Abstract
Nonspecific histone deacetylase (HDAC) inhibition has been shown to facilitate the extinction of drug-seeking behavior in a manner resistant to reinstatement. A key open question is which specific HDAC is involved in the extinction of drug-seeking behavior. Using the selective HDAC3 inhibitor RGFP966, we investigated the role of HDAC3 in extinction and found that systemic treatment with RGFP966 facilitates extinction in mice in a manner resistant to reinstatement. We also investigated whether the facilitated extinction is related to the enhancement of extinction consolidation during extinction learning or to negative effects on performance or reconsolidation. These are key distinctions with regard to any compound being used to modulate extinction, because a more rapid decrease in a defined behavior is interpreted as facilitated extinction. Using an innovative combination of behavioral paradigms, we found that a single treatment of RGFP966 enhances extinction of a previously established cocaine-conditioned place preference, while simultaneously enhancing long-term object-location memory within subjects. During extinction consolidation, HDAC3 inhibition promotes a distinct pattern of histone acetylation linked to gene expression within the infralimbic cortex, hippocampus, and nucleus accumbens. Thus, the facilitated extinction of drug-seeking cannot be explained by adverse effects on performance. These results demonstrate that HDAC3 inhibition enhances the memory processes involved in extinction of drug-seeking behavior.
Keywords: long-term memory, epigenetics, chromatin
Numerous studies have demonstrated that long-term memory mechanisms require transcription (1), likely because gene expression is necessary for the stable changes in neuronal plasticity ultimately driving long-term changes in behavior. A key mechanism by which gene expression profiles are regulated is chromatin modification. One of the best-studied chromatin modifying mechanisms is histone acetylation, carried out by histone acetyltransferases and histone deacetylases (HDACs), which in general facilitate and repress gene expression, respectively (2–4). Several studies have demonstrated that manipulating histone acetyltransferases and HDACs can alter memory processes during initial memory formation (5–13) as well as extinction memory processes (14–17). Extinction is a transcription-dependent process (18–20) through which a previously held conditioned response (such as drug-seeking) is reduced or eliminated.
Recently, it was reported that pharmacologic inhibition of HDACs facilitates extinction of drug-seeking, resulting in rapid and persistent loss of a previously established behavior that is resistant to reinstatement (16, 21). One interpretation of this action is that HDAC inhibition robustly enhances consolidation of extinction memory, suggesting that HDACs normally function as negative regulators of extinction learning, similar to their role in initial memory consolidation. However, the finding that inhibition of HDACs during consolidation of extinction prevents cocaine-induced reinstatement remains open to interpretation. Given that recovery phenomena (i.e., renewal, reconditioning, and reinstatement) are used to demonstrate that the original memory is preserved after extinction (22–27), the ability of HDAC inhibition to eliminate drug-seeking might not reflect an effect on extinction. To our knowledge, there is no drug that blocks consolidation and enhances extinction; however, whether HDAC inhibitors lead to a rapid and persistent decrease of a previously learned behavior by enhancing learning processes during extinction or by negatively affecting performance or disrupting reconsolidation of the original memory is not clear. We examined these possibilities using an innovative behavioral paradigm in which the effect of a pharmacologic treatment on extinction of a previously established drug-seeking behavior can be assessed simultaneously with the effect on memory of an initial learning experience in object recognition. Thus, if HDAC inhibition is enhancing memory processes of extinction, then we predict positive effects on both extinction of a previously learned behavior (measured as decreased drug-seeking) and memory of an initial learning experience (increased discrimination for novelty), even though these performance measures are opposite in nature. Conversely, if HDAC inhibition is disrupting reconsolidation, then there will be a decrease in drug-seeking, with subsequent impaired memory for novelty.
No previous study has examined the role of individual HDACs in extinction of drug-seeking. The HDAC inhibitors (e.g., sodium butyrate) that have been used to enhance extinction target primarily class I HDACs (HDAC1, 2, 3, and 8) (28). Recent work demonstrated that HDAC1 inhibition impairs extinction, whereas HDAC1 overexpression facilitates extinction (17). This finding suggests that HDAC1 may be unique among class I HDACs, and that nonspecific HDAC inhibitors may be facilitating extinction via HDAC2 or 3. HDAC3 is the most highly expressed class I HDAC in the brain (29), and has been identified as a critical negative regulator of learning and memory (13). Thus, we hypothesized that HDAC3 is a negative regulator of memory processes involved in extinction of drug-seeking behavior. In the present study, we used the HDAC3-selective inhibitor RGFP966 to examine the role of HDAC3 in memory processes involved in the consolidation of extinction of drug-seeking behavior.
Results
RGFP966 in the Brain After Dosing.
RGFP966 is an N-(o-aminophenyl)carboxamide HDAC inhibitor (Fig. S1A). As a class, these slow-on/slow-off, competitive tight-binding inhibitors target class I HDACs, with the greatest inhibition of HDAC3 (30–32). A substrate-dependent biochemical assay using recombinant human HDACs preincubated for 2 h with inhibitor (Reaction Biology) found that RGFP966 is specific for HDAC3, with an IC50 of 0.08 μM and no effective inhibition of any other HDAC at concentrations up to 15 μM. To investigate the concentration of RGFP966 in the brain, we treated mice with 10 mg/kg RGFP966 administered s.c., then collected plasma and brain tissue at 15, 30, 60, and 120 min after treatment and analyzed the samples for drug levels. The maximum drug concentration (Cmax) in the brain was 1.25 µg/g (3.15 µM) at 30 min (Fig. S1B). RGFP966 concentration remained high up to 60 min and then declined significantly by 120 min [F(3,16) = 14.48, P < 0.001; Bonferroni post hoc: 30 min vs. 120 min, P < 0.05], but was still 0.57 µg/g (1.5 µM). Distribution of RGFP966 to the CNS was relatively efficient, with a brain:plasma ratio of 0.45. These results confirm that s.c. dosing of RGFP966 at 10 mg/kg resulted in maximal brain exposure between 30 min and 1 h, with brain concentrations in excess of the IC50 of RGFP966 for HDAC3 for the entire sampling period (0.25–2 h).
RGFP966 Treatment Enhances Long-Term Memory for Object Memory.
To examine the ability of this HDAC3 inhibitor to modulate long-term memory consolidation, we treated mice with RGFP966 (3, 10, or 30 mg/kg) or vehicle and trained them in object recognition (ORM) or location-dependent object recognition (OLM), with a subthreshold training period. Systemic delivery of RGFP966 after ORM training (Fig. S2A) significantly increased preference for the novel object in a dose-dependent manner [F(3,21) = 11.15, P < 0.01; Bonferroni post hoc vs. vehicle: 3 mg/kg RGFP966, P < 0.05; 10 mg/kg RGFP966, P < 0.05; 30 mg/kg RGFP966, P < 0.05] (Fig. S2B). Groups did not differ in terms of total exploration time of the two objects [vehicle: 3.8 ± 1.0, n = 6; RGFP966 3 mg/kg: 3.7 ± 0.4, n = 5; RGFP966 10 mg/kg: 4.5 ± 0.4, n = 9; RGFP966 30 mg/kg: 4.4 ± 0.7, n = 5; F(3,17) = 0.35, P = 0.79]. Mice treated with 10 mg/kg RGFP966 either 1 h before (pretraining) or immediately after (posttraining) subthreshold OLM training (Fig. S2C) showed enhanced preference for the object in a novel location during the retention test [F(2,17) = 47.14, P < 0.001; Bonferroni post hoc vs. vehicle: 10 mg/kg RGFP966 posttraining, P < 0.05; 10 mg/kg RGFP966 pretraining, P < 0.05], with no affect on the total exploration time of objects [vehicle: 8.7 ± 2.3, n = 7; RGFP966 10 mg/kg pretraining: 8.0 ± 2.7, n = 6; RGFP966 10 mg/kg posttraining: 7.4 ± 1.9, n = 7; F(2, 17) = 0.59, P = 0.56] (Fig. S2D). These results demonstrate that RGFP966 enhances acquisition/consolidation of memory.
RGFP966 Facilitates Extinction and Prevents Reinstatement of Cocaine- Conditioned Place Preference.
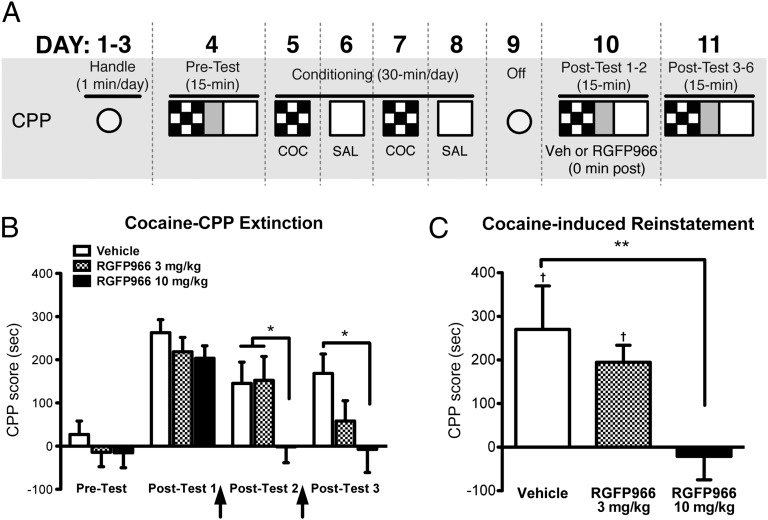
To examine the ability of RGFP966 to facilitate extinction of cocaine-conditioned place preference (CPP), we administered RGFP966 (3 or 10 mg/kg, s.c.) or vehicle immediately after posttest 1 and posttest 2 (Fig. 1A). All mice exhibited a robust preference for the cocaine-paired context after cocaine-CPP training [posttest 1; effect of test: F(1,33) = 135.8, P < 0.001; effect of treatment: F(2,33) = 1.016, P = 0.37] (Fig. 1B). Treatment with RGFP966 immediately after the drug-free preference tests resulted in significant extinction of CPP on posttest 2 and posttest 3 [effect of treatment × test, F(6,99) = 2.68, P < 0.05]. Treatment with 10 mg/kg, but not with 3 mg/kg, resulted in significantly rapid reduction of CPP on the subsequent days (posttest 2: Bonferroni post hoc, 10 mg/kg RGFP966 vs. vehicle, P < 0.05; 10 mg/kg RGFP966 vs. 3 mg/kg RGFP966, P < 0.05; posttest 3: 10 mg/kg RGFP966 vs. vehicle, P < 0.05; 10 mg/kg RGFP966 vs. 3 mg/kg RGFP966, P = not significant; 3 mg/kg RGFP966 vs. vehicle, P > 0.05 for all test days). These results indicate that RGFP966 facilitates extinction of cocaine-seeking behavior.
Fig. 1.
HDAC3 inhibition enhances the rate and persistence of extinction of cocaine-CPP. (A) Schematic of cocaine-CPP training and extinction. (B) Treatment with RGFP966 (10 mg/kg) after posttest 1 significantly reduced cocaine-CPP, and the effect was maintained in subsequent CPP tests. Vehicle, n = 12; RGFP966 3 mg/kg, n = 12; RGFP966 10 mg/kg, n = 12. (C) Mice treated with RGFP966 (10 mg/kg) during extinction consolidation did not reinstate cocaine-seeking behavior; Vehicle, n = 8; RGFP966 3 mg/kg, n = 7; RGFP966 10 mg/kg, n = 8. Data are expressed as mean ± SEM. †Significantly different from 0. *Significantly different from vehicle; P < 0.05.
To investigate the lasting effect of extinction, we subjected mice that had achieved an a priori extinction criterion by posttest 6 [one-sample t test, CPP score vs. 0: vehicle, 46.11 ± 54.85, t(6) = 0.84, P = 0.43; 3 mg/kg RGFP966, −38.36 ± 49.73, t(7) = 0.77, P = 0.47; 10 mg/kg RGFP966, 45.43 ± 40.74, t(7) = 1.11, P = 0.30; one-way ANOVA, F(2,20) = 1.03, P = 0.37] to a reinstatement test. Mice previously treated with vehicle and 3 mg/kg RGFP966 significantly reestablished preference for the cocaine compartment [one-sample t test, CPP score vs. 0: vehicle, 270.2 ± 99.7, t(6) = 2.71, P < 0.05; 3 mg/kg RGFP966, 194.8 ± 39.03, t(7) = 4.99, P < 0.01] (Fig. 1C). However, mice previously treated with 10 mg/kg RGFP966 did not reestablish a preference (one-sample t test, CPP score vs. 0: −21.33 ± 53.54, t(7) = 0.40, P = 0.70; one-way ANOVA, F(2,20) = 5.29, P < 0.05; Bonferroni post hoc, vehicle vs. 3 mg/kg RGFP966, P = not significant; vehicle vs. 10 mg/kg RGFP966, P < 0.05). These results indicate that treatment with 10 mg/kg RGFP966 immediately after extinction completely blocks reinstatement of cocaine-seeking behavior.
Systemic RGFP966 Treatment After Cocaine-CPP Extinction Simultaneously Reduces Drug-Seeking Behavior and Enhances Long-Term Object Memory.
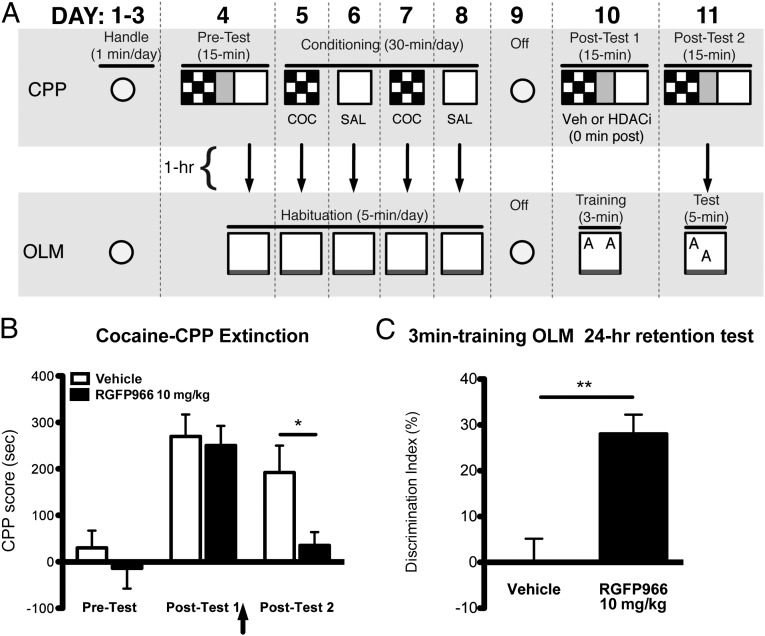
Given that the observed decrease in cocaine-CPP behavior after HDAC inhibitor treatment may be a result of either enhanced extinction or disrupted reconsolidation/performance, we developed a combined behavioral paradigm to assess memory enhancement versus impairment. By combining the extinction of cocaine-CPP and acquisition of OLM, we could assess the effect of a single injection of HDAC inhibitor on memory processes involved in extinction based on performance in both tasks simultaneously. After CPP training and OLM habituation (Fig. 2A), all mice exhibited robust CPP on posttest 1 [effect of test: F(2,20) = 21.65, P < 0.001, Bonferroni post hoc: posttest 1 vs. pretest, P < 0.05] (Fig. 2B). Immediately after this nonreinforced exposure in the previously drug-paired context, mice received vehicle or 10 mg/kg RGFP966 and 1 h later were trained with subthreshold OLM (Fig. 2A), a time point at which RGFP966 is still present in brain (Fig. S1B). On posttest 2, mice treated with 10 mg/kg RGFP966 exhibited significantly reduced CPP for the previously cocaine-paired context compared with controls (Bonferroni post hoc: posttest 2, vehicle vs. RGFP966, P < 0.05) (Fig. 2B), replicating our findings shown in Fig. 1B. After posttest 2, the same RGFP966-treated mice showed significantly increased preference for the object in a novel location compared with controls [t test, t(10) = 4.15, P < 0.01] (Fig. 2C), with no difference in total exploration time (vehicle: 12.68 ± 0.9527, n = 6; RGFP966: 12.56 ± 1.178, n = 6; t = 0.08, P = 0.94). In contrast, when RGFP966 was administered 8 h after training, there was no difference in cocaine-induced CPP or OLM compared with controls (CPP posttest 2: vehicle, n = 7, 127.9 ± 22.87 s, RGFP966 10 mg/kg, n = 8, 127.8 ± 41.61 s, P = not significant; OLM: vehicle, n = 7, −7.05 ± 1.5 s, RGFP966 10 mg/kg, n = 8, 4.6 ± 2.5 s, P = not significant). These findings indicate that the learning event (extinction or OLM) must be temporally contiguous with RGFP966 memory enhancement. Taken together, these results demonstrate that RGFP966 simultaneously enhances extinction consolidation of cocaine-CPP and acquisition of OLM.
Fig. 2.
HDAC3 inhibition simultaneously reduces drug-seeking behavior and enhances memory. (A) Schematic of cocaine-CPP training and extinction, followed in parallel with OLM training. RGFP966 was administered immediately after extinction training, 1 h before OLM training in the same mice. Vehicle, n = 6; RGFP966 10 mg/kg; n = 6. (B) RGFP966 administered immediately after a nonreinforced exposure resulted in loss of CPP the next day. (C) RGFP966 treatment resulted in significantly enhanced object location memory. Data are expressed as mean ± SEM. *Significantly different from vehicle; P < 0.05.
To further examine the efficacy of RGFP966 in reducing drug-seeking behavior after extinction, we used a strong training paradigm (Fig. S3A) to establish robust CPP [posttest 1; vehicle: 246.1 ± 20.6 s, n = 6, one-sample t test, t(5) = 11.94, P < 0.001; RGFP966 10 mg/kg: 242.6 ± 16.1 s, n = 6, one-sample t test, t(5) = 11.94, P < 0.001] (Fig. S3B). Mice were treated with a single injection of RGFP966 (10 mg/kg) after posttest 1 and received subthreshold OLM training. When tested the next day for CPP and OLM, mice previously treated with RGFP966 no longer showed a significant preference for the cocaine-paired compartment by posttest 3, whereas vehicle-treated mice continued to show robust cocaine-CPP [significant effect of test: F(3,30)=14.11, P < 0.001, Bonferroni post hoc: vehicle, pretest vs. posttest 3, P < 0.05; RGFP966, pretest vs. posttest 3, P = not significant] (Fig. S3B), and mice treated with RGFP966 also showed significantly increased preference for the object in a novel location compared with controls [unpaired t test, t(10) = 7.47, P < 0.001], with no difference in total exploration time [vehicle: 9.1 ± 2.1, n = 6; RGFP966: 8.0 ± 1.5, n = 6; t(10) = 0.44, P = 0.67] (Fig. S3C). These results demonstrate that inhibition of HDAC3 can facilitate extinction of robust CPP behavior.
RGFP966 Treatment Promotes Acetylation of Specific Histone Residues After Extinction.
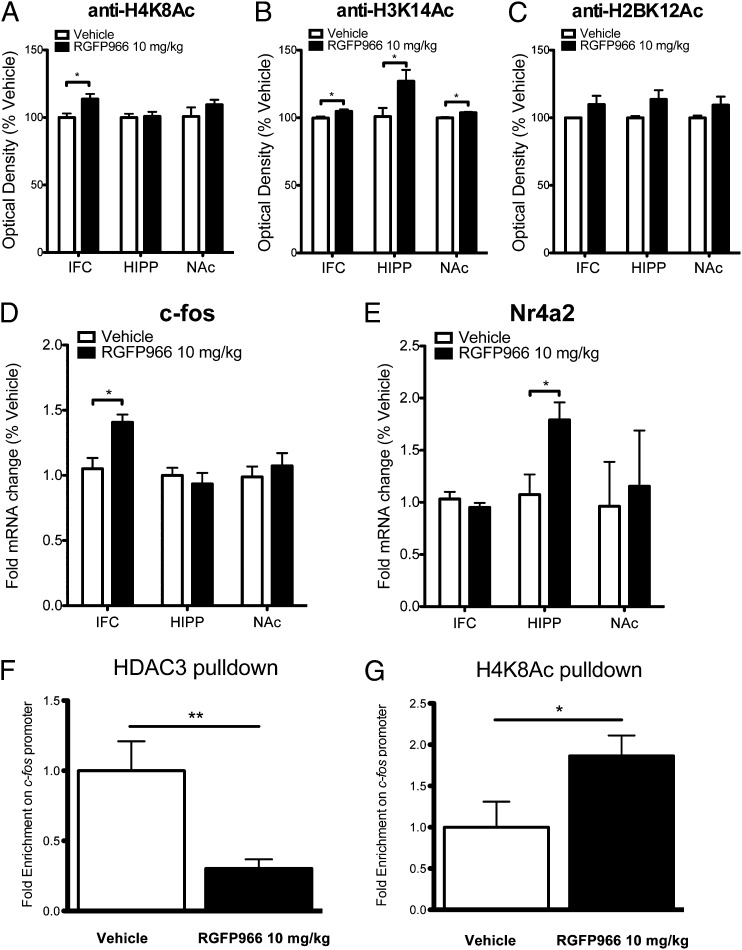
The enhanced extinction of cocaine-CPP resulting from inhibition of HDAC3 during extinction consolidation led us to investigate how RGFP966 affects histone acetylation. We examined the infralimbic cortex (IFC), hippocampus (HIPP), and nucleus accumbens (NAc) at 1 h after the first extinction session with vehicle or 10 mg/kg RGFP966 posttreatment. We first examined the acetylation of lysine 8 on H4 (H4K8Ac), a site regulated by HDAC3 activity in the brain (13), and found it was significantly increased in the IFC, but not in the HIPP or NAc, of the RGFP966-treated mice [one-sample t test: IFC, t(8) = 2.85, P < 0.05; HIPP, t(4) = 0.19, P = 0.86; NAc, t(4) = 1.14, P = 0.32] (Fig. 3A). We then examined the acetylation of lysine 14 on histone H3 (H3K14Ac), which has been shown to correlate with learning (8, 12, 33, 34). We found a significant increase in H3K14Ac within the IFC, HIPP, and NAc in response to RGFP966 treatment [one-sample t test: IFC, t(8) = 2.74, P < 0.05; HIPP, t(8) = 2.49, P < 0.05; NAc, t(9) = 4.65, P < 0.01] (Fig. 3B). We also found that RGFP966 did not alter the acetylation of lysine 12 on histone H2B [H2BK12Ac; one-sample t test: IFC, t(9) = 0.97, P = 0.35; HIPP, t(9) = 1.11, P = 0.30; NAc, t(9) = 0.69, P = 0.51] (Fig. 3C). Taken together, these findings demonstrate that systemic treatment with RGFP966 after extinction training results in specific enhancement of histone acetylation within brain regions implicated in the extinction of drug-seeking behavior (35, 36).
Fig. 3.
HDAC3 inhibition promotes histone acetylation and gene expression during extinction consolidation. (A) RGFP966 treatment promoted acetylation of H4K8 in the IFC (vehicle, n = 5; RGFP966, n = 5), but not in the HIPP (vehicle, n = 3; RGFP966, n = 3) or NAc (vehicle, n = 3; RGFP966, n = 3). (B and C) RGFP966 treatment increased acetylation of H3K14 in the IFC (vehicle, n = 5; RGFP966, n = 5), HIPP (vehicle, n = 5; RGFP966, n = 5), and NAc (vehicle, n = 5; RGFP966, n = 6) during extinction consolidation (B), with no difference in acetylation of H2BK12 (vehicle, n = 6; RGFP966, n = 5 for each brain region) (C). (D) c-FOS expression was significantly increased in the IFC (vehicle, n = 6; RGFP966, n = 5), but not in the HIPP (vehicle, n = 7; RGFP966, n = 6) or NAc (vehicle, n = 9; RGFP966, n = 7), of mice treated with RGFP966. (E) NR4A2 expression was significantly increased in the HIPP (vehicle, n = 5; RGFP966, n = 5), but not in the IFC (vehicle, n = 6; RGFP966, n = 5) or NAc (vehicle, n = 6; RGFP966, n = 5), after RGFP966 treatment during extinction consolidation. (F and G) RGFP966 treatment led to decreased HDAC3 occupancy at the c-fos promoter (vehicle, n = 6; RGFP966, n = 7) (F), with a corresponding increase in acetylation of H4K8 (vehicle, n = 6; RGFP966, n = 8) (G). Data are expressed as mean ± SEM. *Significantly different from vehicle; P < 0.05.
HDAC3 Regulates Gene Expression During Extinction Consolidation.
Acetylation of histones is a key mechanism in transcriptional regulation (4, 37). We predicted that the inhibition of HDAC3 and the resulting increase in histone acetylation would regulate the expression of key genes required for long-term changes in behavior. We examined c-fos and Nr4a2 because these immediate early genes (IEGs) are known to be regulated by HDAC3 in the brain (13). We examined c-fos expression during the consolidation of extinction, at 1 h after extinction training. We found that HDAC3 inhibition promotes expression of the IEG c-fos in the IFC, but not in the HIPP or NAc, during extinction consolidation [one-sample t test: IFC, t(7) = 5.08, P < 0.01; HIPP, t(9) = 0.60, P = 0.70; NAc, t(9) = 0.43, P = 0.68] (Fig. 3D).
We next examined expression of the IEG and transcription factor Nr4a2 during extinction consolidation, which is known to play a key role in initial memory consolidation (38, 39). We found that mice treated with RGFP966 postextinction had increased Nr4a2 expression in the HIPP, but not in the IFC or NAc [one-sample t test: IFC, t(9) = 0.95, P = 0.37; HIPP, t(8) = 2.80, P < 0.05; NAc, t(9) = 0.28, P = 0.78] (Fig. 3E). Taken together, these results demonstrate that systemic treatment with RGFP966 promotes gene expression during extinction consolidation in brain regions implicated in extinction.
Finally, we examined the interaction of HDAC3 at the c-fos promoter in the IFC during extinction consolidation. ChiP analysis showed that RGFP966 significantly decreases HDAC3 occupancy at the c-fos promoter [one-sample t test: t(11) = 3.39, P < 0.01] (Fig. 3F) and promotes acetylation of histone H4K8 [one-sample t test: t(12) = 2.14, P < 0.05] during extinction consolidation (Fig. 3G). These findings indicate that RGFP966 alters the regulation of c-fos via HDAC3 and its acetylation during the consolidation of extinction.
Discussion
Enhancing the extinction of drug-seeking behavior via HDAC inhibition has tremendous therapeutic potential. Whether the rapid and persistent extinction of behavior results from the enhancement of memory processes involved in extinction is unclear, however. In the present study, we validate HDAC3 as a negative regulator of initial memory formation for ORM, and extend this to extinction consolidation of drug-seeking behavior. Our findings demonstrate that HDAC3 inhibition facilitates extinction by enhancing memory processes during consolidation of extinction. HDAC3 inhibition during acquisition or consolidation of a subthreshold ORM learning event resulted in robust long-term memory. Treatment with the HDAC3 inhibitor resulted in rapid and persistent extinction of cocaine-CPP. Using an innovative combination of behavioral paradigms, we have demonstrated that a single treatment with RGFP966 enhances the consolidation of extinction of a previously established cocaine-CPP, while enhancing OLM. Moreover, HDAC3 inhibition promotes the histone acetylation and gene expression necessary for memory formation during extinction consolidation. Thus, the observed blocked reinstatement of drug-seeking behavior cannot be explained simply by performance issues. Our results demonstrate that HDAC3 inhibition enhances memory processes involved in extinction of drug-seeking behavior.
Numerous previous studies have identified HDACs as powerful negative regulators of long-term potentiation and long-term memory (5, 7–9, 14, 15, 40–43). A recent analysis demonstrated that the HDAC inhibitors used in long-term memory studies (e.g., sodium butyrate, valproate) are potent inhibitors of class I HDACs (28). This suggests that class I HDACs (e.g., HDAC2 and HDAC3, but not HDAC1 (7, 13, 17) play a significant role in the pharmacologic enhancement of memory. HDAC1 appears to have a permissive role in extinction of fear-related memories, but no role in initial memory formation (17). HDAC3 is the most highly expressed class I HDAC in the brain (29). In the present study, we found that the HDAC3 inhibitor RGFP966 transforms a subthreshold learning event into a robust long-term memory and facilitates the extinction of cocaine-seeking behavior in a persistent form that is refractive to reinstatement, indicating that HDAC3 normally has a repressive role in extinction processes.
The removal of acetylation by HDAC3 represses transcription by increasing the affinity of histone tails for DNA and recruiting complementary enzymes (e.g., methyltransferases, phosphatases) that further modify chromatin, reducing accessibility to DNA. Low levels of acetylation reduce the recruitment of transcriptional coactivators that typically bind acetyl-lysine motifs (44). Notably, HDAC3 can deacetylate, and thus inhibit the function of, the acetyltransferase CBP (45), which was recently shown to be necessary for the acquisition of cocaine-CPP (11). In a complex with corepressors, HDAC3 can interact with transcription factors, keeping their target genes in an inactive state (46, 47). Based on these findings, HDAC3 can be predicted to negatively regulate long-term memory. However, its role in extinction processes is not as predictable, in light of findings demonstrating that specific HDACs can play unique roles in memory processes (7, 13, 17), highlighting the significance of our present findings. In this study, we found that inhibition of HDAC3 results in reduced interaction with the c-fos promoter, leading to increased levels of histone acetylation, which promotes the gene expression necessary for extinction processes in the IFC.
In a previous study using a genetic and pharmacologic approach, we found that HDAC3 is a critical negative regulator of long-term memory formation (13). Focal deletion of HDAC3 in the dorsal CA1 region of the HIPP resulted in increased histone acetylation, gene expression, and long-term memory (13). HDAC3-dependent modulation of memory was found to be dependent on the transcription factor Nr4A2 (13). Using a selective HDAC3 inhibitor called RGFP136, we demonstrated parallel effects on histone acetylation and long-term memory formation to the genetic deletion of HDAC3 (13). In the present study, we could not use a focal deletion of HDAC3, because this could have effects on acquisition/consolidation of CPP that would confound the interpretation of extinction data. Thus, we only examined the effect of the HDAC3 inhibitor RGFP966 on extinction. RGFP966 is a more selective inhibitor than RGFP136 with a longer elimination half-life, significantly improved exposure, and better blood-brain barrier penetrance (with s.c. dosing producing a fourfold increase in overall brain exposure at the time when maximum brain concentration was reached for RGFP966 compared with RGFP136, with an improved brain:plasma ratio of 0.5 for RGFP966 vs. 0.2 for RGFP136 in mice), which makes it a more attractive compound for therapeutic use.
An alternative explanation for the absence of drug-seeking behavior after HDAC inhibition may be disrupted reconsolidation (e.g., memory erasure) or performance. In the present study, we examined this possibility by combining the extinction of drug-seeking and acquisition of a new memory. Because extinction is measured as a reduction in behavior and ORM is observed as increased exploratory behavior of novelty, we were able to assess the effects on memory and performance. We found that selective inhibition of HDAC3 simultaneously eliminates drug-seeking behavior and enhances memory of a separate learning event, demonstrating that HDAC inhibition does not simply impair performance. Moreover, the effect of HDAC3 inhibition on extinction of drug-seeking behavior was temporally restricted. Taken together, these behavioral findings support the idea that inhibition of HDAC3 enhances memory processes involved in extinction consolidation. The persistent effect of HDAC inhibition on the extinction of cocaine-seeking behavior is consistent with the idea that specific HDACs are negative regulators of memory processes (48). It is hypothesized that pharmacologic inhibition of these negative regulators sets up a permissive state for the transcription processes required for consolidation, generating robust long-lasting memories, including persistent extinction that is refractive to reinstatement. In line with this hypothesis, RGFP966 reduced HDAC3 repression of c-fos and Nr4a2 and allowed increased acetylation of H4K8 and H3K14, two sites implicated in memory processes, during extinction consolidation.
In summary, HDAC3 appears to be a key negative regulator of memory formation, including the extinction of established memories. In the case of maladaptive behaviors, such as drug-seeking behavior, HDAC3 inhibition during extinction learning or cognitive behavioral therapy in drug abuse clinics and research centers may be a powerful cotherapy. This approach also could be applied to other cognitive disorders, such as posttraumatic stress disorder or anxiety disorders, that could potentially benefit from more robust extinction memory. With regard to basic research, there are many open questions regarding mechanisms, including the exact nature of the corepressor complex, what genes are being coordinately regulated, and where this regulation is most important in terms of extinction of drug-seeking behavior. The present study has begun to elucidate a specific pattern of histone acetylation and gene expression within the IFC, HIPP, and NAc that correlates with enhanced extinction. Future studies will be pivotal to our understanding of the HDAC3-dependent mechanisms important in changing cell function and ultimately leading to stable changes in behavior.
Materials and Methods
Male C57BL/6J mice were given the HDAC3 inhibitor RGFP966 to assess the effect on ORM, OLM, and extinction of cocaine-induced CPP. In brief, mice were treated with RGFP966 or vehicle and trained on ORM or OLM with a subthreshold training period. For extinction of CPP, RGFP966 or vehicle was given immediately after posttest 1 and posttest 2, and once all mice achieved an a priori extinction criterion, they received a reinstatement test. The effect of RGFP966 was examined by combining the extinction of cocaine-CPP and acquisition of OLM, separated by 1 h, in the same mice. The distribution of RGFP966 in brain and plasma was determined in a substrate-dependent biochemical assay using recombinant human HDACs at 15, 30, 60, and 120 min after systemic treatment. Histone acetylation, gene expression, and ChiP were examined during the consolidation of extinction in conjunction with RGFP966 treatment, with α levels were held at 0.05. See SI Materials and Methods for more detailed information.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Mental Health Grant R01 MH081004 (to M.A.W.), National Institute on Drug Abuse Grants R01 DA025922 and R21 DA031989 (to M.A.W.),National Research Service Award Predoctoral Fellowship F31DA29368 (to M.M.), T32 Institutional Training Grant NS045540 (to G.A.R.), and Postdoctoral Fellowship F32DA031520 (to G.A.R.).
Footnotes
Conflict of interest statement: G.A.R. and M.A.W. received research support from a Sponsored Research Agreement from Repligen Corp. V.J., S.C., and J.R.R. are employed by Repligen Corp. M.M. and S.C.M. report no financial disclosures.
See Commentary on page 2442.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213364110/-/DCSupplemental.
References
- 1.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yacubian J, et al. Gene–gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104(19):8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Alarcón JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: A model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 9.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27(23):6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13(5):609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. J Neurosci. 2011;31(47):16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett RM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36(8):1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McQuown SC, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31(2):764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121(5):1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14(4):268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67(1):36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahari-Javan S, et al. HDAC1 regulates fear extinction in mice. J Neurosci. 2012;32(15):5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci. 2010;21(1):1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lattal KM, Radulovic J, Lukowiak K. Extinction: Does it or doesn’t it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry. 2006;60(4):344–351. doi: 10.1016/j.biopsych.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Zhang Y, Qing H, Liu M, Yang P. The extinction of morphine-induced conditioned place preference by histone deacetylase inhibition. Neurosci Lett. 2010;483(2):137–142. doi: 10.1016/j.neulet.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 22.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 23.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 24.Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13(5-6):397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12(3):270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlov IP, Anrep GV. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford Univ Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rescorla RA. Retraining of extinguished Pavlovian stimuli. J Exp Psychol Anim Behav Process. 2001;27(2):115–124. [PubMed] [Google Scholar]
- 28.Kilgore M, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35(4):870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broide RS, et al. Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci. 2007;31(1):47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- 30.Chou CJ, Herman D, Gottesfeld JM. Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J Biol Chem. 2008;283(51):35402–35409. doi: 10.1074/jbc.M807045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, et al. Chemical probes identify a role for histone deacetylase 3 in Friedreich’s ataxia gene silencing. Chem Biol. 2009;16(9):980–989. doi: 10.1016/j.chembiol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai M, et al. Two new pimelic diphenylamide HDAC inhibitors induce sustained frataxin up-regulation in cells from Friedreich’s ataxia patients and in a mouse model. PloS ONE. 2010;5(1):e8825. doi: 10.1371/journal.pone.0008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27(46):12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13(3):322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17(4):168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87(5):953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 38.Colón-Cesario WI, et al. Knockdown of Nurr1 in the rat hippocampus: implications to spatial discrimination learning and memory. Learn Mem. 2006;13(6):734–744. doi: 10.1101/lm.407706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña de Ortiz S, Maldonado-Vlaar CS, Carrasquillo Y. Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem. 2000;74(2):161–178. doi: 10.1006/nlme.1999.3952. [DOI] [PubMed] [Google Scholar]
- 40.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodeling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 41.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106(23):9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haettig J, et al. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18(2):71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15(1):39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L, Zhou MM. Bromodomain: An acetyl-lysine binding domain. FEBS Lett. 2002;513(1):124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 45.Chuang HC, Chang CW, Chang GD, Yao TP, Chen H. Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucleic Acids Res. 2006;34(5):1459–1469. doi: 10.1093/nar/gkl048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinzel T, et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387(6628):43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 47.Lutterbach B, et al. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol. 1998;18(12):7176–7184. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McQuown SC, Wood MA. HDAC3 and the molecular brake pad hypothesis. Neurobiol Learn Mem. 2011;96(1):27–34. doi: 10.1016/j.nlm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.