Abstract
The Hippo signaling pathway inhibits cell growth and regulates organ size through a kinase cascade that leads to the phosphorylation and nuclear exclusion of the growth-promoting transcriptional coactivator Yes-associated protein (YAP)/Yorkie. It mediates contact inhibition of cell growth downstream of cadherin adhesion molecules and other cell surface proteins. Contact inhibition is often antagonized by mitogenic growth factor signaling. We report an important mechanism for this antagonism, inhibition of Hippo pathway signaling by mitogenic growth factors. EGF treatment of immortalized mammary cells triggers the rapid translocation of YAP into the nucleus along with YAP dephosphorylation, both of which depend on Lats, the terminal kinase in the Hippo pathway. A small-molecule inhibitor screen of downstream effector pathways shows that EGF receptor inhibits the Hippo pathway through activation of PI3-kinase (PI3K) and phosphoinositide-dependent kinase (PDK1), but independent of AKT activity. The PI3K-PDK1 pathway also mediates YAP nuclear translocation downstream of lysophosphatidic acid and serum as a result of constitutive oncogenic activation of PI3K. PDK1 associates with the core Hippo pathway-kinase complex through the scaffold protein Salvador. The entire Hippo core complex dissociates in response to EGF signaling in a PI3K-PDK1–dependent manner, leading to inactivation of Lats, dephosphorylation of YAP, and YAP nuclear accumulation and transcriptional activation of its target gene, CTGF. These findings show that an important activity of mitogenic signaling pathways is to inactivate the growth-inhibitory Hippo pathway and provide a mechanism for antagonism between contact inhibition and growth factor action.
Keywords: MCF-10A, MCF-7, Wortmannin
Much is known about the roles of mitogenic growth factors in regulating cell proliferation. More recently, there has been progress in understanding the mechanisms underlying physical constraints on cell growth, such as contact inhibition, mechanotransduction, and the role of cell polarity. In particular, the Hippo signaling pathway is thought to mediate growth inhibition through some of these processes.
The Hippo signaling pathway is an evolutionarily conserved pathway that inhibits cell proliferation, and loss of Hippo signaling leads to organ overgrowth and cancer development (1–4). The core of the pathway consists of a kinase cascade and associated coactivators and scaffold proteins. The Mst/Hippo kinase in complex with Sav1 phosphorylates and activates the downstream kinase Lats. Lats in turn phosphorylates the growth-promoting transcriptional activator Yes-associated protein (YAP) on Ser127, leading to its cytoplasmic retention (5). When the Hippo pathway is inactive, YAP accumulates in the nucleus and activates the transcription of several growth-promoting genes (5).
Cadherin adhesion proteins have been proposed to mediate contact inhibition of growth by antagonizing growth signaling of receptor tyrosine kinases (RTKs). Cadherins may interact directly with RTKs (6, 7) or via tumor-suppressor proteins merlin/NF2 and NHERF (8). We previously reported that inhibition of EGF receptor (EGFR) phosphorylation by Src family kinases might play a role in cadherin-mediated contact inhibition of growth (9). We recently discovered that cadherin-mediated contact, independent of other cell interactions, inhibits cell growth through activation of the Hippo signaling pathway (10). Given that our earlier work implicated inhibition of RTK signaling activity in the cadherin-mediated contact inhibition of growth, we wondered whether the two growth inhibitory activities are related. In the present study, we found a direct relationship between mitogenic growth factor pathways and the growth-inhibitory Hippo pathway, and identified the general mechanism for this interaction.
Results
EGF Treatment Inhibits Hippo Pathway in Confluent MCF-10A Cells.
MCF-10A is an immortalized human mammary epithelial cell line that is contact-inhibited at high cell density via the Hippo signaling pathway (5, 10, 11). In serum-starved, contact-inhibited MCF-10A cells, the nuclear effector of the Hippo pathway, YAP, is excluded from the nucleus. However, EGF treatment triggered rapid YAP nuclear accumulation in most cells within 30 min (Fig. 1A). This phenomenon was not unique to MCF10A and mammary cells, but was observed for A431 epidermoid and HeLa cervical carcinoma cell lines as well (Fig. S1A).
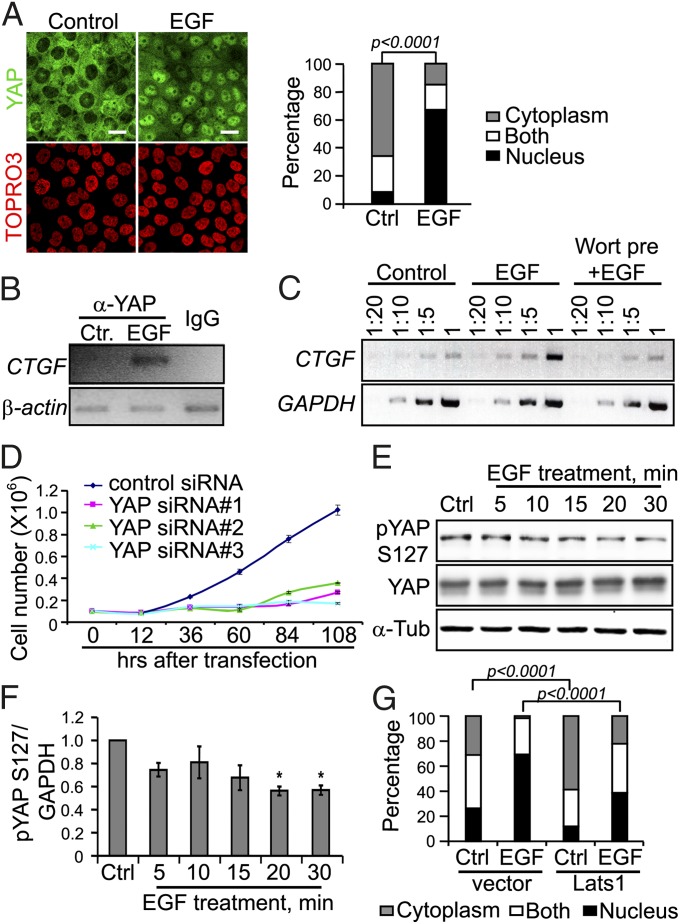
Fig. 1.
EGF treatment inhibits Hippo signaling pathway in confluent serum-starved MCF-10A cells. (A) EGF treatment for 30 min induces YAP nuclear accumulation in confluent serum-starved MCF-10A cells, observed by confocal immunofluoresence microscopy. Nuclear staining with TOPRO3 is shown below for each panel. (Scale bar: 20 μm.) The percentage of cells with nuclear staining in the control and EGF groups was compared using the two-sided Fisher’s exact test. (B) ChIP assay demonstrates that EGF treatment induces YAP binding to the CTGF promoter region. PCR shows that CTGF promoter fragment is enriched in the cells treated with EGF for 30 min compared with the control treatment. Mouse IgG serves as the antibody control, and β-actin gene serves as the internal control. (C) EGF treatment increases CTGF mRNA expression in confluent serum-starved MCF-10A cells, as measured by RT-PCR. Equal amounts of total RNA were serially diluted as the template for cDNA synthesis and RT-PCR using CTGF primer or GAPDH primer. (D) MCF-10A cells depleted of YAP by siRNA treatment proliferate less than control MCF-10A cells after EGF treatment. Error bar represents mean ± SEM; n = 6. (E) EGF treatment reduces phospho YAP Ser127 in MCF-10A cells, as shown by Western blot analysis, using α-tubulin as an internal loading control. (F) Quantification of Western blot intensity shown in E by ImageJ. Statistical significance was calculated using the Student t test. Error bar represents mean + SEM; n = 3. *P ≤ 0.0005. (G) Lats1 overexpression inhibits EGF-mediated YAP nuclear accumulation in MCF-10A cells. GFP vector or GFP-Lats1 was transiently transfected into MCF-10A cells by electroporation. The percentage of cells with YAP nuclear staining was compared in indicated groups. Statistical significance was calculated using the two-sided Fisher exact test.
To determine whether YAP nuclear translocation is associated with its transcriptional activity, we performed ChIP analysis for promoter-binding activity. YAP is known to associate with the DNA-binding protein TEAD to regulate expression of the connective tissue growth factor (CTGF) gene (12). EGF treatment triggered YAP binding to the CTGF promoter relative to control treatment (Fig. 1B). EGF treatment also increased CTGF mRNA expression compared with control treatment, as determined by RT-PCR (Fig. 1C). To determine whether YAP has a role in EGF-stimulated growth of MCF-10A cells, we knocked down the expression of YAP by siRNA in MCF-10A cells. Three different YAP siRNAs effectively depleted YAP expression for several days compared with control siRNA (Fig. S2A). EGF failed to increase cell numbers in the YAP knockdown group compared with control (Fig. 1D). Taken together, these results show that EGF treatment triggers YAP nuclear accumulation and transcriptional activity, which is critical for EGF-stimulated cell proliferation in MCF-10A cells.
We next examined whether EGF regulates YAP nuclear accumulation through the Hippo pathway. The core Hippo pathway kinase Lats phosphorylates YAP directly at Ser127, which causes YAP cytoplasmic retention (5). EGF treatment reduced overall YAP phosphorylation and YAP phosphorylation at Ser127 in a time-dependent manner in confluent serum-starved MCF-10A cells (Fig. 1 E and F and Fig. S2B), suggesting that EGF treatment regulates YAP through inhibition of Lats activity. To test whether a decrease in Lats activity mediates EGF regulation of YAP localization, we overexpressed Lats in MCF-10A cells. EGF treatment induced YAP nuclear accumulation in 69% of control vector-transfected cells, whereas only 39% of Lats1-transfected cells showed nuclear YAP staining (Fig. 1G). These data indicate that EGF treatment causes a decrease in Lats-mediated phosphorylation of YAP and stimulates YAP nuclear accumulation in a Lats-dependent manner. Given that Lats is a defining core kinase of the Hippo pathway, EGF treatment regulates YAP, at least in part, through inhibition of the Hippo pathway.
EGF Inhibits Hippo Pathway Through PI3K and PDK1, but Independent of AKT Activity.
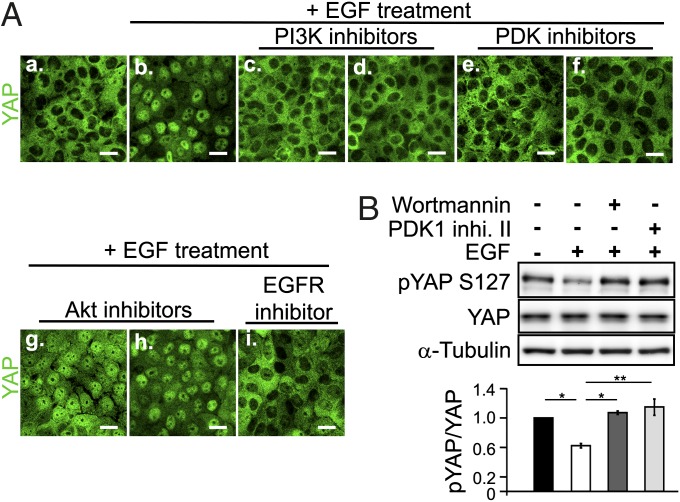
We used numerous specific pharmacologic inhibitors of its downstream pathways (13) to examine how EGFR affects the Hippo pathway. Confluent, serum-starved MCF-10A cells were pretreated with different inhibitors for 30 min, followed by EGF treatment for another 30 min, and YAP nuclear accumulation was determined by confocal microscopy. Inhibitors of PI3K significantly blocked EGF-mediated YAP nuclear accumulation as effectively as the EGFR inhibitor Iressa (Fig. 2A and Fig. S3B). Inhibitors of the PI3K downstream effector PDK1 were also able to block YAP nuclear accumulation (Fig. 2A and Fig. S3B). Importantly, dose-dependence studies showed that both sets of inhibitors acted at low concentrations expected for specific effects on these enzymes (Fig. S4). In contrast, inhibitors of another major kinase downstream of PI3K, AKT, had no effect on YAP nuclear accumulation (Fig. 2A). None of the other inhibitors, including Src family kinase inhibitor, MEK inhibitors, JAK2 inhibitor, PKC inhibitors, Ras/farnesyltransferase inhibitor, and PKA inhibitors had any effect on EGF-mediated YAP nuclear accumulation (Fig. S3). Moreover, both the PI3K inhibitor and the PDK1 inhibitor blocked the reduction in YAP Ser127 phosphorylation caused by EGF treatment (Fig. 2B). These results demonstrate that EGFR regulates the Hippo pathway through PI3K-PDK1 activity.
Fig. 2.
EGF inhibits the Hippo pathway through the PI3K-PDK1 pathway, independent of AKT activity. (A) Inhibitor screening of the EGF signaling pathway in MCF-10A cells by confocal microscopy of YAP nuclear accumulation. Cells in c–i received a 30-min inhibitor pretreatment followed by a 30-min EGF treatment. a, no treatment; b, EGF treatment alone; c, Wortmannin; d, LY294002; e, PDK1 inhibitor II; f, BX795; g, AKT inhibitor V; i, Iressa. (Scale bar: 20 μm.) (B) Pretreatment with PI3K (Wortmannin) or PDK1 inhibitor (PDK1 inhibitor II) blocks the effect of EGF on phospho-YAP Ser127 by Western blot analysis. The bar graph indicates the Western blot band intensity. Statistical significance was calculated using the Student t test. Error bar represents mean ± SEM; n = 4. *P < 0.0001; **P < 0.005.
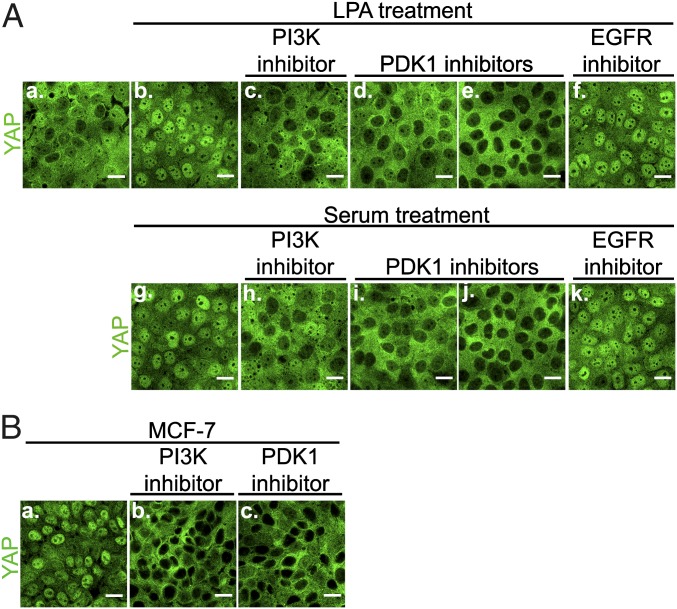
Because PI3K can be activated by growth factors other than EGF, we tested whether this PI3K-PDK1–mediated regulation of the Hippo pathway is specific to EGFR signaling. Lysophosphatidic acid (LPA) or horse serum, which can activate PI3K signaling independent of EGFR signaling (14), both caused YAP nuclear accumulation within 30 min of treatment of MCF-10A cells (Fig. 3A). Importantly, PI3K and PDK1 inhibitors blocked YAP nuclear accumulation caused by LPA or serum treatment. Pretreatment of the cells by the EGFR inhibitor Iressa had no effect on YAP nuclear accumulation induced by LPA or serum, ruling out an indirect effect via EGFR (Fig. 3A) (15). A different human mammary cell line, MCF-7, harbors a constitutive active E545K mutation in the PI3K gene, PIK3CA (16). In contrast to MCF-10A cells, YAP remained in the nucleus in confluent MCF-7 cells even after serum starvation (Fig. 3B). Importantly, the PI3K or PDK1 inhibitors caused YAP to accumulate in the cytoplasm of MCF-7 cells, demonstrating that constitutive activation of PI3K and PDK1 activity was responsible for YAP nuclear accumulation in confluent, serum-starved MCF-7 cells. Two colon cancer cell lines, HCT-116 and HT29, also carry activating mutations in the PIK3CA gene (17), and both the PI3K and PDK1 inhibitors similarly caused accumulation of YAP in the cytoplasm of both cell lines (Fig. S1B). These data suggest that many, if not all, extracellular stimuli or oncogenic mutations that activate PI3K-PDK1 signaling will inhibit Hippo pathway signaling and trigger YAP nuclear accumulation.
Fig. 3.
PI3K activated by different growth factors or constitutive mutation causes YAP nuclear accumulation. (A) Similar to EGF treatment (Fig. 1), LPA treatment or serum treatment induced YAP nuclear accumulation through PI3K-PDK1 pathway in confluent serum-starved MCF-10A cells, independent of EGFR activity. Cells in c–f received a 30-min inhibitor treatment, followed by a 30-min LPA treatment. a, no treatment; b, LPA treatment only; c, Wortmannin; d, PDK1 inhibitor II; e, BX795; f, Iressa. Cells in h–k received a 30-min inhibitor treatment, followed by a 30-min serum treatment. g, serum treatment only; h, Wortmannin; i, PDK1 inhibitor II; j, BX795; k, Iressa. YAP intracellular localization was determined by confocal microscopy. (Scale bar: 20 μm.) (B) Inhibition of PI3K or PDK1 activity blocks YAP nuclear accumulation caused by PIK3CA constitutive mutation in MCF-7 cells. Serum-starved MCF-7 cells were treated with PI3K inhibitor (Wortmannin) or PDK1 inhibitor (BX795) for 4 h, after which YAP intracellular localization was determined by confocal microscopy. (Scale bar: 20 μm.)
PDK1 Forms a Complex with Hippo Pathway Molecules in Confluent Serum-Starved Cells, and EGF Treatment Dissociates the PDK1-Hippo Complex and the Entire Hippo Pathway-Kinase Complex.
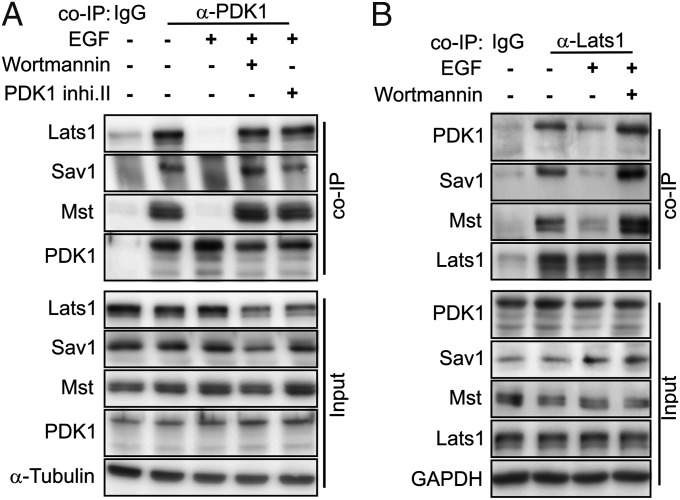
Components of the Hippo kinase cascade, including Mst, Sav1, and Lats, form a complex (2, 18–20). These proteins coimmunoprecipitate with endogenous PDK1 in confluent, serum-starved cells (Fig. 4A), demonstrating that PDK1 is in the same complex as Hippo pathway kinases. EGF treatment for 30 min disrupted the PDK1 interaction with these Hippo pathway components, as evidenced by the loss of Lats1, Mst, or Sav1 in the PDK1 immunoprecipitates. Importantly, the PI3K inhibitor and PDK1 inhibitor blocked the effect of EGF on dissociation of PDK1 from the Hippo complex. We also performed a reciprocal coimmunoprecipitation (co-IP) using anti-Lats1 antibody and found that PDK1, as well as Mst and Sav1, coimmunoprecipitated with endogenous Lats1 in serum-starved cells. The interaction of Lats with all of these components was disrupted after 30 min of EGF treatment (Fig. 4B), and pretreatment with the PI3K inhibitor blocked the effect of EGF on complex dissociation. In contrast, the Sav1–Mst interaction assessed by co-IP was not affected by EGF treatment (Fig. S5). These co-IP experiments identify PDK1 as a component of the Hippo pathway complex. They also show that brief EGF treatment causes dissociation of the complex in a way that would be expected to disrupt the kinase cascade owing to the disruption of Lats from Mst and Sav. Finally, these data show that EGF-induced complex dissociation depends on the activities of PI3K and PDK1, suggesting that PDK1 regulates dissociation.
Fig. 4.
PDK1 forms a complex with Hippo pathway components, and EGF treatment dissociates the Hippo-kinase complex. Confluent serum-starved MCF-10A cells were untreated, treated with EGF for 30 min, or treated with EGF after inhibitor pretreatment as indicated. (A) EGF treatment disrupts endogenous PDK1 binding to the Hippo complex proteins (Lats1, Mst, and Sav1) in MCF-10A cells, as determined by co-IP. The precipitated PDK1 immunocomplex and corresponding input samples were subjected to Western blot analysis with the indicated antibody. (B) EGF treatment disrupts endogenous Lats1 binding to PDK1, Mst, and Sav1 in MCF-10A cells, as determined by co-IP. The precipitated Lats1 immunocomplexes and corresponding input samples were subjected to Western blot analysis with the indicated antibody.
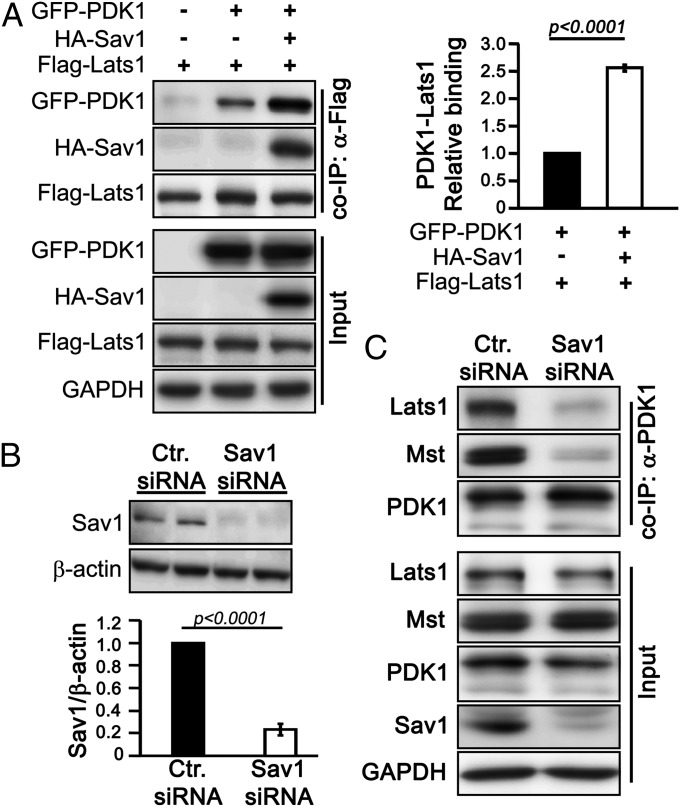
To better understand how PDK1 interacts with the Hippo complex, we studied the PDK1–Lats interaction by deletion mutagenesis and co-IP analysis (Fig. S6A). Deletion of the Lats PPxY motif completely disrupted the Lats–PDK1 association in HEK293T cells (Fig. S6B). However, the PPxY motif binds to molecules containing the WW domain, which is absent in PDK1. Thus, Lats may associate with PDK1 indirectly via the WW domain-containing molecule Sav1. Indeed, we found that overexpression of Sav1 increased the binding between PDK1 and Lats by 2.6-fold compared with vector control (Fig. 5A). Sav1 binds to Mst through their SARAH domains (18), and we found that Sav1 increased the PDK1–Mst association in a SARAH domain-dependent manner (Fig. S6C), suggesting that Sav1 mediates the association between PDK1 and Mst.
Fig. 5.
PDK1 interacts with Lats1 and Mst through scaffold protein Sav1. (A) Sav1 expression facilitates Lats1–PDK1 interaction, determined by coexpression of exogenous proteins in HEK293T cells and co-IP. The graph shows quantification of Western blots. Error bar represents mean ± SEM; n = 4. Statistical significance was calculated using the Student t test. (B) siRNA- mediated knocked down of Sav1 used in C. in MCF-10A cells. The bar graph shows Sav1 levels normalized to β-actin (n = 5). Error bar represents mean ± SEM. Statistical significance was calculated using the Student t test. (C) Sav1 mediates PDK1–Hippo complex binding in MCF-10A cells. Lysates from control siRNA or Sav1 siRNA-treated cells was incubated with anti-PDK1 antibody. Co-IP and input samples were subjected to Western blot analysis with indicated antibodies.
To further test the idea that Sav1 is the central molecule that mediates the interaction of PDK1 with other Hippo components, we knocked down Sav1 by siRNA in MCF-10A cells. Although PDK1–Lats1 and PDK1–Mst associations were detected in control siRNA-transfected cells, loss of Sav1 abolished the PDK1–Lats and PDK1–Mst associations (Fig. 5 B and C). Analysis of the interactions of a series of Sav1 deletion mutants with PDK1 in HEK293T cells showed that amino acid residues 145–162 of Sav1 molecule are critical for the PDK1–Sav1 interaction (Fig. S7). Thus, Sav1 is the central molecule that mediates the interaction of PDK1 with the Hippo complex.
PDK1 Pleckstrin Homology Domain Is Important for EGF-Mediated Hippo Inhibition.
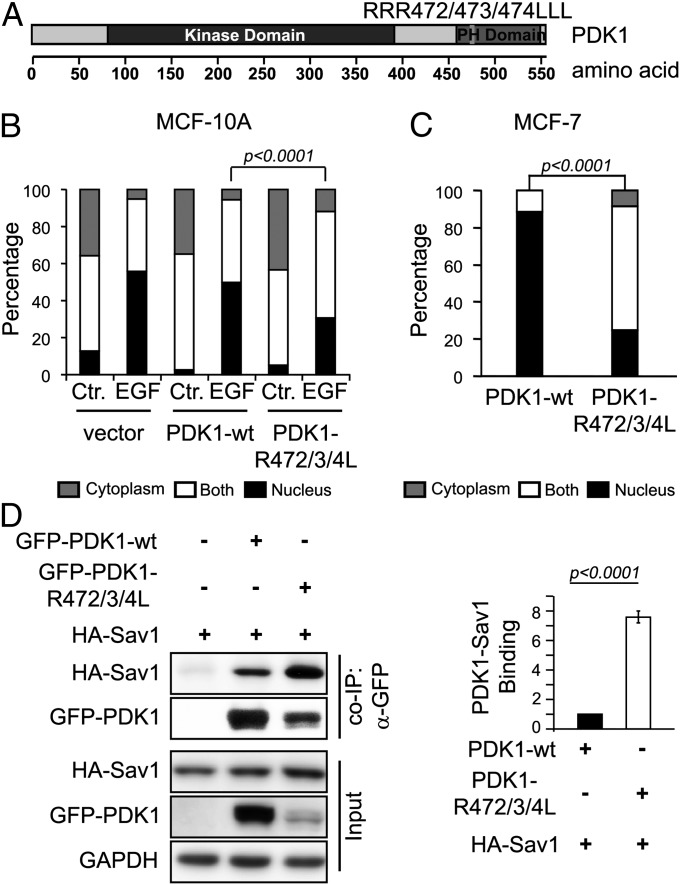
PDK1 comprises an N-terminal kinase domain and a C-terminal pleckstrin homology (PH) domain, and is recruited to the plasma membrane on growth factor stimulation through PH domain binding to PtdIns(3,4,5)P3, which leads to PDK1 activation (21–23). To determine whether PDK1 PH domain-mediated membrane recruitment and activation is important for the function of PDK1 in EGF regulation of the Hippo pathway, we generated a PDK1 PH domain mutant, PDK1-RRR472/473/474LLL (PDK1-R472/3/4L), which cannot bind PtdIns(3,4,5)P3 and shows impaired kinase activity (21, 23, 24). Expression of the PDK1-R472/3/4L in confluent serum-starved MCF-10A cells reduced YAP nuclear accumulation in response to EGF treatment compared with WT PDK1 (Fig. 6). PDK1-R472/3/4L was expressed at only 10% of the level of WT PDK1 in MCF-10A cells (Fig. S8), consistent with observations in a previous study (24), which explains its only partial effect on EGF-stimulated YAP nuclear accumulation.
Fig. 6.
PDK1 PH domain is important for EGF-mediated Hippo pathway inactivation. (A) Schema showing overall PDK1 structure and the PDK1 PH domain mutant (RRR472/473/474LLL). (B) PDK1 PH domain defective mutant (PDK1-R472/3/4L) inhibits EGF-stimulated YAP nuclear accumulation in MCF-10A cells. GFP control vector, GFP-PDK1-wt, or GFP-PDK1-R472/3/4L was transfected into MCF-10A cells by electroporation. Serum-starved cells were treated with EGF. The statistical significance of differences in YAP nuclear localization was calculated using the two-sided Fisher exact test. (C) PDK1 PH domain-defective mutant (PDK1-R472/3/4L) causes YAP cytoplasmic retention in MCF-7 cells. GFP-PDK1-wt or GFP-PDK1-R472/3/4L was transfected into MCF-7 cells by electroporation. The statistical significance of differences in YAP nuclear localization was calculated using the two-sided Fisher exact test. (D) PDK1 PH domain-defective mutant binds to Sav1 more abundantly than WT PDK1 in HEK293T cells, determined by co-IP analysis of coexpressed exogenous proteins. Statistical significance was calculated using the Student t test. Error bar represents mean ± SEM; n = 4.
We also performed this experiment in MCF-7 cells, and found that PDK1-R472/3/4L significantly decreased YAP nuclear staining compared with WT PDK1 (Fig. 6C). We used co-IP to examine how the PH domain influences the interaction of PDK1 with the Hippo complex. Although PDK1-R472/3/4L was expressed at only 20% of the level of WT PDK1 in HEK293T cells, more Sav1 bound to PDK1-R472/3/4L than to WT PDK1 (Fig. 6D). We obtained similar results for the PDK1-R472/3/4L mutant and Lats1 association (Fig. S8B). These findings suggest that PDK1 affects the Hippo pathway complex as a result of membrane recruitment on EGF treatment.
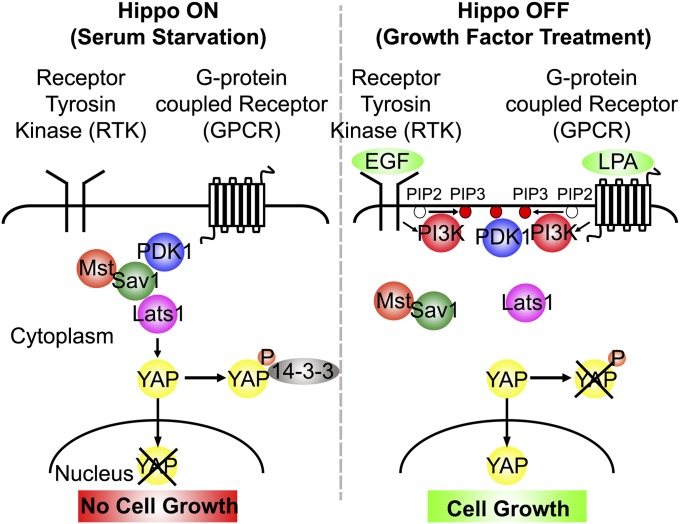
We propose a model to explain our findings (Fig. 7). In serum-starved confluent cells, PDK1 remains in the cytoplasm and forms a complex with active Hippo pathway kinases that phosphorylate YAP to retain it in the cytoplasm. When growth factors are added, PDK1 is recruited onto plasma membrane, leading to Hippo complex dissociation. Complex dissociation inactivates Lats, leading to YAP dephosphorylation and nuclear accumulation.
Fig. 7.
Model for growth factor regulation of the Hippo pathway. In confluent cells in the absence of growth factors, PDK1 forms a complex with Hippo pathway proteins (Lats, Mst, and Sav1), and the Hippo pathway is active. Mst phosphorylates Mob and Lats, which phosphorylates YAP. YAP is retained in the cytoplasm, and cell growth is arrested. In the presence of growth factors (EGF, LPA, or serum), PDK1 is recruited on to the membrane, and the PDK1-Hippo complex dissociates, preventing regulation of Lats by Mst, which eventually leads to YAP nuclear accumulation and cell proliferation.
Discussion
Our findings demonstrate that mitogenic growth factor signaling rapidly inactivates the Hippo signaling pathway in confluent contact-inhibited cells. EGFR signaling, as well as signaling stimulated by serum and LPA, caused rapid nuclear accumulation of the YAP transcriptional activator. Although nuclear accumulation of YAP is also known to be regulated by Hippo pathway-independent mechanisms (25, 26), we used several other criteria to establish the Hippo pathway itself as an important target of EGFR signaling. We found that dephosphorylation of YAP at Ser127, a target for Lats, a key component of the Hippo kinase cascade, is associated with its nuclear accumulation. Moreover, YAP nuclear accumulation in response to EGF depends in part on Lats. Finally, we observed rapid dissociation of the core Hippo pathway complex of kinases and accessory/scaffolding proteins in response to EGFR signaling, demonstrating that the core Hippo pathway complex is a target of EGFR signaling action.
Extracellular mediators that regulate the Hippo pathway include the Daschous/Fat system (27) and polarity proteins, such as crumbs (28–30) in Drosophila. Cell contact is known to activate Hippo signaling, and we previously identified cadherin-mediated contacts as responsible (10); evidence for roles of other junction-associated proteins has been reported as well (26, 31, 32). Our current findings show that growth factor receptors are upstream negative regulators of the Hippo pathway. Similarly, in Drosophila, insulin-like growth factor (IGF) regulates growth through Yorkie/YAP (33). Soluble factors in serum have recently been reported to act through G protein-coupled receptors to either activate or inhibit the Hippo pathway, although the mechanisms were not identified (34). Clearly, the factors controlling the Hippo pathway activity are numerous and varied.
We discovered a common mechanism by which various types of mitogenic pathways inactivate the Hippo pathway. Although we used EGFR as a model, we found that both serum and LPA, which works through a G protein-coupled receptor, stimulated rapid nuclear accumulation of YAP. Indeed, our discovery that this activity is mediated by PI3K and PDK1 suggests that many, if not all, of the various signaling pathways that activate PI3K signaling will inhibit the Hippo pathway. We even found that a constitutively active oncogenic mutation in PI3K drives YAP nuclear localization in mammary tumor cells. Although the PI3K inhibitor Wortmannin was not found to affect YAP phosphorylation in response to FBS (34), the effects of Wortmannin and other PI3K and PDK1 inhibitors in our system were very robust and reproducible. In agreement with our findings, IGF regulation of Hippo signaling in Drosophila was found to be mediated by PI3K and PDK1 (33).
Despite the key roles of PI3K and PDK1 in growth factor regulation of Hippo signaling, AKT, the major effector kinase that typically acts downstream of these proteins, does not appear to have a role in Hippo pathway regulation in MCF-10A cells. This suggests that alternative effectors are important. PDK1 is known to have other substrates (35), but our findings implicate PDK1 directly in control of the Hippo pathway. The protein target phosphorylated by PDK1 important for control of Hippo signaling is not yet clear; we have not been able to identify known components of the Hippo complex as direct targets. Nonetheless, our finding that PDK1 forms a protein complex with the core Hippo complex through its interaction with Sav1 indicates that the Hippo pathway is an important target of PDK1 activity.
The mechanism by which growth factor-induced PI3K-PDK1 activity inhibits the Hippo pathway appears to be related to dissociation of the core Hippo complex containing Mst, Sav1, Lats, and PDK1. Complex dissociation is rapid, occurring in a similar time frame as nuclear accumulation and dephosphorylation of YAP. Like YAP nuclear accumulation, hippo complex dissociation depends on the activities of PI3K and PDK1. Formation of this complex is well known to be critical for Hippo pathway activity, given that the complex and the scaffolding protein Sav1 are required for the phosphorylation of Lats and Mob by Hippo/Mst (2, 19, 20, 36). Thus, complex dissociation is expected to result in a loss of Lats activation, leading to dephosphorylation of YAP. Although the formation of the core complex is known to be important for Hippo activity, our findings demonstrate that active dissociation of the complex by regulatory factors plays a role in control of Hippo pathway activity.
Exactly how PI3K and PDK1 activities control the state of the core Hippo complex is not yet clear, but likely involves PH domain-mediated recruitment of PDK1 to the plasma membrane by phosphoinositide products of PI3K. Because a mutant form of PDK1 lacking the PH domain associates more abundantly with the Hippo complex, membrane recruitment of PDK1 may be involved in the control of complex dissociation.
Inhibition of the growth-inhibitory Hippo pathway by mitogenic growth factor signaling raises interesting ideas about integration of various types of growth control mechanisms. Inactivation of the Hippo pathway may be an important requirement for growth factors to trigger proliferation. Indeed, we found that YAP is required for EGF stimulation of cell proliferation in MCF-10A cells. This is surprising and important, because it suggests that, for this cell type at least, the other branches of the EGF pathway may be insufficient on their own to stimulate growth. Similarly, YAP and Yorkie were found to be required for IGF-stimulated growth in Drosophila (33), and serum GPR stimulated growth in HEK293A cells (34). Moreover, these pathways likely create important autocrine and other feedback loops via transcriptional targets of YAP, including CTGF, amphiregulin, and upstream components of the IGF pathway (12, 33, 37, 38). Amphiregulin seems to inhibit the Hippo pathway in MCF-10A cells, as demonstrated by the need to add neutralizing antibodies for effective serum starvation. The interactions between these pathways may be important for understanding tissue growth in vivo.
Oncogenic activation of RTKs, as well as PI3K, are known to contribute to tumor formation (39–41). Our findings raise the possibility that nuclear YAP is an important downstream effector of these pathways that contributes to tumor growth and has been shown to have oncogenic activity when activated by mutations that block its regulation by the upstream Hippo pathway (4). It will be important to investigate this relationship between RTK and Hippo pathways in tumorigenesis experimentally in animals and in human tumors.
Materials and Methods
Cell Culture and Treatment.
Confluent MCF-10A cells were serum-starved with basal medium supplemented with 1 μg/mL anti–amphiregulin-neutralizing antibody for 24 h. Cells were pretreated with indicated inhibitors for 30 min, and then treated with 20 ng/mL EGF (PeproTech), 25 μM lysophosphatidic acid (LPA; Sigma-Aldrich), or 5% horse serum in basal DMEM/F12 medium (Invitrogen). Other cells were cultured as described previously (10). Antibodies, reagents, plasmids, and transfections are described in detail in SI Materials and Methods.
Immunofluoresence Staining and Confocal Microscopy.
Cells were fixed with 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100. Primary and secondary antibodies were diluted in blocking buffer (PBS with 3% BSA). TOPRO3 was added for nuclear staining. Slides were analyzed with a Nikon Eclipse TE2000 confocal microscope.
Immunoprecipitation and Western Blot Analysis.
MCF-10A cells were lysed with hypotonic buffer [10 mM Hepes (pH 7.4), 1 mM EDTA, 150 mM NaCl] supplemented with protease and phosphatase inhibitors. HEK293T cells were lysed with Nonidet P-40 buffer [150 mM NaCl, 1.0% Nonidet P-40, 50 mM Tris (pH 8.0)] at 24 h after transfection. Immunoprecipitation and Western blot analyses are described in detail in SI Materials and Methods.
ChIP-PCR.
ChIP was performed as described by Braunstein et al. (42) and as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ken Irvine for sharing his related findings in Drosophila with us before publication. This work was supported by National Institutes of Health Grant R01 GM098615 (to B.M.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216462110/-/DCSupplemental.
References
- 1.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103(33):12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Suzuki K. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp Cell Res. 1996;226(1):214–222. doi: 10.1006/excr.1996.0221. [DOI] [PubMed] [Google Scholar]
- 8.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18(6):2013–2025. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108(29):11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 15.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379(6565):557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 16.Stemke-Hale K, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikediobi ON, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5(11):2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273(18):4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 19.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 20.Tapon N, et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8(12):684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 22.Filippa N, Sable CL, Hemmings BA, Van Obberghen E. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol Cell Biol. 2000;20(15):5712–5721. doi: 10.1128/mcb.20.15.5712-5721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toker A, Newton AC. Cellular signaling: Pivoting around PDK-1. Cell. 2000;103(2):185–188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 24.McManus EJ, et al. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 2004;23(10):2071–2082. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38(10):1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 28.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20(7):582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20(7):573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 30.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 2010;19(6):831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Silvis MR, et al. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4(174):ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367(2):187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27(8):1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11(12):1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 40.Slamon DJ, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 41.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229(4717):974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 42.Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7(4):592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.