Abstract
Dysregulation of the “intrinsic” apoptotic pathway is associated with the development of cancer and autoimmune disease. Bak and Bax are two proapoptotic members of the Bcl-2 protein family with overlapping, essential roles in the intrinsic apoptotic pathway. Their activity is critical for the control of cell survival during lymphocyte development and homeostasis, best demonstrated by defects in thymic T-cell differentiation and peripheral lymphoid homeostasis caused by their combined loss. Because most bak−/−bax−/− mice die perinatally, the roles of Bax and Bak in immunological tolerance and prevention of autoimmune disease remain unclear. We show that mice reconstituted with a Bak/Bax doubly deficient hematopoietic compartment develop a fatal systemic lupus erythematosus-like autoimmune disease characterized by hypergammaglobulinemia, autoantibodies, lymphadenopathy, glomerulonephritis, and vasculitis. Importantly, these mice also develop a multiorgan autoimmune disease with autoantibodies against most solid glandular structures and evidence of glandular atrophy and necrotizing vasculitis. Interestingly, similar albeit less severe pathology was observed in mice containing a hematopoietic compartment deficient for only Bak, a phenotype reminiscent of the disease seen in patients with point mutations in BAK. These studies demonstrate a critical role for Bak and an ancillary role for Bax in safeguarding immunological tolerance and prevention of autoimmune disease. This suggests that direct activators of the intrinsic apoptotic pathway, such as BH3 mimetics, may be useful for treatment of diverse autoimmune diseases.
Keywords: apoptosis, Bcl-2 family
Several mechanisms account for the establishment of immunological tolerance and prevention of autoimmunity. They include removal of autoreactive B and T lymphocytes by apoptotic cell death (deletion), the dampening of immunoreceptor signaling in potentially dangerous lymphocytes (anergy), and the immunoregulatory actions of regulatory T cells (1). Apoptosis regulates immune cell homeostasis at almost every stage of development. Most importantly for tolerance, T and B cells expressing antigen receptors that recognize self-antigens with high avidity are eliminated by apoptosis (2). Moreover, during contraction of immune responses, after the successful elimination of a pathogen, many activated T and B lymphocytes die to make space for subsequent immune responses and to limit collateral damage to healthy tissues (3). Defects in apoptosis at any of these developmental checkpoints may allow the escape of potentially dangerous cells, engendering the risk that they cause lympho-proliferative and/or autoimmune diseases.
In mammals, apoptosis proceeds through two distinct but ultimately converging pathways (4): the “extrinsic” pathway, activated by “death receptors” on the cell surface (5), and the “intrinsic” pathway that is initiated by developmental cues or a broad range of stress stimuli, such as cytokine deprivation (6).
The intrinsic apoptotic pathway is regulated by the Bcl-2 family of proteins, which can be divided into three subgroups according to their structure and function (6). The prosurvival members (Bcl-2, Bcl-xL, Mcl-1, A1/Bfl-1, and Bcl-w) are essential for cell survival. The proapoptotic BH3-only proteins (Bim, Bid, Puma, Bad, Bmf, Hrk, Bik, and Noxa) are activated transcriptionally, posttranscriptionally, and/or posttranslationally by a broad range of cytotoxic stimuli and are required for initiation of apoptosis signaling. The third subgroup, the so-called multi-BH domain proapoptotic proteins, including Bak and Bax [plus possibly Bok (7)], are essential for the mitochondrial outer membrane permeabilization that is necessary to unleash caspase-mediated cell demolition (8). Bak and Bax are thought to be activated either directly through binding of certain BH3-only proteins (e.g., Bim, tBid) (8) or indirectly when they are released from their restraint by the prosurvival Bcl-2 proteins when the latter are neutralized by the BH3-only proteins (9).
Mice lacking Bak appear largely normal (10), and those deficient for Bax show only relatively mild abnormalities, including minor splenomegaly and male infertility (11). Remarkably, combined loss of Bak and Bax causes severe developmental abnormalities and renders a range of cell types (including lymphoid ones) profoundly resistant to diverse apoptotic stimuli and even enforced expression of BH3-only proteins (10, 12–14). Investigations into the long-term consequences of Bak/Bax compound deficiency have been severely limited by the high incidence of perinatal lethality (thought to be due to neuronal abnormalities) of bak−/−bax−/− mice (10). Experiments using lethally irradiated WT mice in which the hematopoietic system had been reconstituted with bone marrow cells from bak−/−bax−/− embryos showed gross defects in intrathymic T-cell development and peripheral T-cell homeostasis (12). Notably, deletion of self-superantigen specific TCRVβ5+CD4+ T cells was substantially reduced in these animals. Moreover, inducible deletion of loxP flanked bax genes on a bak−/− background using the inflammatory compound poly I:C to activate the Mx1-Cre transgene resulted in profound defects in T-cell apoptosis and, ultimately, glomerulonephritis (GN), but no organ-specific autoimmune manifestations were described (15). Although these studies were informative, no long-term analysis of health was performed. Here we report that the combined loss of Bak and Bax in hematopoietic cells caused progressive lymphadenopathy, hypergammaglobulinemia, and accumulation of antinuclear autoantibodies, precipitating a fatal systemic erythematosus (SLE)-like autoimmune GN. Importantly, these mice also developed autoantibodies against a broad range of organs and in many cases severe autoimmune destruction of the blood vessels (necrotizing vasculitis) in the spleen, submandibular gland, and pancreas. Surprisingly, these systemic and organ-specific autoimmune manifestations were also observed in mice with a bak−/− hematopoietic system (albeit, at a lower severity compared with the bak−/−bax−/− chimeras) but were never observed in Bax-deficient mice. Our mouse studies, together with data from clinical observations (16), therefore identify Bak and, to a lesser extent Bax, as critical barriers against autoimmune disease.
Results
Mice Reconstituted with a bak−/−bax−/− Hematopoietic System Die Abnormally Early.
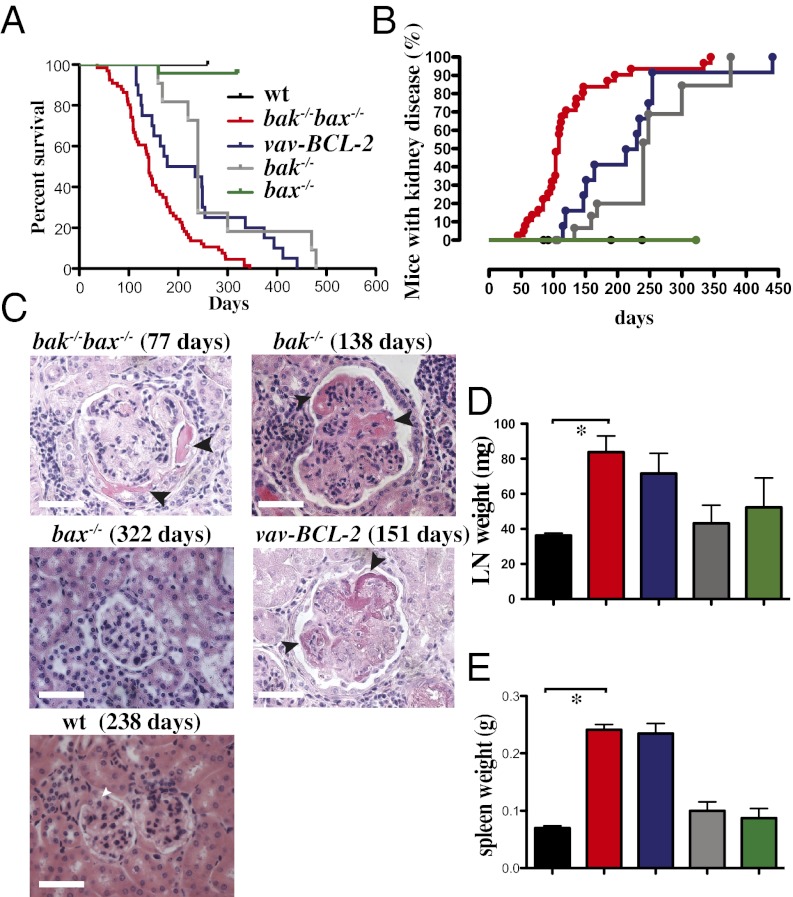
Consistent with previous results (10, 12), our intercrosses of bak−/−bax+/− mice produced significantly fewer bak−/−bax−/− offspring than would be expected from Mendelian inheritance, with only 0.98% present at weaning instead of the expected 25%. Given the low numbers of bak−/−bax−/− mice available for long-term observation, we investigated the roles of Bak and Bax in preventing autoimmunity by using a hematopoietic reconstitution model. Lethally irradiated C57BL/6-Ly5.1 (Ly5.1, WT) mice were injected with fetal liver cells (FLC; a rich source of hematopoietic stem/progenitor cells) from embryonic day (E)13.5 bak−/−bax−/− embryos or embryos of the relevant control genotypes (WT, bak−/− or bax−/−; all on a C57BL/6-Ly5.2 background). Recipients of FLC from bak−/−bax−/− mice had a dramatically reduced lifespan (median survival of 141 d, range 36–334 d) compared with mice reconstituted with a WT hematopoietic system (hereafter called control mice), which were healthy at the time of analysis (238 d after reconstitution; Fig. 1A). Consistent with these findings, blocking the intrinsic apoptotic pathway by overexpression of Bcl-2 also reduced lifespan. Ly5.1 mice reconstituted with FLC from vav-BCL-2 transgenic mice (17) also died significantly earlier compared with control mice (vav-BCL-2 mice; median survival 206 d, range 112–252 d; survival WT vs. vav-BCL-2 P = 0.0053) (Fig. 1A).
Fig. 1.
Fatal SLE-like GN, lymphadenopathy, and splenomegaly in mice reconstituted with a bax−/−bak−/− hematopoietic system. (A) Survival curves for lethally irradiated C57BL/6-Ly5.1 mice reconstituted with FLC from WT (n = 22), bak−/− bax−/− (n = 67; P < 0.0001 WT vs. bak−/− bax−/−), bak−/− (n = 11; P = 0.0065), bax−/− (n = 24; P = 0.6171), or vav-BCL-2 transgenic (n = 20, P = 0.0053) embryos. (B) Incidence of severe autoimmune kidney disease (refer to legend in A) when mice were sick or at the end of the experiments (up to 238 d for WT and 322 d for bax−/− reconstituted mice) in Ly5.1 mice reconstituted with WT (total mice analyzed n = 25), bak−/−bax−/− (n = 39), vav-BCL-2 transgenic (n = 13), bak−/− (n = 21), or bax−/− FLC (n = 7 analyzed after 322 d). Control WT vs. bak−/− bax−/−: P < 0.0001; bak−/− bax−/− vs. vav-BCL-2: P = 0.0016; bak−/− bax−/− vs. bak−/−: P < 0.0001; bak−/− vs. bax−/−: P = 0.002; control WT vs. vav-BCL-2: P < 0.0001; control WT vs. bax−/−: P = ns. (C) Representative images of H&E-stained sections of kidneys from Ly5.1 mice reconstituted with FLC of the indicated genotypes (with the time after transplantation indicated in brackets). Arrows indicate fibrinoid necrosis. (Scale bar: 128 μm.) (D) Lymph node and (E) spleen weights at necropsy in Ly5.1 mice reconstituted with FLC from embryos of the indicated genotypes (refer to legend in A). Data represent mean ± SEM (*P < 0.05).
Interestingly, Ly5.1 mice reconstituted with bak−/− FLC also had a reduced lifespan compared with control mice (bak−/−; 50% dead/sick at 240 d, compared with controls, which all remained healthy at a similar age). Bak was shown to interact with the prosurvival Bcl-2 family member Mcl-1 (18); loss of Bak may therefore lead to alterations in the levels of Mcl-1. Mcl-1 levels were, however, comparable in the spleen and thymus of bak−/−bax−/− and control reconstituted mice (Fig. S1). Mice reconstituted with bax−/− E13.5 FLC (n = 18) had a lifespan indistinguishable from that of control animals (P = 0.6015; Fig. 1A). These results show that in hematopoietic tissues Bak plays a major and Bax an ancillary role in allowing a normal mouse lifespan.
Mice Reconstituted with bak−/−bax−/− FLC Develop Fatal Autoimmune GN and Vasculitis with Polyclonal Hypergammaglobulinemia and Antinuclear Antibodies.
With increasing age, bak−/−bax−/− chimeras showed signs of weight loss, lethargy, and hematuria (>200 red blood cells per μL, normal range <3 cells per μL) and GN [mean age of GN onset (grade 3–4) 109 d, a pathology that was not detected in control mice; bak−/−bax−/− vs. WT P < 0.0001; Fig. 1B]. Histological examination of kidney sections from sick mice reconstituted with bak−/−bax−/− FLC revealed signs of SLE-like autoimmune pathology, including glomerular enlargement, hypercellularity, thickening of capillary loops, and fibrinoid necrosis (Fig. 1C). Mice reconstituted with vav-BCL-2 transgenic FLC and, unexpectedly, those reconstituted with bak−/− (but not those with bax−/−) FLC also showed evidence of GN (Fig. 1 B and C).
In mice with a bak−/−bax−/−, vav-BCL-2 transgenic, or bak−/− hematopoietic system, immunofluorescent staining revealed the deposition of IgM, IgA, and IgG antibodies in the renal glomeruli (particularly at the capillary loops), which may be indicative of severe renal disease (Fig. S2). Interestingly, although fatal GN had not previously been documented in bak−/− mice, our bak−/− reconstituted animals developed GN, had a reduced lifespan (median age of GN development 350 d; survival bak−/− vs. WT P = 0.0084; Fig. 1A) and demonstrated significant Ig deposition in their kidneys (Fig. S2). In contrast, such pathological changes were not observed in mice reconstituted with WT or bax−/− FLC.
We also noted the development of GN in four rare bak−/−bax−/− mice from our colony that survived to adulthood. These animals had to be killed owing to malocclusion, vaginal prolapse, or overt GN (hematuria) (at postnatal days 126, 126, 133, or 160, respectively). Consistent with a previous study (10), these mice displayed persistent interdigital webbing, a hallmark of defective apoptosis. In these sick bak−/−bax−/− animals we found evidence of autoimmune GN on histological analysis [GN; grade +2 (n = 2) and +3 (n = 2); scale 0–4; Fig. S3A], akin to the bak−/−bax−/− reconstituted mice. Moreover, immunofluorescence analysis showed evidence of IgG, IgM, and IgA deposition on the renal glomerular capillary loops. In contrast, no significant immune complex deposition was observed in kidneys from age-matched WT mice (Fig. S3B).
Impaired Lymphocyte Homeostasis and Immunological Tolerance Underlie the Autoimmune Disease in Mice Reconstituted with a bak−/−bax−/− Hematopoietic System.
To investigate why hematopoietic deficiency in both Bak and Bax caused the premature death of mice, we analyzed their lymphoid organs at necropsy. Ly5.1 mice reconstituted with bak−/−bax−/− FLC displayed splenomegaly and lymphadenopathy at the time of necropsy (Fig. 1 D and E). Such abnormalities were not observed in similarly aged mice transplanted with FLC from WT mice or mice singly deficient for either Bak or Bax (Fig. 1 D and E). Flow cytometric analysis of spleens and lymph nodes from mice reconstituted with bak−/−bax−/−, bak−/−, bax−/−, or WT FLC revealed similar frequencies of all major lymphocyte subsets (Fig. S4 A and B), indicating that combined loss of Bak and Bax caused an expansion of all these cell types, because their overall spleen and lymph node cellularity were abnormally elevated. Analysis of peripheral blood demonstrated significant (P < 0.05) lymphocytosis in the bak−/−bax−/− reconstituted animals, whereas platelet counts were lower compared with both WT (17) and bax−/− reconstituted mice (P < 0.05 or P < 0.005, respectively) (Fig. S4 C and D).
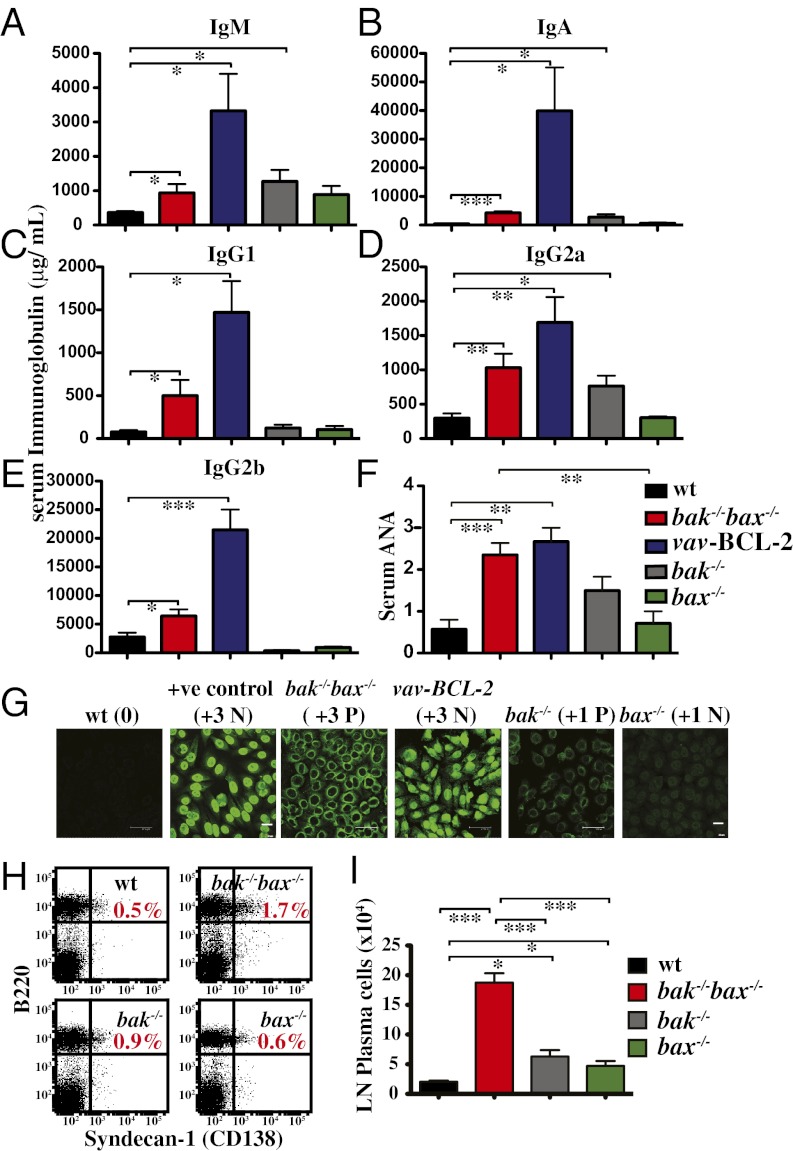
We next investigated the effecter mechanisms that cause SLE. Significant elevations in all serum Ig isotypes tested were observed in mice reconstituted with bak−/−bax−/− FLC compared with control animals (Fig. 2 A–E). Mice reconstituted with vav-BCL-2 transgenic FLC that became sick also developed severe hypergammaglobulinemia, with levels of IgM, IgA, IgG1, IgG2a, and IgG2b even higher than those seen in bak−/−bax−/− FLC reconstituted mice (P < 0.05; Fig. 2 A–E). This enhanced hypergammaglobulinemia may reflect the longer survival of mice reconstituted with vav-BCL-2 FLC compared with the bak−/−bax−/− FLC reconstituted animals (Fig. 1A), which would allow more time for accumulation of immunoglobulins.
Fig. 2.
Mice reconstituted with a bak−/−bax−/− hematopoietic system develop hypergammaglobulinemia and ANA. (A–E) Serum levels of the Ig isotypes indicated in Ly5.1 mice reconstituted with FLC of the indicated genotypes were determined by ELISA, n = 6. (F) Levels of ANA in the sera of Ly5.1 mice reconstituted with FLC of the indicated genotypes (n = 5–17 per genotype) were quantified by indirect immunofluorescence staining of slides covered with human HEp2 epithelial cells. (G) Representative examples of ANA quantification in sera of Ly5.1 mice reconstituted with FLC of the indicated genotypes. Immunofluorescence intensity score is indicated in brackets. Data represent mean ± SEM. ANA designation: P, peripheral; N, nuclear. (Scale bars: 47.62 μm or 20 μm, as indicated.) (H) Representative flow cytometric plots of B220 plus CD128 staining of lymph node cells from Ly5.1 mice reconstituted with FLC of the indicated genotypes. (I) Graph depicting the total numbers of B220+CD128+ plasma cells in the mice described in H (n = 3/genotype). Data represent mean ± SEM (*P < 0.05; **P < 0.005; ***P < 0.0005).
Polyclonal hypergammaglobinemia is a characteristic of chronic inflammatory conditions, such as SLE (19). Accordingly, significant levels of antinuclear antibodies (ANA) were detected in sera from sick bak−/−bax−/−, vav-BCL-2, and bak−/− hematopoietic chimeras but not in mice reconstituted with WT or bax−/− FLC (Fig. 2 F and G). Notably, sera from sick bak−/−bax−/− or vav-BCL-2 reconstituted mice showed characteristic patterns of ANA staining on HEp2 epithelial cells (peripheral, homogenous, and nucleolar), whereas sera from sick bak−/− reconstituted mice produced mainly a peripheral staining pattern (Fig. 2G). Peripheral and homogenous ANA staining patterns indicate the presence of autoantibodies specific for dsDNA, ssDNA, nuclear DNA, antideoxyribonucleoprotein (DNP), and histones, all of which are associated with SLE.
In accordance with their polyclonal hypergammaglobinemia and elevated ANA levels, mice reconstituted with bak−/−bax−/− FLC had an almost threefold increase and those transplanted with bak−/− FLC an ∼1.7-fold increase in the percentages of Ig producing (B220+CD138+) plasma cells in their lymph nodes, compared with WT and bax−/− reconstituted mice (Fig. 2H). The total numbers of plasma cells in lymph nodes were significantly augmented in both the bak−/−bax−/− (∼10-fold) as well as the bak−/− (∼fourfold) reconstituted mice compared with the WT reconstituted mice (Fig. 2I). Plasma cell numbers in bax−/− reconstituted mice were mildly elevated compared with WT controls (∼twofold) but significantly lower (P < 0.0005) compared with the bak−/−bax−/− reconstituted animals.
bak−/−bax−/− Chimeras Develop Fatal GN More Rapidly than vav-BCL-2 Transgenic Chimeras.
To determine why mice reconstituted with bak−/−bax−/− FLC die earlier than those transplanted with vav-BCL-2 transgenic FLC (Fig. 1 A and B), we compared cohorts of mice at 8 wk after reconstitution, a time before severe GN is manifest. Mice reconstituted with bak−/−bax−/− or vav-BCL-2 FLC showed similarly elevated serum levels of IgM, IgG1, IgG2a, and IgG2b (Fig. S5A) and ANA (Fig. S5 B and C). However, histological analysis revealed that kidneys of both vav-BCL-2 and WT FLC reconstituted mice were normal, whereas those from bak−/−bax−/− reconstituted mice already showed features of GN (Fig. S5 D and E). Accordingly, significant Ig deposition on the glomerular basement membrane associated with severe disease was observed in mice reconstituted with bak−/−bax−/− FLC but not in the vav-BCL-2 or WT FLC reconstituted animals, where Ig deposition was predominantly associated with mesangial cells (Fig. S5 F and G). These results suggest that bak−/−bax−/− reconstituted mice die earlier than vav-BCL-2 reconstituted animals because SLE-like autoimmune kidney damage develops more rapidly in the former.
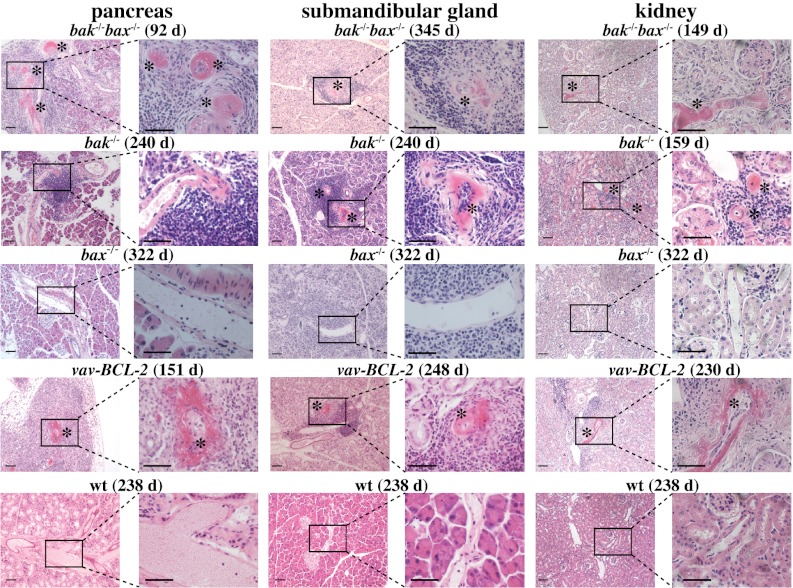
bak−/−bax−/− Chimeras Develop Autoimmune Pathology and Necrotizing Vasculitis in Multiple Organs.
The data presented above show that Bak and Bax are necessary for immunological tolerance of ubiquitous self-antigens. To assess whether Bak/Bax are also required for tolerance of tissue-specific antigens, we searched for signs of autoimmunity in a range of organs (scored 1–4; Fig. S6). Sick bak−/−bax−/− and vav-BCL-2 chimeras all exhibited moderate perivascular and periductal lymphocytic infiltrates, most notably in the liver, pancreas, and submandibular glands, that were significantly increased compared with controls (Fig. S6 A and B). Occasional focal areas of parenchymal chronic inflammation and epithelial atrophy were also observed, particularly in the pancreas of bak−/−bax−/−, vav-BCL-2, and bak−/− reconstituted mice but were not detected in bax−/− or WT reconstituted animals. Most bak−/−bax−/−, vav-BCL-2, and bak−/− reconstituted mice also had multiorgan necrotizing vasculitis that was accompanied with serum antineutrophil cytoplasmic autoantibodies (Fig. S6 C and D). The vasculitis, chiefly involved the kidney, pancreas, spleen, submandibular glands, and occasionally the lymph nodes or thymus but spared the heart and liver (Table S1). Vasculitis involving the pancreas was invariably accompanied by focal or extensive inflammation and acinar cell destruction (Fig. 3 and Fig. S6A). Complete acinar cell obliteration was only observed in the vav-BCL-2 reconstituted mice, although similar (albeit less severe) pathology was also detected in bak−/−bax−/− and bak−/− but never in control or bax−/− reconstituted mice. It seems likely that, because the bak−/−bax−/− reconstituted mice died earlier of GN, they did not have time to progress to end-stage exocrine pancreatitis.
Fig. 3.
Mice reconstituted with a vav-BCL-2 and to a lesser extent those reconstituted with a bak−/− bax−/− or bak−/− hematopoietic system develop necrotizing vasculitis. Representative photomicrographs depicting necrotizing vasculitis in various organs from mice reconstituted with FLC of the indicated genotypes. Numbers in brackets indicate the days after reconstitution. (Magnification: ×10 or ×40; scale bar: 128 mm.)
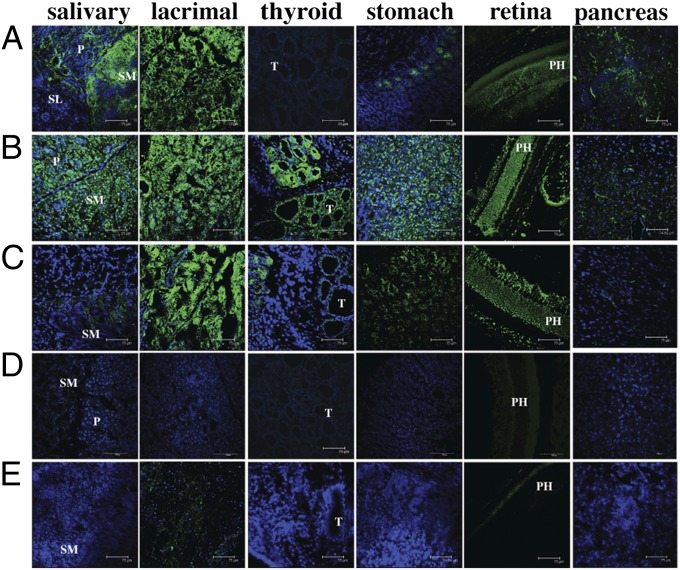
To assess whether the multiorgan lymphocytic infiltration observed in sick bak−/−bax−/− or vav-BCL-2 reconstituted mice was associated with accumulation of autoantibodies to these tissues, we analyzed sera from these animals by immunostaining an array of frozen sections from organs of rag1−/− mice. We found that sera from sick bak−/−bax−/− and vav-BCL-2 reconstituted mice contained autoantibodies that targeted a plethora of organs (Fig. 4 A–E, summarized in Table S2). Indeed, at 8 wk after reconstitution the bak−/−bax−/− reconstituted mice showed a more extensive array of organ-specific autoantibodies compared with the vav-BCL-2 chimeras (Fig. S7 A and B). Interestingly, this autoantibody-mediated staining was mainly confined to discrete secretory tissues within these organs, such as the parietal cells in the stomach (Fig. 4 A–E). This profile of multiple organ autoimmune attack resembles that described for the aire−/− mouse model of the human disease autoimmune polyglandular syndrome 1 (APS1) (20), demonstrating that combined loss of Bak and Bax in hematopoietic cells can cause autoimmune pathology in a broad range of organs.
Fig. 4.
Mice reconstituted with a bak−/−bax−/− or vav-BCL-2 hematopoietic system develop autoantibodies against various tissues. Representative photomicrographs of immunofluorescent staining of cryo-sections of salivary gland, lacrimal gland, stomach, retina, and prostate from Rag-1−/− mice with sera from mice reconstituted with a hematopoietic system of the genotypes indicated. Sera were harvested at the time when mice were sick or at termination of the experiment (bax−/−: 322 d; WT: 190–238 d). n = 4–7 genotype: (A) bak−/− bax−/−, (B) vav-BCL-2, (C) bak−/− (D) bax−/−, and (E) WT reconstituted mice. P, parotid; PH, photoreceptors; SL, sublingual gland; SM, submandibular gland; T, thyroid gland. (Scale bars: 75 μm.)
bak−/−bax−/− Chimeras Have Abnormal T-Cell Repertoire Selection.
To provide mechanistic insight into the pathogenesis in the bak−/−bax−/− reconstituted mice, we examined their T cell receptor (TCR) repertoire. Although the representation of TCRvβ use in mature, peripheral (lymph node) CD4+ and CD8+ (Fig. S8A) T cells was comparable between mice reconstituted with a bak−/−bax−/− or WT hematopoietic system, there was a significant increase in the percentages of CD4+8− thymocytes bearing Vβ4, Vβ5, or Vβ6 containing TCRs in the former (Fig. S8B). In contrast, the TCR repertoire in both the thymus and lymph nodes of bak−/− and bax−/− reconstituted mice was comparable to that of WT reconstituted mice.
To examine whether autoimmunity was accompanied by a particular effector T-cell cytokine profile, we measured hallmark Th1, Th2 and Th17 cytokines and chemokines. Sera of sick bak−/−bax−/−, vav-BCL-2, or bak−/− reconstituted mice contained similar levels of the 23 cytokines and chemokines examined as healthy WT and bax−/− reconstituted mice (Fig. S9). This indicates that a major imbalance in serum cytokines or chemokines is not critical for the autoimmune pathologies caused by combined loss of Bak and Bax, although imbalances in specialized tissue sites cannot be ruled out.
Defects in so-called regulatory lymphocytes (21, 22) or in FasL (5) have been shown to cause lymphadenopathy and autoimmune disease. An examination of the rare B10 (CD19+CD5+CD1d+) B regulatory cells by flow cytometry revealed that mice reconstituted with a hematopoietic system of all genotypes tested (bak−/−bax−/−, bak−/−, bax−/−, WT) had similar frequencies of these cells in their spleens (Fig. S8C). Similarly, there were no marked differences in frequencies of CD4+CD25+Foxp3+ T regulatory cells in peripheral lymphoid organs between the mice with hematopoietic systems of the different genotypes (Fig. S8D). Finally, we found that there were no differences in the levels of FasL on the surface of freshly isolated (not stimulated in vitro) mature, peripheral CD4+ and CD8 T cells between mice with hematopoietic systems of the different genotypes (Fig. S8E).
Collectively, these results show that abnormalities in selection and homeostasis of T and B lymphoid cells, but not defects in cytokine/chemokine production, FasL expression, or frequencies of regulatory T and B cells, contribute to the systemic and organ-specific autoimmune disease pathology seen in the mice with a bak−/−bax−/− or a bak−/− hematopoietic system.
Discussion
Bax and Bak are known to be essential for apoptosis of hematopoietic and many other cell types triggered by diverse developmental cues and stress stimuli (10, 12). Our results demonstrate that Bak/Bax-mediated apoptosis is critical for immunological tolerance of both systemic as well as organ-specific antigens. Furthermore, our data cement the notion that combined Bak plus Bax deficiency only in hematopoietic cells is sufficient to precipitate fatal autoimmune disease. Interestingly, the long-term observation of bak−/− and bax−/− hematopoietic chimeras revealed that loss of Bak alone, but not loss of Bax, in hematopoietic cells shortens animal lifespan owing to autoimmune pathology. Combined loss of both Bak and Bax causes more severe disease and at a substantially accelerated rate, demonstrating that Bak plays a major and Bax a more ancillary role in hematopoietic cells as guardians against autoimmune disease.
Hypergammaglobulinemia or excessive autoantibody levels have not previously been documented in bak−/− mice (11) or the few bak−/−bax−/− mice that survived into early adulthood, for the latter most likely because they could not be followed for sufficient time owing to premature death for reasons other than autoimmune disease (10). Mice with constitutive loss of Bak and conditional loss of Bax only in B cells (CD19-Cre;bak−/−baxfl/- mice) developed B-cell hyperplasia and hypergammaglobinemia (15), demonstrating that some defects elicited by combined Bak/Bax loss are B-cell intrinsic. However, autoantibodies were not reported as a feature of these CD19-Cre;bak−/−baxfl/- animals and were only observed 30 wk after poly I:C/Mxl-Cre–mediated deletion of the loxP flanked bax gene in mice that were constitutively deficient for Bak (Mx1-Cre;bak−/−baxfl/- mice) (15). These findings, in conjunction with the data presented here, indicate that Bak and Bax must be lost not only in B cells but also in other hematopoietic cell subsets to cause severe, fatal autoimmune disease.
Importantly, we found that many organs come under autoimmune attack in bak−/−bax−/− hematopoietic chimeras. The profile of this pathology somewhat resembles that described for the aire−/− mouse model of the human disease APS1 (20). The AIRE protein is expressed in thymic medullary epithelial cells and drives the transcription of a range of organ-specific genes to impose tolerance to the corresponding proteins upon developing thymocytes (23). In aire−/− mice, both the organs targeted and the severity of autoimmunity are affected by genetic background (e.g., relatively mild on a C57BL/6 but severe on the NOD background) (20). It is therefore remarkable that ∼40% of bak−/−bax−/− hematopoietic system reconstituted mice on a C57BL/6 background showed destruction of the exocrine pancreas, either as focal demolition or because of vasculitis, and that 100% of these animals contained autoantibodies to multiple tissues. These findings demonstrate that the intrinsic pathway of apoptosis within hematopoietic cells is critical to prevent organ-specific autoimmune disease.
Organ-specific autoimmune disease was also seen in bak−/− (but not bax−/−) hematopoietic chimeras, albeit at a later time point and with reduced severity compared with the bak−/−bax−/− reconstituted mice. The striking dependency on Bak (but lesser requirement for Bax) for immunological tolerance may be due to differences in the functional interactions of Bak vs. Bax with the prosurvival Bcl-2 family members (18). Thus, loss of Bak may lead to heightened Mcl-1 prosurvival activity (but not expression), which promotes enhanced survival of lymphoid, including activated B cells (24) and myeloid cells (25, 26). However, transgenic mice overexpressing Mcl-1 display considerably less lymphadenopathy, hypergammaglobulinemia, and symptoms of autoimmunity than the bak−/−bax−/− reconstituted or even the bak−/− reconstituted mice (26), Therefore, the loss of Bak and certainly the combined loss of Bak and Bax must impair the intrinsic apoptotic pathway more extensively in hematopoietic cells than Mcl-1 overexpression.
A role for Bak and Bax in the prevention of T-cell malignancies has recently been demonstrated in mice lacking Bak or Bax only in T cells (27). Similar to Bcl-2 overexpression (28, 29), combined loss of Bak and Bax is expected to promote lymphomagenesis by keeping cells alive that would normally be programmed to die, thereby increasing their risk of acquiring additional oncogenic mutations. It would be interesting to know whether mice lacking both Bax and Bak only in T cells (27) also developed autoimmune pathology. Absence of such pathology in these animals would indicate that loss of Bak and Bax not only in T cells but probably also in B cells (and possibly other hematopoietic cell subsets) is required for development of autoimmune disease. We did not observe B- or T-cell lymphomas/leukemias in our bak−/−bax−/− reconstituted animals; however, one bak−/− reconstituted mouse presented with a lymphoma in the pancreas, and one vav-BCL-2 reconstituted mouse developed histiocytic sarcoma. The absence of lymphomas in our bak−/−bax−/− reconstituted mice may be due to the fact that they succumbed early to autoimmune disease before the leukemic precursors had sufficient time to acquire the oncogenic lesions required for malignant transformation.
Mutations in BAK have been associated with some forms of human autoimmune disease (16). Our observation of autoimmune disease in bak−/− chimeras is consistent with these findings and extends them to show that hematopoietic deficiency in Bak is sufficient to cause autoimmunity. Pertinently, in humans polymorphisms in BAK have been associated with Sjögren syndrome (16), and several point mutations in BAK have been associated with vasculitis, particularly in aortic aneurysms (30). In contrast, to date no polymorphisms or mutations in BAX have been associated with autoimmune diseases in humans.
In conclusion, this study highlights the prominent hematopoietic cell intrinsic role of the proapoptotic multi-BH domain Bcl-2 family members Bak and to a lesser extent Bax in the prevention of multiorgan system as well as organ-specific autoimmune disease. Our present and previous findings (31, 32) that defects in the intrinsic apoptotic pathway can cause autoimmune pathology provide support for the idea that these diseases may benefit from treatment with BH3 mimetics, such as ABT-737 (33) or ABT-263 (Navitoclax) (34), which induce apoptosis by antagonizing prosurvival Bcl-2, Bcl-xL, and Bcl-w. Consistent with this idea, the prosurvival proteins Bcl-2 and Bcl-xL are often elevated in lymphoid and myeloid cells in autoimmune diseases, and ABT-737 was shown to trigger apoptosis in certain B as well as T lymphoid cell populations. This enabled ABT-737 to curtail humoral as well as cellular immune responses (33) and reduce autoimmune pathology in several mouse models of SLE-like and other autoimmune diseases (35).
Materials and Methods
Mice and Hematopoietic Reconstitution.
The bak−/− (10) and bax−/− mice (11) were backcrossed for >10 generations onto a C57BL/6 background, then intercrossed to generate bak−/−bax+/− mice. E13.5 embryos were harvested from intercrosses of bak−/−bax+/− mice and FLC suspensions prepared to reconstitute the hematopoietic compartment of lethally irradiated (2 × 5.5 Gy, 2 h apart) C57BL/6-Ly5.1 mice (2 × 106 FLC injected i.v. per recipient). The vav-BCL-2 transgenic mice (generated on an inbred C57BL/6-Ly5.2 background) have been previously described (36).
Histological Analysis.
Histological analysis is detailed in SI Materials and Methods.
Flow Cytometric Analysis, Immunofluorescent Staining, and Confocal Microscopy.
Single cell suspensions of spleen, lymph nodes (pooled axillary, brachial, inguinal, mesenteric), and peripheral blood were stained as previously described (37).
Immune complex deposits and ANA were stained as detailed in SI Materials and Methods. Confocal microscopic detection of organ-specific autoantibodies in sera of sick mice was performed as previously described (37) and in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank C. Thompson (Memorial Sloan-Kettering Cancer Center, New York) for bak−/− mice; the late S. Korsmeyer (Harvard University) for bax−/− mice; L. Lee for technical assistance; G. Siciliano, J. Coughlin, K. McKenzie, F. Dabrowski, S. Ross, S. Green, C. Evans, K. Trueman, E. Lanera, H. Donatucci, and D. Cooper for animal care; J. Corbin for automated blood analysis; B. Helbert, C. Young, and A. Georgiou for genotyping; and S. Mihajlovic, E. Tsui, A. Hasanein, V. Babo, and K. Weston for preparation of histological sections. This work was supported by fellowships and grants from the National Health and Medical Research Council (NHMRC) [Canberra; programs 461221, 461219, fellowships; Australia Fellowship (to A.S.), 637353 (to D.H.D.G.), 516701; and Project Grant 637309 (to A.W.R.), 575535 (to B.T.K.), 637332 (to D.H.D.G.), and 1009145 (to L.A.O.)], an NHMRC infrastructure grant, Independent Research Institutes Infrastructure Support Scheme Grant 361646, the Victorian State Government Operational Infrastructure Support (OIS) grant, the Leukemia and Lymphoma Society [Specialized Centre of Research (SCOR) Grant 7413, fellowship to E.C.J.], National Institutes of Health Grants CA043540-18 and CA80188-6, the Juvenile Diabetes Research Fund (JDRF)/NHMRC (A.S.), the Association for International Cancer Research, the Victorian Cancer Agency (K.D.M. and A.W.R.), the Australian Cancer Research Foundation, the Sylvia and Charles Viertel Charitable Foundation, and the Leukemia Foundation Australia (B.T.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215097110/-/DCSupplemental.
References
- 1.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 3.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25(11):610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14(24):6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 7.Ke F, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19(6):915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis SN, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 10.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 12.Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3(10):932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 13.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi O, et al. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA. 2005;102(32):11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado-Vega AM, et al. Bcl-2 antagonist killer 1 (BAK1) polymorphisms influence the risk of developing autoimmune rheumatic diseases in women. Ann Rheum Dis. 2010;69(2):462–465. doi: 10.1136/ard.2008.100818. [DOI] [PubMed] [Google Scholar]
- 17.Josefsson EC, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208(10):2017–2031. doi: 10.1084/jem.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis SN, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santamaria P. Effector lymphocytes in autoimmunity. Curr Opin Immunol. 2001;13(6):663–669. doi: 10.1016/s0952-7915(01)00276-x. [DOI] [PubMed] [Google Scholar]
- 20.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 24.Vikstrom I, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330(6007):1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89(2):630–643. [PubMed] [Google Scholar]
- 26.Campbell KJ, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116(17):3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas S, et al. A role for proapoptotic Bax and Bak in T-cell differentiation and transformation. Blood. 2010;116(24):5237–5246. doi: 10.1182/blood-2010-04-279687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 29.Strasser A, Harris AW, Cory S. E mu-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene. 1993;8(1):1–9. [PubMed] [Google Scholar]
- 30.Gottlieb B, et al. BAK1 gene variation and abdominal aortic aneurysms. Hum Mutat. 2009;30(7):1043–1047. doi: 10.1002/humu.21046. [DOI] [PubMed] [Google Scholar]
- 31.Strasser A, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 33.Carrington EM, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA. 2010;107(24):10967–10971. doi: 10.1073/pnas.1005256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tse C, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 35.Bardwell PD, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182(12):7482–7489. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]
- 36.Ogilvy S, et al. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96(26):14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’ Reilly LA, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461(7264):659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.