with an individuals’ unfair behavior. Follow-up research in these BLA-damaged subjects with learning paradigms in a social context is necessary to answer that question.
In conclusion, we provide evidence for a neural foundation of dual-process frameworks of human social decision making at the amygdala subregion level (3, 4). Our findings are in line with the idea that a primary impulsive response in humans may be to help and cooperate (28), whereas the execution of calculative-instrumental—that is, selfish—behaviors are learned from interactions with the social environment (24–28) and require the BLA.
Materials and Methods
Participants.
UWD is a rare genetic developmental disorder wherein selective bilateral amygdala brain calcifications occur (33). We selected four female subjects without any history of secondary psychopathology or epilepsy from a previously described group of UWD subjects in South Africa (44) where this genetic disease is most prevalent (see also ref. 45). In this group, we formerly described selective bilateral BLA damage, and a functional CMA using structural and functional neuroimaging methods (15, 38). For the behavioral assessment, patients with UWD were compared against a group of healthy volunteers (n = 12) matched for sex, age, and IQ, and living in the same area of South Africa (i.e., mountain-desert villages near the Namibian border). One UWD subject was not able to comprehend the probabilities of the economic tasks during instruction and was excluded from further participation in the experiment. Demographic data are summarized in Tables S1 and S2, including age and IQ (Wechsler Abbreviated Scale of Intelligence) (46). For details and issues regarding IQ testing in this non-Western sample, see Neuropsychological Assessment in SI Text.
Trust Game.
In the trust game, both investor and trustee subjects receive an initial endowment of 12 ZAR. The investor can send 0, 4, 8 or 12 ZAR to the trustee. The experimenter triples each ZAR that the investor transfers. After the investor's decision is made, the trustee is informed about the investor's transfer. Then the trustee has the option of sending any amount between zero and the total amount available back to the investor. The experimenter does not triple the back-transfer. The investor's final payoff corresponds to the initial endowment minus the transfer to the trustee, plus the back-transfer from the trustee. The trustee's final payoff is given by his initial endowment plus the tripled transfer of the investor, minus the back-transfer to the investor. Each subject made five decisions in the same player role while they were saliently made to believe that they were paired with five different, randomly selected interaction partners. The investors did not receive feedback about the trustee’s decisions until the end of the experiment.
Risk Task.
The risk task was presented to the subjects in the form of four distinct lotteries. All four lotteries were wheel-of-fortune type lotteries whose winning probabilities and outcomes approximated the risk associated with investor risk in the trust game (Fig. 2). Subjects had to buy a total of five lottery tickets with the different prices for the tickets corresponding to the four different types of lotteries. Prices were 0, 4, 8, and 12 ZAR, with higher prices corresponding to lotteries with higher variance in return and, thus, to higher risk. Therefore, ticket prices correspond directly to the transfer the investor can make in the trust game.
After subjects read the instructions of the trust game and risk task, control questions were used to check whether they had properly understood the rules. All subjects that were included in data analysis answered these questions correctly.
Facial Trustworthiness Task.
The stimuli in the trustworthiness task consisted of 150 grayscale pictures of unfamiliar faces with neutral emotional expressions (36), of which 100 were adapted from Adolphs et al. (33) and 50 were taken from the Psychological Image Collection at Stirling (http://pics.psych.stir.ac.uk/). During the task, directly below the facial stimulus, a visual analog scale was presented ranging from (left to right) “very untrustworthy” to “neutral” to “very trustworthy.” For each stimulus, subjects were presented with the question “How trustworthy do you think this person is?” and asked to answer by clicking on the scale with a mouse cursor. After the response to each trial, a button appeared with the word “next”; the subject’s response to the scale could be adjusted until this button was clicked. For each presentation trial, the scale was reset to the neutral position. The stimuli were presented using software written in E-Prime (Psychology Software Tools). For data analysis, the scale positions were coded from −100 (very untrustworthy) to 0 (neutral) to +100 (very trustworthy) in steps of 1. These scores were averaged for each subject to obtain an individual measure of trustfulness.
Supplementary Material
Acknowledgments
This work was supported by Hope For Depression Research Foundation Grant RGA 9-015; the Netherlands Society of Scientific Research (Brain and Cognition Grant 056-24-010); and the University of Cape Town (Brain Behavior Initiative).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Klumpers F, The neuroanatomy of human fear conditioning: new evidence from patients with bilateral basolateral amygdala lesions, Fourth European Meeting on Human Fear Conditioning, May 5, 2012, Rauischholzhausen, Germany.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217316110/-/DCSupplemental.
References
- 1.Becker GS. The Economic Approach to Human Behavior. Chicago: Univ of Chicago Press; 1976. [Google Scholar]
- 2.Hollis M, Nell EJ. Rational Economic Man: A Philosophical Critique of Neo-Classical Economics. Cambridge, UK: Cambridge Univ Press; 1975. [Google Scholar]
- 3.Fehr E, Camerer CF. Social neuroeconomics: The neural circuitry of social preferences. Trends Cogn Sci. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Camerer C, Loewenstein G, Prelec D. Neuroeconomics: How neuroscience can inform economics. J Econ Lit. 2005;43(1):9–64. [Google Scholar]
- 5.Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games Econ Behav. 1995;10(1):122–142. [Google Scholar]
- 6.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Xiao E, Houser D, Montague PR. Neural responses to sanction threats in two-party economic exchange. Proc Natl Acad Sci USA. 2009;106(39):16835–16840. doi: 10.1073/pnas.0908855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt MA, Lohrenz T, Camerer CF, Montague PR. Distinct contributions of the amygdala and parahippocampal gyrus to suspicion in a repeated bargaining game. Proc Natl Acad Sci USA. 2012;109(22):8728–8733. doi: 10.1073/pnas.1200738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. What motivates repayment? Neural correlates of reciprocity in the trust game. Soc Cogn Affect Neurosci. 2009;4(3):294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King-Casas B, et al. Getting to know you: Reputation and trust in a two-person economic exchange. Science. 2005;308(5718):78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 11.Fusar-Poli P, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- 12.Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 14.Balleine BW, Killcross S. Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Terburg D, et al. Hyper-vigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tye KM, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388(6640):377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 19.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: Parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27(6):543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8(4):375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- 21.Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- 22.Macedo CE, Cuadra G, Molina V, Brandão ML. Aversive stimulation of the inferior colliculus changes dopamine and serotonin extracellular levels in the frontal cortex: Modulation by the basolateral nucleus of amygdala. Synapse. 2005;55(1):58–66. doi: 10.1002/syn.20094. [DOI] [PubMed] [Google Scholar]
- 23.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18(2):159–165. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Warneken F, Tomasello M. Altruistic helping in human infants and young chimpanzees. Science. 2006;311(5765):1301–1303. doi: 10.1126/science.1121448. [DOI] [PubMed] [Google Scholar]
- 25.Warneken F, Tomasello M. Extrinsic rewards undermine altruistic tendencies in 20-month-olds. Dev Psychol. 2008;44(6):1785–1788. doi: 10.1037/a0013860. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg N. The Caring Child. Cambridge, MA: Harvard Univ Press; 1992. [Google Scholar]
- 27.Eisenberg N, Fabes RA, Spinrad T. Prosocial development. In: Eisenberg N, editor. Handbook of Child Psychology: Social, Emotional, and Personality Development. Hoboken, NJ: Wiley; 2006. pp. 646–718. [Google Scholar]
- 28.Rand DG, Greene JD, Nowak MA. Spontaneous giving and calculated greed. Nature. 2012;489(7416):427–430. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- 29.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 30.Bohnet I, Zeckhauser R. Trust, risk and betrayal. J Econ Behav Organ. 2004;55(4):467–484. [Google Scholar]
- 31.Fehr E, Rockenbach B. Human altruism: Economic, neural, and evolutionary perspectives. Curr Opin Neurobiol. 2004;14(6):784–790. doi: 10.1016/j.conb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Bohnet I, Greig F, Herrmann B, Zeckhauser R. Betrayal aversion: Evidence from Brazil, China, Oman, Switzerland, Turkey, and the United States. Am Econ Rev. 2008;98(1):294–310. [Google Scholar]
- 33.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 34.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54(1):51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21(1):34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bos PA, Terburg D, van Honk J. Testosterone decreases trust in socially naive humans. Proc Natl Acad Sci USA. 2010;107(22):9991–9995. doi: 10.1073/pnas.0911700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baas D, et al. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage. 2008;40(2):719–727. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Morgan B, Terburg D, Thornton HB, Stein DJ, van Honk J. Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS ONE. 2012;7(6):e38116. doi: 10.1371/journal.pone.0038116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakley B, Knafo A, Madhavan G, Sloan Wilson D. Pathological Altruism. Oxford: Oxford Univ Press; 2011. [Google Scholar]
- 40.O’Doherty J. Can’t learn without you: Predictive value coding in orbitofrontal cortex requires the basolateral amygdala. Neuron. 2003;39(5):731–733. doi: 10.1016/s0896-6273(03)00525-7. [DOI] [PubMed] [Google Scholar]
- 41.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39(5):855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 42.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain. 2007;130(4):898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 43.Becker B, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72(1):70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Thornton HB, et al. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsychiatry Clin Neurosci. 2008;20(1):86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- 45.Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: Evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- 47.Amunts K, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 48.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.