Abstract
Gastrin releasing-peptide (GRP) is a potent growth factor in many malignancies. Benign prostatic hyperplasia (BPH) is a progressive age-related proliferation of glandular and stromal tissues; various growth factors and inflammatory processes are involved in its pathogenesis. We have demonstrated that potent antagonists of GRP inhibit growth of experimental human tumors including prostate cancer, but their effect on models of BPH has not been studied. Here, we evaluated the effects of GRP antagonist RC-3940-II on viability and cell volume of BPH-1 human prostate epithelial cells and WPMY-1 prostate stromal cells in vitro, and in testosterone-induced BPH in Wistar rats in vivo. RC-3940-II inhibited the proliferation of BPH-1 and WPMY-1 cells in a dose-dependent manner and reduced prostatic cell volume in vitro. Shrinkage of prostates was observed after 6 wk of treatment with RC-3940-II: a 15.9% decline with 25 μg/d; and a 18.4% reduction with 50 μg/d (P < 0.05 for all). Significant reduction in levels of proliferating cell nuclear antigen, NF-κβ/p50, cyclooxygenase-2, and androgen receptor was also seen. Analysis of transcript levels of genes related to growth, inflammatory processes, and signal transduction showed significant changes in the expression of more than 90 genes (P < 0.05). In conclusion, GRP antagonists reduce volume of human prostatic cells and lower prostate weight in experimental BPH through direct inhibitory effects on prostatic GRP receptors. GRP antagonists should be considered for further development as therapy for BPH.
Keywords: bombesin, cell volume measurement, prostatic inflammation, rodent benign prostatic hyperplasia model, targeted therapy
Hormonal polypeptides, bombesin (BN) and gastrin-releasing peptide (GRP), can act as autocrine and paracrine growth factors, and regulate cellular growth, differentiation, and apoptosis (1, 2). Tetradecapeptide bombesin was isolated from the skin of the European fire-bellied toad (Bombina bombina) (3), and subsequently, two mammalian bombesin-like peptides were characterized: GRP and neuromedin B (NMB) (4). GRP and BN influence the secretion of gastrointestinal hormones, stimulate the release of gastrin and somatostatin, induce pancreatic exocrine secretion (5), and induce contractions of the smooth muscle of the stomach, gall bladder, uterus, urinary bladder, and prostate (6). GRP was first shown to be a mitogen for Swiss 3T3 murine embryonal fibroblasts and, subsequently, for a number of normal cell types and cancers in vivo and in cell culture (7). GRP is now recognized as the prototypical autocrine growth factor, based on the detection of GRP and its receptors in small cell lung carcinoma and other tumors (8). GRP was shown to stimulate growth of several other types of carcinomas including those of prostate, breast, colon, and pancreas (9). Three different receptor subtypes for the BN/GRP family of peptides have been described in mammals (10). Receptor subtype 1 (termed GRP-R or BB2) binds BN, GRP, and GRP antagonist RC-3940-II with high affinity (11). Subtype 2 (termed NMB-R or BB1) prefers neuromedin B but also shows moderate affinity for GRP; subtype 3 (BRS-3 or BB3) is classified as an orphan receptor because its natural ligand is not yet identified (10).
We and others have developed antagonists of GRP for the treatment of various malignancies (12, 13). These antagonists act by blocking the GRP receptors (GRP-Rs). Potent GRP antagonists, including RC-3940-II, have been shown to inhibit the growth of human experimental prostatic cancers and numerous other cancers and to suppress tumoral growth factors such as EGF, VEGF, and basic FGF, and to down-regulate their receptors (10, 13–16). GRP receptor subtype 1 has been also detected in normal, hyperplastic, and malignant human prostate tissues and prostatic cancer cell lines (17–20).
Benign prostatic hyperplasia (BPH) is a progressive age-related pathologic proliferation of prostatic glandular and stromal tissues (21). BPH is clinically characterized by prostatic enlargement and lower urinary tract symptoms. There is no completely effective treatment for BPH. Medical therapies include α-adrenergic blockers (lower adrenergic tone), 5α-reductase inhibitors (5-ARIs) (decrease levels of dihydrotestosterone), and combinations. Surgery, usually transurethral prostate resection, is the most effective intervention (22). New therapies are clearly needed. Despite the vast burden on public health, the pathogenesis of BPH remains undetermined. A variety of growth factors and inflammatory processes are inculpated in its pathogenesis (23).
BPH can be induced by injections of testosterone (TE) in male Wistar rats (24). This dominantly epithelial hyperplastic model has been adapted for several studies (25, 26), including our own (27–29). We have previously discussed the limitations of these models of BPH (29). We also showed that neurohormones including growth hormone-releasing hormone (GHRH) and luteinizing hormone-releasing hormone (LHRH) can act as growth factors in experimental BPH. Potent synthetic antagonists of GHRH and of LHRH cause marked shrinkage of experimental BPH by direct inhibition of prostatic GHRH and LHRH and by suppression of proinflammatory IL-1β, NF-κβ/p65, and COX-2 and various other growth factors and inflammatory cytokines (27–30).
In this study, we evaluated the effect of the potent GRP antagonist RC-3940-II on cell proliferation and cell volume in vitro and in a testosterone-induced model of BPH. We investigated mechanisms of action of RC-3940-II including in vivo effects on expression of various molecules involved in pathogenesis and at transcriptional and protein levels. Morphological analyses of the prostates treated with RC-3940-II were also performed.
Results
Expression of BN/GRP Receptors and Their Ligands in Rat Prostate and Human Prostate Cell Lines.
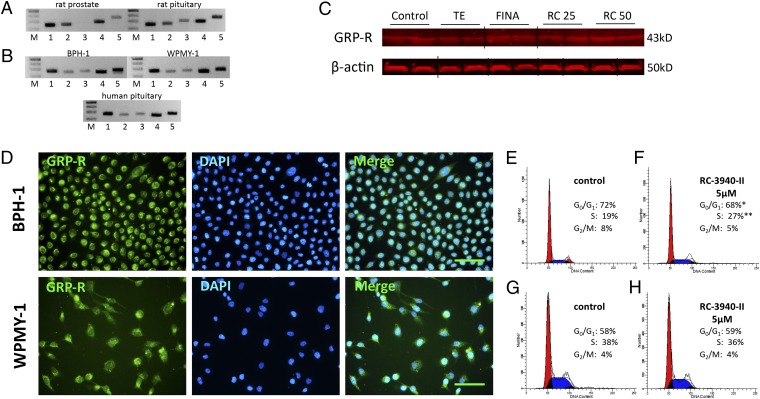
We observed mRNA for GRP-Rs (NMB-R and GRP-R) and their respective ligands (NMB and GRP) in rat prostates, but the orphan receptor BRS-3 was not present (Fig. 1A). The protein of GRP-R in rat prostates was detected by Western blot as well (Fig. 1C). Finasteride (0.07 mg⋅kg–1⋅d–1) and RC-3940-II at doses of 25 and 50 µg/d significantly elevated GRP-R protein by 43.9%, 38.2%, and 50.7%, respectively (P < 0.05 for all), whereas TE did not cause any change in GRP-R levels (Fig. 1C and Fig. S1). Both the human BPH-1 prostate epithelial cell line and WPMY-1 prostate stromal cell line expressed all three subtypes of GRP receptors and ligands (Fig. 1B). We confirmed the expression of GRP-R in BPH-1 and WPMY-1 by immunofluorescent staining (Fig. 1D). Radioligand binding assays using [Tyr4]BN revealed a single class of specific high-affinity binding sites in prostates of untreated control rats (Table S1). We also found a single class of specific, high-affinity binding sites for GRP-R in untreated samples of cultured BPH-1 and WPMY-1 cells. The concentrations of GRP receptors and the binding affinities are shown in Table S1.
Fig. 1.
Expression of BN/GRP-type receptors and their ligands in rat prostate and human prostatic cell lines BPH-1 and WPMY-1. (A) Real-time RT-PCR analysis of (1) bombesin-type receptors neuromedin-B receptor (NMB-R), (2) gastrin-releasing peptide receptor (GRP-R), (3) bombesin-like receptor 3 (BRS-3), and ligands (4) NMB and (5) GRP in rat prostates. Rat pituitary was used as positive control. DNA molecular weight marker is shown in lane M. (B) Real-time RT-PCR analysis of bombesin-type receptors (1) NMB-R, (2) GRP-R, (3) BRS-3, (4) NMB, and (5) GRP in BPH-1 human prostate epithelial cells derived from a BPH patient and WPMY-1 normal human prostate stromal cells. Human pituitary was used as positive control. (C) Western blot analysis of GRP-R in rat prostates. Representative blots of three independent experiments are presented and include β-actin as an internal standard; corresponding signal intensity values are shown in Fig. S1. Grouping of representative bands for each experimental group was performed digitally. DNA molecular weight marker is shown in lane M. FINA, finasteride (0.07 mg/kg); RC 25, RC-3940-II (25 µg/d); RC 50, RC-3940-II (50 µg/d). (D) Presence of GRP receptor (green) in BPH-1 and WPMY-1 cells. (Scale bar, 50 µm.) (E–H) Cell cycle distribution analysis of BPH-1 cells (E and F) and WPMY-1 cells (G and H) by flow cytometry. (F) In BPH-1 cells exposed to 5 µM RC-3940-II, a significant increase in the number of cells blocked in S phase (27%), whereas the number of cells with G0/G1 DNA content decreased (68%), compared with E (control). The histograms represent three independent experiments. *P < 0.05; **P < 0.05.
Inhibition of Cell Proliferation and Reduction of Cell Volume in BPH-1 and WPMY-1 Human Prostate Cell Lines.
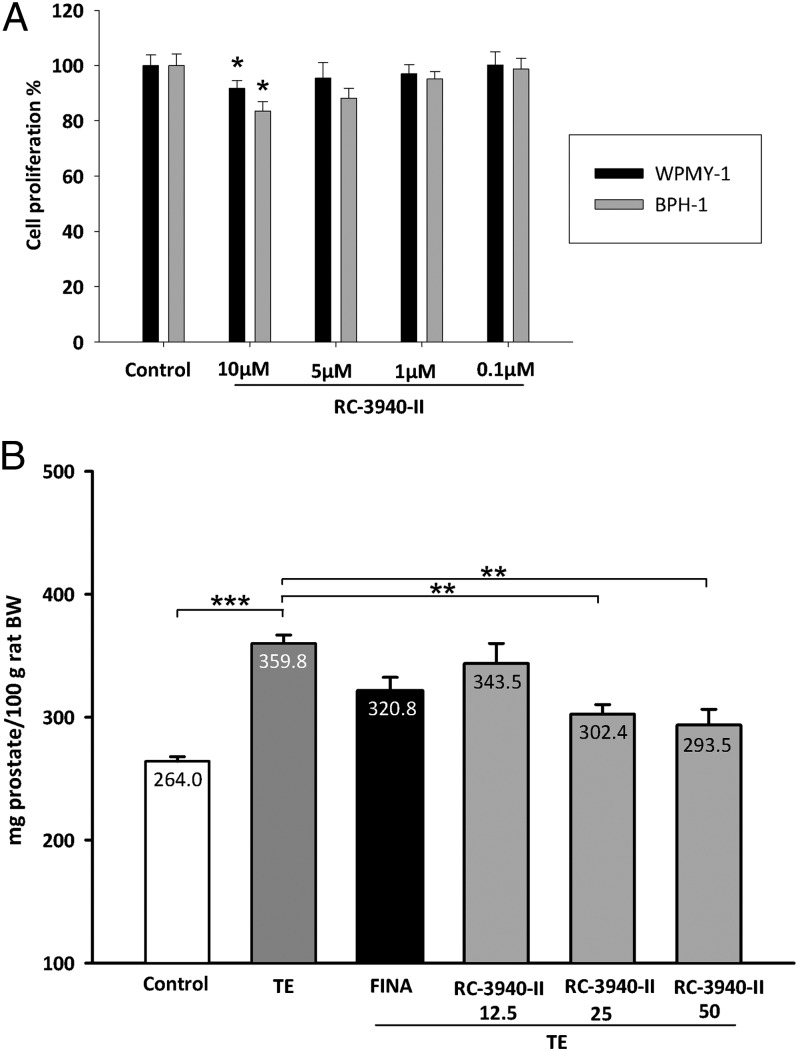
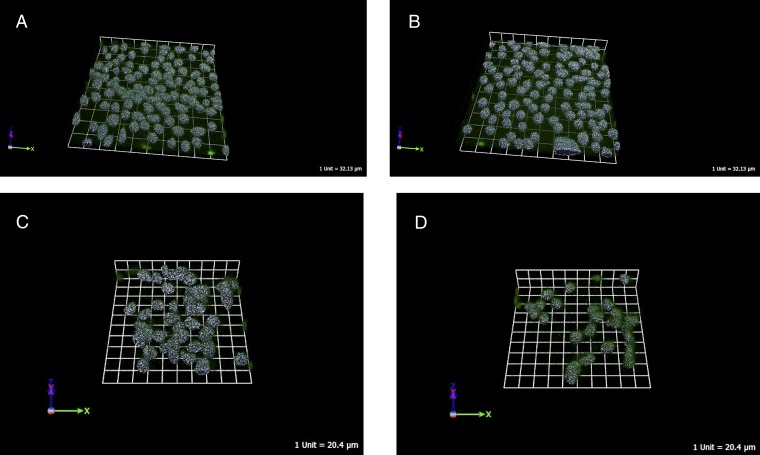
In vitro, treatment with the GHRH antagonist RC-3940-II inhibited cell proliferation of BPH-1 and WPMY-1 human prostate cell lines in a dose-dependent manner. In the MTS assays, the growth of BPH-1 and WPMY-1 cells was suppressed significantly by 10 µM RC-3940-II (P < 0.05) after 72 h, compared with control (Fig. 2A). Using flow cytometry, we observed that 5 µM RC-3940-II increased the number of BPH-1 cells with S-phase DNA content (P < 0.05) (Fig. 1 E and F). The GRP antagonists decreased not only the number of cell divisions but also the volume of BPH-1 and WPMY-1 cells. As a direct index of the cell volume, the intracellular water space was measured after 6 h of treatment with 10 µM RC-3940-II (Table S2). The antagonist reduced the volume of BPH-1 and WPMY-1 cells by 20.9% and 21.7%, respectively (P < 0.05 for all). We used 3D GRP receptor immunofluorescent microscopy on fixed BPH-1 and WPMY-1 cells to confirm these results and found that 10 µM RC-3940-II significantly reduced cell volumes by 15.5% and 15.6%, respectively (P < 0.05 for all) (Table S2, Fig. 3, and Movies S1–S4).
Fig. 2.
Effect of GRP antagonist RC-3940-II on proliferation of human prostatic epithelial BPH-1 and human prostatic myofibroblast stromal WPMY-1 cell lines, and on testosterone-induced BPH. (A) Cell viabilities were measured by an MTS assay and were expressed as percentage of untreated cells. The results are the mean ± SEM of three independent experiments performed in quadruplicate; *P < 0.05. (B) Effect of TE and treatment with finasteride or GRP antagonist RC-3940-II on relative prostate weight (n = 10), evaluated 42 d after the start of treatments. Statistical analysis was performed by one-way ANOVA, followed by Bonferroni t test. Significant differences are marked by asterisks (*P < 0.05, **P < 0.01, and ***P < 0.001).
Fig. 3.
Representative scans of BPH-1 and WPMY-1 cells, control and treated with RC-3940-II for 6 h, stained with GRP-receptor antibody. Cell size was measured using confocal laser-scanning microscopy. (A) Control BPH-1 cells, (B) BPH-1 cells treated with 10 µM RC-3940-II, (C) control WPMY-1 cells, and (D) WPMY-1 cells treated with 10 µM RC-3940-II. The scale is depicted.
Dose-Dependent Reduction of Prostate Size by GRP Antagonist RC-3940-II After 42 d of Treatment.
Control prostates weighed 264.0 ± 3.8 mg per 100g of rat body weight (BW), whereas in TE controls, prostates were enlarged by 36.4% to 359.8 ± 6.9 mg per 100 g of rat BW (P < 0.001; Fig. 2B, Table 1). Shrinkage of the rat prostate in response to RC-3940-II occurred in a dose-dependent manner: a nonsignificant 4.5% decrease with 12.5 µg/d; a 15.9% decline at the doses of 25 µg/d (P < 0.01); and an 18.4% reduction with 50 µg/d (P < 0.01) (Fig. 2B). These reductions in prostate weight were superior to the nonsignificant 10.8% reduction obtained with finasteride at 0.07 mg⋅kg–1⋅d–1. Furthermore, RC-3940-II lowered prostatic content of DNA (Table 1).
Table 1.
Effect of GRP antagonists RC-3940-II on body weight and prostatic parameters in rats
| Body weight, g |
Relative prostate weight, mg/100 g rat |
Prostatic DNA content, ng DNA/mg tissue |
||
| Group | Day −28 | Day 42 | Day 42 | Day 42 |
| Control | 197.4 ± 8.3 | 493.0 ± 7.4 | 264.0 ± 16.7 | 222.7 ± 12.5 |
| TE | 197.5 ± 3.5 | 464.6 ± 17.0* | 359.8 ± 20.3† | 291.0 ± 8.5 |
| TE/finasteride (0.07 mg⋅kg–1⋅d–1) | 194.0 ± 3.5 | 427.0 ± 10.0* | 320.8 ± 26.0 | 262.3 ± 5.5 |
| TE/RC-3940-II (12.5 µg/d) | 195.8 ± 4.1 | 447.5 ± 18.2 | 343.5 ± 26.0 | 276.5 ± 9.2 |
| TE/RC-3940-II (25 µg/d) | 195.5 ± 3.5 | 440.0 ± 6.6 | 302.4 ± 18.1§ | 246.2 ± 13.4 |
| TE/ RC-3940-II (50 µg/d) | 190.6 ± 3.3 | 440.3 ± 10.4 | 293.5 ± 17.3§ | 241.6 ± 6.7‡ |
Statistical analysis was performed by one-way ANOVA, followed by Bonferroni t test. *P < 0.05 and †P < 0.01 compared with control; ‡P < 0.05 and §P < 0.01 compared with TE.
Molecular Changes in the Prostate After Treatment with RC-3940-II.
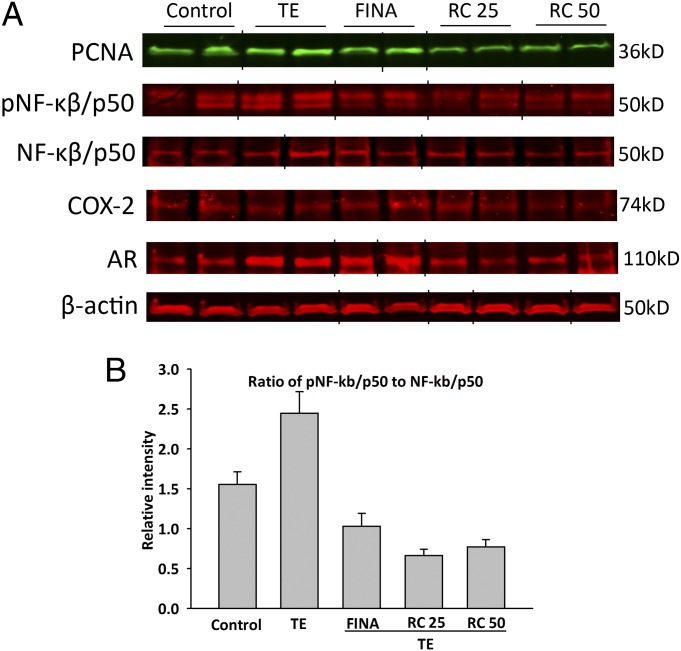
Levels of prostatic androgen receptor (AR) protein were significantly elevated by 104.0% in TE-induced BPH (P < 0.05); treatment with RC-3940-II at the doses of 25 or 50 µg/d significantly suppressed AR protein level by 66.0% and 63.8%, respectively (P < 0.05) (Fig. 4). The relative expression of phosphorylated NF-κβ/p50 (pNF-κβ/p50) compared with unphosphorylated NF-κβ/p50 markedly decreased after finasteride and RC-3940-II treatment. The relative intensity (RI) of pNF-κβ/p50 decreased by 57.8% after finasteride, whereas RC-3940-II at doses of 25 or 50 µg/d, caused 72.9% and 68.5% suppression, respectively (Fig. 4 A and B, and Fig. S1). The RI of pNF-κβ/p50 was increased by 57.4% in the TE control prostates.
Fig. 4.
Treatment with GRP antagonist RC-3940-II suppressed expression of PCNA, phosphorylated and total NF-κβ/p50, COX-2, and AR. (A) Western blot analysis of PCNA, phospho–NF-κβ/p50 (pNF-κβ/p50), NF-κβ/p50, COX-2, and AR in rat prostates. Representative blots of three independent experiments are presented and include β-actin as an internal standard; corresponding signal intensity values are shown in Fig. S1. Grouping of representative bands for each experimental group was performed digitally. (B) Bars represent average relative intensity of phosphorylated NF-κβ/p50. FINA, finasteride (0.07 mg/kg); RC25, RC-3940-II (25 µg/d); RC50, RC-3940-II (50 µg/d).
GRP Antagonist RC-3940-II Reduces Epithelial Compartment, Inhibits Cell Division, and Induces Apoptosis in Rat Prostates.
H&E slides revealed that the mean epithelial area in the ventral prostates of TE-treated BPH control animals was significantly increased by 111% compared with untreated controls; finasteride and GRP antagonist RC-3940-II at doses of 25 and 50 µg/d markedly reduced this increase by 35%, 25%, and 33%, respectively, compared with TE-treated controls (P < 0.05 for all; Table 2). Mitotic cells were fewer after treatment with RC-3940-II at the dose of 25 µg/d compared with TE-treated controls (P < 0.05). Both the finasteride group and the RC-3940-II (25 and 50 µg/d)-treated groups showed significantly higher apoptotic indices by 98%, 279%, and 398% (P < 0.05), respectively, compared with TE-treated animals; the group given RC-3940-II at 50 µg/d had an even higher apoptotic index (201% increase; P < 0.05) than those treated with finasteride (Table 2). No significant change in prostatic proliferating cell nuclear antigen (PCNA) protein levels occurred after TE treatment. The GRP antagonist RC-3940-II at the doses of 25 or 50 µg/d reduced PCNA protein by 49.9% and 45.7% (P < 0.05 for all) (Fig. 4A).
Table 2.
Effect of GRP antagonist RC-3940-II on prostatic epithelium, cell proliferation, and apoptosis in ventral prostates of rats
| Group | Mean epithelial area in view fields, % | No. of mitoses in one theoretical field composed entirely of epithelial cells | No. of apoptotic cells in one theoretical field composed entirely of epithelial cells |
| Control | 15.1 ± 1.2* | 1.10 ± 0.23 | 0.82 ± 0.50 |
| TE | 31.8 ± 1.6 | 1.04 ± 0.25 | 1.06 ± 0.17 |
| TE/finasteride (0.07 mg⋅kg–1⋅d–1) | 20.6 ± 0.2* | 0.40 ± 0.21 | 2.10 ± 0.15* |
| TE/RC-3940-II (25 µg/d) | 23.9 ± 2.0* | 0.14 ± 0.14* | 2.96 ± 0.29* |
| TE/RC-3940-II (50 µg/d) | 21.2 ± 1.6* | 0.52 ± 0.32 | 4.22 ± 1.01*† |
The data were evaluated by one-way ANOVA, followed by the Student–Newman–Keuls method. *P < 0.05 compared with TE group. †P < 0.05 compared with TE/finasteride group.
GRP Antagonist RC-3940-II Suppresses Multiple Genes Involved in Growth, Inflammatory Response, and Signaling.
Growth factors, molecules involved in inflammatory response, and signal transduction factors were evaluated in control rats, rats with TE-induced BPH, and rats with TE-induced BPH treated with finasteride or GRP antagonist RC-3940-II using rat real-time RT-PCR arrays. We identified important molecules altered by treatment with RC-3940-II; these selected genes are potentially related to prostate shrinkage. Almost 100 genes were significantly altered after treatment with TE and RC-3940-II (P < 0.05; Tables S3–S6).
Transcriptional levels of several growth factors, including angiogenic factors (Bmp4, Ereg, Figf, and Vegfa) were lowered by GRP antagonist RC-3940-II (P < 0.05; Table S3). Prostatic expression of mRNA for apoptosis regulating growth factors (Gdf5, Igf1, Il4, and Il6) were also down-regulated after treatment with RC-3940-II. The antagonist suppressed the levels of growth factors affecting cell differentiation such as Bmp3, Bmp4, Bmp5, Fgf5, Lep, Mdk, and Tff1. Levels of mRNA for other growth factors including Bmp10, Csf3, Fgf3, Fgf7, Fgf11, Fgf18, Hgf, and Igf2 were also lowered (P < 0.05 for all) (Table S3).
Expression of inflammatory chemokines including Ccl2, Ccl3, Ccl5-7, Ccl11, Ccl12, Ccl17, Ccl19, Ccl20, Ccl25, Cxcl1, Cxcl3, Cxcl5, Cxcl9-11, and Cxcl12 were decreased by RC-3940-II (P < 0.05) (Table S4). Chemokine receptors, Ccr1, Ccr3-7, Ccr10, and Cxcr3 were also suppressed (P < 0.05; Table S4). Numerous inflammatory cytokines, including Ifng, Il1b, Il1f5, Il5, Il6, Il10, Il11, Il13, Il15, and Il17b, were markedly decreased by the antagonist RC-3940-II (P < 0.05; Table S4). Among receptors for cytokines, Ilr1, Il6r, Il8ra, and Il13ra1 were also down-regulated after combination therapy (P < 0.05 for all) (Table S4).
Using quantitative PCR arrays for signal transduction (Table S5), we found several putative downstream pathways responsible for effects of GRP antagonist RC-3940-II on prostate shrinkage in this model of BPH. Expression of Wnt pathway target genes, Birc5, Vegfa, and Wisp1, was significantly reduced by RC-3940-II (P < 0.05). mRNAs for Hedgehog pathway genes Bmp2 and Foxa2 were significantly down-regulated by treatment with RC-3940-II (P < 0.05; Table S5). Levels of TGF-β pathway-related genes including Cdkn1a and Cdkn2b were suppressed by treatment with RC-3940-II (P < 0.05). The expression of the following NF-κβ pathway genes was also markedly lowered by GRP antagonist treatment: Cxcl1, Nos2, and Vcam1. Transcriptional suppression of Jak–Stat pathway genes Irf1 and Mmp10 occurred after treatment with RC-3940-II. The expression of LDL pathway genes Ccl2 and Sele was also lowered by RC-3940-II treatment (P < 0.05 for all; Table S5)
Discussion
The main finding of our study is that the GRP antagonist RC-3940-II reduces prostate size in a testosterone-induced model of BPH. In addition to prostate shrinkage in rats, the cell volume and the proliferation of human prostatic cell lines BPH-1 and WPMY-1 were reduced in vitro. When BPH-1 cells were treated with RC-3940-II, an accumulation of cells in S phase occurred. In contrast, in WPMY-1 cells, RC-3940-II treatment did not cause a significant change in cell cycle distribution. Thus, GRP antagonist RC-3940-II appears to affect cell cycle traverse in the disease-modeling prostate epithelial cell line BPH-1 to a greater extent than in WPMY-1 normal prostatic myofibroblast. The accumulation of cells in S phase after GRP antagonist treatment indicates a slowing of BPH-1 cells through mitotic transit, so that more cells remain in S phase, suggesting an inhibition of cell division. Antiproliferative and proapoptotic effects of RC-3940-II were demonstrated in vitro as well as in vivo. In addition, multiple factors related to growth and inflammation, which are crucial in the pathogenesis and progression of BPH (31, 32), were markedly reduced by treatment with the GRP antagonist. The expression of GRP receptor in rat prostate was demonstrated by RT-PCR and Western blot. The ligand competition assay also detected specific high-affinity receptors for GRP in rat prostate. In BPH-1 human prostate epithelial cells and WPMY-1 normal prostate stromal cells, the expression of GRP-R was confirmed by RT-PCR, immunofluorescent microscopy, and ligand competition assay.
Androgen signaling is significantly elevated in BPH relative to the normal prostate (33). In addition, prostate epithelial AR function seems to be important for macrophage-mediated epithelial–mesenchymal transition and proliferation of prostate epithelial cells (34). A role for stromal AR/CCL3 signaling pathways in macrophage-induced prostate stromal cell proliferation was also implicated (35). Antagonistic selective androgen receptor modulators were reported to be beneficial in experimental BPH as well (36). In our study, levels of AR were markedly elevated in TE-treated prostates and decreased after treatment with GRP antagonist RC-3940-II.
We showed that GRP antagonist RC-3940-II caused a pronounced decrease in activated (i.e., phosphorylated) NF-κβ/p50 and COX-2. The activation of NF-κβ is an early event in chronic inflammation (28). COX-2 overexpression was reported in human BPH samples (28); GRP antagonists suppress COX-2 in experimental lung (37) and colon cancer (38).
We used real-time PCR arrays to investigate the beneficial molecular mechanisms of GRP antagonists in a BPH model. Our investigations revealed that several growth factors were up-regulated in TE-induced BPH control rats and down-regulated in animals treated with GRP antagonist RC-3940-II. Growth factors can control the response of cells to injury and mediate the processes of cell growth, differentiation, and apoptosis. Many growth factors use autocrine or paracrine pathways to signal stromal and epithelial cells in the microenvironment (39). The role of major growth factors that are affected by GRP antagonist was recently reviewed (28, 30).
Our finding of transcriptional activation of inflammatory chemokines and cytokines in TE-induced BPH prostates is consistent with clinical and experimental findings and our previous observations (27). We found that RC-3940-II markedly suppressed several of these proinflammatory molecules including many Ccl- and Cxcl-type chemokines and interleukins, which is in accordance with recent clinical studies with septic patients, where a GRP antagonist decreased NF-κβ, IL-6, and TLR-4 among many other inflammatory factors (40). These proinflammatory molecules are part of an inflammatory network in BPH (28, 30).
The effects of RC-3940-II were greater than those of finasteride especially on shrinkage and suppression of prostatic AR, growth factors, and proinflammatory cytokines as well as inhibition of proliferation and increase in apoptosis. Finasteride was introduced in the 1990s for the treatment of BPH. Finasteride decreases prostate volume by 15–25% because of apoptosis of the glandular epithelial compartment in both the transition and peripheral zones of the prostate (41). In our experimental setting, the reduction of prostates obtained with RC-3940-II is comparable with the above-mentioned clinical outcomes by finasteride therapy. Therefore, we assume that GRP antagonist RC-3940-II will be efficient in later clinical trials on BPH patients. A great advantage of GRP antagonist is the lack of known adverse effects, contrary to finasteride. Because our GRP antagonist seems to have similar effects on the prostate as finasteride (prostate shrinkage through reduction in the epithelial compartment, increase in apoptosis), a combination of GRP antagonist with α-adrenoreceptor blockers (ABs) such as doxasosin would be a good choice based on information emerging from clinical trials on combinations of an 5-ARI and an AB (MTOPS, CombAT) (23).
In summary, in this study, we demonstrated that the GRP antagonist RC-3940-II reduces prostate size in an experimental BPH model and shrinks human prostatic epithelial and stromal cells in vitro. Our findings suggest that this reduction in prostate volume and cell volume is induced by direct inhibitory effects of GRP antagonist exerted through prostatic GRP receptors, as well as by transcriptional suppression of growth factors and proinflammatory cytokines. In addition to lowered levels of prostatic PCNA and AR, we also showed a strong decrease in proinflammatory NF-κβ activation and COX-2. Antiproliferative and proapoptotic effects of RC-3940-II also have been demonstrated. These findings hint at mechanisms of action of GRP antagonists in BPH. The adverse effects of 5-ARIs and α-adrenergic blockers may encourage the development of GRP antagonists as an alternative therapy for BPH alone or in combination with other agents.
Materials and Methods
Peptide and Chemicals.
The GRP antagonist RC-3940-II with the structure of [Hca6, Leu13 ψ(CH2N)-Tac14]BN (6–14) is based on the sequence of BN and contains a reduced peptide bond at its COOH terminus. RC-3940-II, originally designed and synthesized in our laboratory (42), was made and provided by Æterna/Zentaris. For daily injection, RC-3940-II was dissolved in 0.1% DMSO in 10% (vol/vol) aqueous propylene glycol solution.
Animal Study.
BPH was induced in experimental groups of male Wistar rats (about 200 g) by daily s.c. injection of TE (2 mg/d), for 4 wk before study treatments from day −28 to day 0 (induction phase) as reported (27–30). Negative controls (n = 10) received s.c. corn oil injections. Dosage, duration, and administration of testosterone, and finasteride, were previously described (29). Experimental groups (n = 10 each) consisted of the following: (i) TE only, (ii) TE/finasteride (0.07 mg⋅kg–1⋅d–1), (iii) TE/RC-3940-II (12.5 µg/d), (iv) TE/RC-3940-II (25 µg/d), and (v) TE/RC-3940-II (50 µg/d). The dosage of GRP antagonist RC-3940-II was based on prior experimental oncological use, where we used RC-3940-II at the doses of 10 and 20 µg/d in nude mice bearing human xenografts (10, 15). TE-only positive control animals received 0.1% DMSO in 10% aqueous propylene glycol solution instead of finasteride or RC-3940-II on the same schedule. Rats were killed under anesthesia on day 42; whole prostates were removed, weighed, and snap frozen. The study scheme is depicted in Fig. S2. Animal care was in accordance with institutional guidelines and complied with National Institutes of Health policy.
Additional information is provided in SI Text.
Supplementary Material
Acknowledgments
We thank Mr. Gabriel Gaidosh for assistance in confocal microscopy. This work was supported in part by a grant from the American Urological Association (AUA) Foundation Research Scholars Program and the AUA Southeastern Section (to F.G.R.). Our study was also supported by the Medical Research Service of the Veterans Affairs, Departments of Pathology and Medicine, Division of Hematology/Oncology of the Miller Medical School, University of Miami, and South Florida Veterans Affairs Foundation for Research and Education (all A.V.S.), and TÁMOP-4.2.2.A-11/1/KONV-2012-0025 Project. This project was cofinanced by the European Union and the European Social Fund (G.H.), and The L. Austin Weeks Endowment for Urologic Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222355110/-/DCSupplemental.
References
- 1.Aprikian AG, et al. Neuroendocrine differentiation and the bombesin/gastrin-releasing peptide family of neuropeptides in the progression of human prostate cancer. Prostate Suppl. 1998;8:52–61. [PubMed] [Google Scholar]
- 2.Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42(2):150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 4.Spindel ER, Giladi E, Segerson TP, Nagalla S. Bombesin-like peptides: Of ligands and receptors. Recent Prog Horm Res. 1993;48:365–391. doi: 10.1016/b978-0-12-571148-7.50017-8. [DOI] [PubMed] [Google Scholar]
- 5.Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides and their receptors. Int J Dev Biol. 2005;49(2–3):293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- 6.Watts SW, Cohen ML. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12(5):1057–1062. doi: 10.1016/0196-9781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- 7.Sausville EA, Trepel JB, Moyer JD. Inhibitors of bombesin-stimulated intracellular signals: Interruption of an autocrine pathway as a therapeutic strategy in small cell lung carcinoma. Prog Clin Biol Res. 1990;354A:193–207. [PubMed] [Google Scholar]
- 8.Cuttitta F, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 9.Hohla F, Schally AV. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle. 2010;9(9):1738–1741. doi: 10.4161/cc.9.9.11347. [DOI] [PubMed] [Google Scholar]
- 10.Rick FG, et al. Combination of gastrin-releasing peptide antagonist with cytotoxic agents produces synergistic inhibition of growth of human experimental colon cancers. Cell Cycle. 2012;11(13):2518–2525. doi: 10.4161/cc.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halmos G, Wittliff JL, Schally AV. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res. 1995;55(2):280–287. [PubMed] [Google Scholar]
- 12.Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61(9):1042–1068. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stangelberger A, Schally AV, Djavan B. New treatment approaches for prostate cancer based on peptide analogues. Eur Urol. 2008;53(5):890–900. doi: 10.1016/j.eururo.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Pinski J, Reile H, Halmos G, Groot K, Schally AV. Inhibitory effects of somatostatin analogue RC-160 and bombesin/gastrin-releasing peptide antagonist RC-3095 on the growth of the androgen-independent Dunning R-3327-AT-1 rat prostate cancer. Cancer Res. 1994;54(1):169–174. [PubMed] [Google Scholar]
- 15.Szepeshazi K, et al. Powerful inhibition of in-vivo growth of experimental hepatic cancers by bombesin/gastrin-releasing peptide antagonist RC-3940-II. Anticancer Drugs. 2012;23(9):906–913. doi: 10.1097/CAD.0b013e328354bd25. [DOI] [PubMed] [Google Scholar]
- 16.Stangelberger A, et al. Inhibition of human androgen-independent PC-3 and DU-145 prostate cancers by antagonists of bombesin and growth hormone releasing hormone is linked to PKC, MAPK and c-jun intracellular signalling. Eur J Cancer. 2005;41(17):2735–2744. doi: 10.1016/j.ejca.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766(1):23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate. 2000;42(4):295–303. doi: 10.1002/(sici)1097-0045(20000301)42:4<295::aid-pros7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999;59(5):1152–1159. [PubMed] [Google Scholar]
- 20.Reile H, Armatis PE, Schally AV. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate. 1994;25(1):29–38. doi: 10.1002/pros.2990250105. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs JT. Etiology of benign prostatic hyperplasia. Eur Urol. 1994;25(Suppl 1):6–9. doi: 10.1159/000475324. [DOI] [PubMed] [Google Scholar]
- 22.Ventura S, et al. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH) Br J Pharmacol. 2011;163(5):891–907. doi: 10.1111/j.1476-5381.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rick FG, et al. Hormonal manipulation of benign prostatic hyperplasia. Curr Opin Urol. 2013;23(1):17–24. doi: 10.1097/MOU.0b013e32835abd18. [DOI] [PubMed] [Google Scholar]
- 24.Maggi CA, Manzini S, Giuliani S, Meli A. Infravesical outflow obstruction in rats: A comparison of two models. Gen Pharmacol. 1989;20(3):345–349. doi: 10.1016/0306-3623(89)90271-1. [DOI] [PubMed] [Google Scholar]
- 25.Altavilla D, et al. Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br J Pharmacol. 2012;167(1):95–108. doi: 10.1111/j.1476-5381.2012.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yang D, et al. Mechanisms of prostate atrophy after LHRH antagonist cetrorelix injection: An experimental study in a rat model of benign prostatic hyperplasia. J Huazhong Univ Sci Technolog Med Sci. 2012;32(3):389–395. doi: 10.1007/s11596-012-0067-x. [DOI] [PubMed] [Google Scholar]
- 27.Rick FG, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71(7):736–747. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- 28.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108(9):3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rick FG, et al. Combining growth hormone-releasing hormone antagonist with luteinizing hormone-releasing hormone antagonist greatly augments benign prostatic hyperplasia shrinkage. J Urol. 2012;187(4):1498–1504. doi: 10.1016/j.juro.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 30. Rick FG, et al. (2013) Mechanisms of synergism between antagonists of growth hormone-releasing hormone and antagonists of luteinizing hormone-releasing hormone in shrinking experimental benign prostatic hyperplasia. Prostate, 10.1002/pros.22633. [DOI] [PubMed]
- 31.Robert G, et al. Biomarkers for the diagnosis of prostatic inflammation in benign prostatic hyperplasia. Prostate. 2011;71(15):1701–1709. doi: 10.1002/pros.21387. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23(1):5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley KJ, et al. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69(16):1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu T, et al. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26(10):1707–1715. doi: 10.1210/me.2012-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): Role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J Biol Chem. 2012;287(22):18376–18385. doi: 10.1074/jbc.M112.355164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nejishima H, et al. Anti-androgenic effects of S-40542, a novel non-steroidal selective androgen receptor modulator (SARM) for the treatment of benign prostatic hyperplasia. Prostate. 2012;72(14):1580–1587. doi: 10.1002/pros.22511. [DOI] [PubMed] [Google Scholar]
- 37.Hohla F, et al. Growth inhibition of non-small-cell lung carcinoma by BN/GRP antagonist is linked with suppression of K-Ras, COX-2, and pAkt. Proc Natl Acad Sci USA. 2007;104(47):18671–18676. doi: 10.1073/pnas.0709455104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corral RS, Iñiguez MA, Duque J, López-Pérez R, Fresno M. Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells. Oncogene. 2007;26(7):958–969. doi: 10.1038/sj.onc.1209856. [DOI] [PubMed] [Google Scholar]
- 39.Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: Basic science implications. Curr Urol Rep. 2008;9(4):272–278. doi: 10.1007/s11934-008-0048-6. [DOI] [PubMed] [Google Scholar]
- 40.Petronilho F, et al. Gastrin-releasing peptide receptor antagonism induces protection from lethal sepsis: Involvement of Toll-like receptor 4 signaling. Mol Med. 2012;18(1):1209–1219. doi: 10.2119/molmed.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev Urol. 2005;7(Suppl 9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- 42.Cai RZ, Reile H, Armatis P, Schally AV. Potent bombesin antagonists with C-terminal Leu-psi(CH2-N)-Tac-NH2 or its derivatives. Proc Natl Acad Sci USA. 1994;91(26):12664–12668. doi: 10.1073/pnas.91.26.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.