Abstract
Squids have used their tunable iridescence for camouflage and communication for millions of years; materials scientists have more recently looked to them for inspiration to develop new “biologically inspired” adaptive optics. Iridocyte cells produce iridescence through constructive interference of light with intracellular Bragg reflectors. The cell’s dynamic control over the apparent lattice constant and dielectric contrast of these multilayer stacks yields the corresponding optical control of brightness and color across the visible spectrum. Here, we resolve remaining uncertainties in iridocyte cell structure and determine how this unusual morphology enables the cell’s tunable reflectance. We show that the plasma membrane periodically invaginates deep into the iridocyte to form a potential Bragg reflector consisting of an array of narrow, parallel channels that segregate the resulting high refractive index, cytoplasmic protein-containing lamellae from the low-index channels that are continuous with the extracellular space. In response to control by a neurotransmitter, the iridocytes reversibly imbibe or expel water commensurate with changes in reflection intensity and wavelength. These results allow us to propose a comprehensive mechanism of adaptive iridescence in these cells from stimulation to color production. Applications of these findings may contribute to the development of unique classes of tunable photonic materials.
Keywords: Doryteuthis opalescens, iridophore, structural color
Although structural color is widespread across both the animal and plant kingdoms (1–4), there are very few cases in which the photonic structures are tunable and adaptive (5–7). Many cephalopods exhibit iridescence, but only a few squid species can tune this iridescence for adaptive camouflage and communication (8, 9) by modulating the periodicity of multilayer reflectors (7) in specialized cells classically called iridocytes. [Earlier workers have referred to these cells variously as iridocytes, iridophores, or reflective cells. We use here the unambiguous convention of contemporary cell biology, referring to them as iridocytes (literally “iridescent cells”), with no specific photonic mechanism implied.] For this reason, the tunable photonics of squids have long been a source of intrigue and inspiration to materials scientists (10–12). Although it is known that the neurotransmitter acetylcholine (ACh) activates a signal-transduction cascade to drive the changes in periodicity of the reflectors (7, 13), and the nerve cells delivering this signal recently have been identified (14), details of the molecular mechanisms and cellular architectures governing the biophotonic processes themselves have remained elusive.
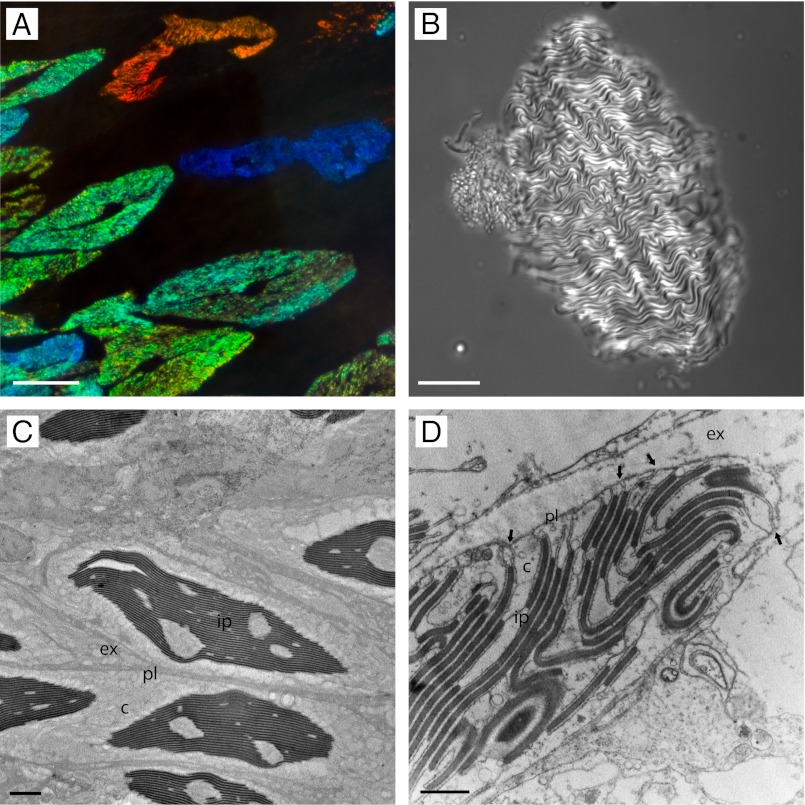
Morphology of cephalopod iridocytes has traditionally been interrogated by light microscopy (Fig. 1 A and B) and transmission electron microscopy (TEM) (Fig. 1 C and D), revealing the membrane-bound subcellular lamellae that constitute a Bragg reflector. Whereas these analyses by optical imaging are fundamentally limited because the subcellular platelet structure of this reflector is ordered at length scales commensurate with the wavelengths of visible light, TEM of thin sections reveals further details of the iridocyte Bragg reflector. A unique feature observed in TEM cross-sections of the iridocytes of some squids is the peculiar invaginations of the plasma membranes that extend deep into the cell and create lamellae of the high refractive index, protein-rich platelets (15, 16). These invaginations separate the low refractive index space continuous with the exterior of the cell from the protein-rich Bragg lamellae. Similar membrane invagination/lamination morphology has been observed in photophore (i.e., bioluminescent organ) iridocytes of Pterygioteuthis microlampas (17) and the dermal iridocytes of Octopus dolfini (16) (but here, the Bragg stacks are arranged outside the cell body). It has been unclear, however, whether these unusual invaginations are artifactual (18), and recent articles have suggested that the low refractive index space of the tunable iridocytes may instead be wholly intracellular (19–21). Although TEM analyses have provided great insights, efforts to extrapolate a 3D model from such 2D images have been unsuccessful (18), and a full understanding of the tunable reflective structure through optical microscopy and TEM alone has remained elusive (15, 18, 22).
Fig. 1.
Iridophores viewed by light and electron microscopy. (A) Several iridocytes within the dermis (dark-field illumination). (B) A single isolated iridocyte in brightfield illumination. (C) Transmission electron microscopy of several iridocytes in cross-section. (D) A small portion of the edge of an iridocyte, highlighting several key characteristics. The plasma membrane invaginates deep into the cell (arrows) laminating both sides of the cytoplasm-containing platelets and separating them with channels of extracellular space (Scale bars: A, 50 µm; B, 30 µm; C, 3 µm; D, 1 µm.) Ultrastructural features are labeled as c, cytoplasm; ex, extracellular space; ip, iridocyte platelets; pl, plasma membrane.
Results and Discussion
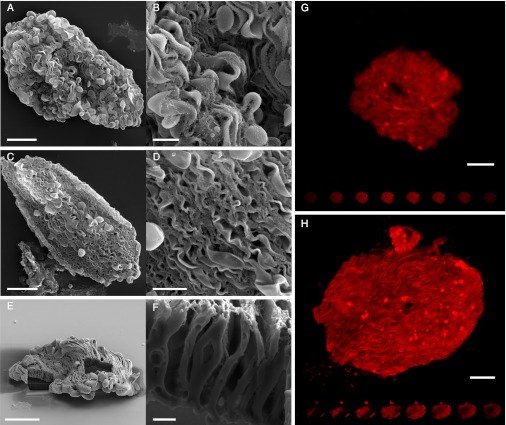
We sought to resolve this ambiguity of plasma membrane morphology in the squid tunable iridocytes through 3D dissection by focused ion beam milling (FIB) in conjunction with scanning electron microscopy (SEM). We began by isolating single adaptive iridocytes from the squid, Doryteuthis opalescens, by careful dissection and enzymatic digestion to liberate the tunably reflective cells from the surrounding connective tissue (Fig. 1B); methods are similar to those reported for the related squid, Alloteuthis subulata (21). The isolated iridocyte cells were confirmed to be intact via the exclusion of trypan blue and propidium iodide. We imaged the cell surface of both dorsal (Fig. 2 A and B) and ventral (Fig. 2 C and D) iridocytes by SEM. From these images, it is evident that the plasma membrane indeed folds into the cell, forming numerous channels that flute the cell surface. To confirm these findings, we used a FIB for subcellular dissection, removing portions of the cell to provide another means to visualize the invaginations by SEM (Fig. 2 E and F). To further corroborate these results, we used a plasma membrane-specific stain to image the cells with confocal microscopy, thereby highlighting the membrane infolding throughout the entirety of the cell (Fig. 2 G and H).
Fig. 2.
Scanning electron and confocal imaging reveal the complex surface topology of enzymatically isolated iridocytes. (A) Single iridocyte from the dorsal region of the mantle. (B) Close-up of the same cell showing the complicated folding and invaginations of the plasma membrane. (C) Isolated iridocyte from the ventral mantle. (D) Close up of the same cell. (E) To expose the membrane folds, we cut out a portion of a cell with a focused ion beam. (F) Close up of the cut region. Confocal images of iridocytes from the dorsal (G) and ventral (H) mantle stained with a fluorescent plasma-membrane stain. (Scale bars: A, C, and H, 20 µm; B and D, 5 µm; E and G, 10 µm; F, 1 µm.).
The observed membrane morphology gives the iridocytes a large surface and a morphology similar to the outer segment of vertebrate photoreceptor cells (23). The color changing ability of these squid cells is much more pronounced in the dorsal iridocytes, which can change sequentially from nonreflecting to red, then green, and finally blue. The ventral cells have a much weaker response, showing only minor changes, if any at all (7, 21). Regularity of the membrane folding is more clearly seen in iridocytes from the ventral dermis than from the dorsal dermis because the platelets of the ventral iridocytes are oriented perpendicular to the flat plane of the cells (21), have greater thicknesses and spacing, and are less convoluted (Fig. 2) (18, 24). The large, thick platelets and spacing are likely used more for scattering light rather than for constructive interference. The dorsal iridocyte membrane clearly folds deep into the cells, although the ultimate path of these folds is not easily followed. The platelets in dorsal iridocytes are parallel to the plane of the cell, more abundant, much thinner (providing periodicity for constructive interference in the visible wavelength range), and more sinuous (18, 20). Our laboratory's direct measurements confirm the correlation between the number of periodic platelets and brightness of these iridocytes, consistent with the higher level of invagination (i.e., the number of lamellae) of these dorsal iridocytes.
Brocco et al. (16) suggested that one function of these membrane invaginations is to establish the highest refractive index contrast by using extracellular fluid instead of cytoplasm as the low index layer, thereby maximizing reflectance intensity (3). We hypothesize that two additional functional roles for the invaginations in the adaptive cells may be (i) to provide high surface area access of the entire photonic apparatus to extracellular signal molecules such as ACh, and (ii) a high surface area route for the rapid exchange of water between the high index platelets and the cell’s exterior. Both of these roles would facilitate rapid changes of the apparent lattice constant and dielectric contrast of the photonic structure, consequently changing both the intensity and color of reflectance. These hypotheses are in agreement with Sutherland’s mathematical model in which he suggested that shifts in reflected wavelength from the adaptive iridocytes are directly related to the volume fraction of water contained in the platelets (20).
To ascertain directly whether water is exchanged with the exterior of the iridocyte during stimulation and relaxation, we used seawater spiked with D2O to track water movement into and out of isolated iridocyte tissue (see Materials and Methods for details). We measured the increase in D2O tracer inside the cells after cycles of stimulation and washing (Fig. 3A). Because the high index platelets are composed of reflectin proteins (13, 25), each sample was normalized to the reflectin concentration. As expected, D2O did not increase within the iridocytes until after the acetylcholine stimulus was washed away and the platelets relaxed to their swollen state (swelling with the uptake of exterior water) (Fig. 3B). A second stimulation then diminished the intracellular D2O content, providing direct support for the water expulsion hypothesis. To control for proton exchange and leakiness of the cells, all of the samples were exposed to D2O for the same amount of time. During this waiting period, the cells were stored with or without ACh to match the final conditions of the sample and suppress extraneous changes in platelet hydration. Second, we performed the reverse of the previous experiment; instead of measuring D2O uptake, we directly measured expulsion from the cells. We equilibrated tissue samples in D2O-labeled seawater, washed away external D2O and then measured the D2O increase in the label-free bath after stimulation (Fig. 3C). In this case, we observed significant water expulsion from the dorsal iridocytes, whereas the ventral iridocytes showed relatively poor response compared with the control. Correspondingly, the dorsal iridocytes displayed the most significant photonic response, changing color from the initial nonreflecting state to red and then progressively to blue, whereas the ventral cells showed only small changes (7, 21, 26).
Fig. 3.
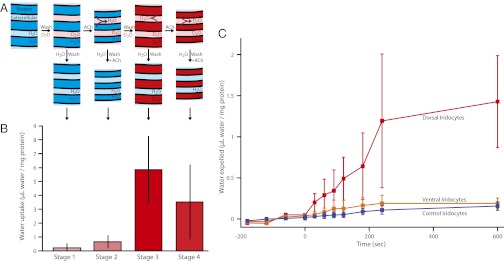
Iridocytes exchange water with their external environment commensurate with change in iridescence. (A) Diagram of the iridocyte platelet swelling and shrinkage by fluxes of water, as revealed by the first experiment. The samples begin in a relaxed state (hydrated with unlabeled seawater). Immersion of the sample in seawater spiked with isotopically labeled water allows diffusion of D2O into the interplatelet space (stage 1). Stimulation by ACh drives expulsion of the previously loaded unlabeled water, collapsing the platelets (stage 2). After washing away the ACh, the platelets relax back to a hydrated state, swelling by uptake of the isotope enriched seawater from the cell’s exterior (stage 3). Finally, a second stimulation with ACh expels some of the labeled water from the platelets (stage 4). Finally, all samples are washed in isotope-free seawater. (B) Data showing the amount of water taken up at each stage of the experiment calculated from the enrichment in deuterium signal and normalized to the protein content of each sample, as described in Materials and Methods (n = 4). Error bars show one SD. (C) Data showing water released from the cells after stimulation by ACh at t = 0. Tissues were first equilibrated in D2O-enriched water and then stimulated with ACh; the increase in D2O in the surrounding seawater was measured. The red line represents dorsal samples, the orange line represents ventral samples, and the blue line represents control (no ACh) samples. The deuterium signal was normalized to protein content and used to calculate the total movement of water; error bars represent SD.
Several important assumptions and approximations are made in analyzing the water exchange experiments of this study. Although the principal conclusion that water is exchanged with the extracellular space commensurate with color change is sound, quantitative uncertainties prevent our determination of the exact volume of water that enters and leaves the lamellae. First, there is a wide range of responsiveness of reflectance to ACh stimulation in the excised tissue samples; variable fractions of the iridocytes respond, and the extent and timing of their responsiveness varies as well. We also have not corrected for the minimal proton exchange with other molecules in the sample, passive diffusion of water in or out of the cells, and changes in response over time. Nevertheless, the observed water uptake and expulsion appear to be in the right range to effectively produce the observed changes in iridescence. The actual protein concentration of the iridocyte platelets is still not clear, but assuming the condensed platelets have a refractive index as high as 1.42 (16) and a standard protein refractive index increment of 0.2097 mL/g (27), the platelet protein concentration would be 381 mg/mL. With a uniquely high protein concentration approaching that of heavily concentrated lens crystalins (28), a change of 1.27 ± 0.56 μL of water per mg of protein would yield the necessary change in volume fraction of water to produce the observed spectral changes (20).
The membrane invaginations provide a high surface area interface between all of the protein lamellae and the extracellular space. We believe that the unique morphology of the iridocyte cells is functionally important, not only for maximizing the refractive index contrast as discussed earlier, but also by providing a route for the rapid exchange of water facilitated by the large surface area between the protein lamellae and extracellular space. Water can be exchanged directly with the outside of the cell, quickly mitigating potential osmotic stress and disruption of intracellular chemical equilibrium. Similarly, direct and rapid expulsion from the reflectin-containing platelets into the extracellular space is important for color production; uniformity of the multilayer reflector is critical for the generation and color tuning of iridescence, whereas uneven shrinkage of the platelets would reduce both the brightness and color purity of the reflected light (20).
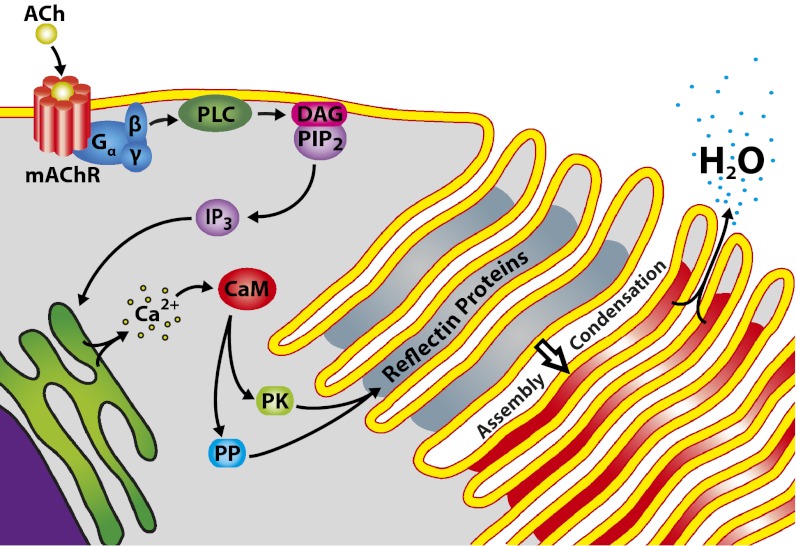
Our observations allow us to envision a more complete mechanism of adaptive iridocyte iridescence (Fig. 4): ACh is known to bind a muscarinic type G protein-coupled receptor (7). The G protein is then released and activates the signaling cascade (as confirmed by stimulation of brightness and color with cholera toxin) (13), resulting in calcium release from the endoplasmic reticulum (21). Ca2+ acts as a second messenger, binding calmodulin (24) and activating protein kinases and phosphatases (29). The resulting differential phosphorylation and dephosphorylation of specific reflectins, as observed (13), apparently overcomes Coulombic repulsion, driving the observed condensation of the reflectins to a dense, compressible, hydrogel-like network (26). This condensation of the reflectin proteins, before any change in the dimensions of the Bragg reflector, is first manifested optically as the previously observed increase in the refractive index contrast between the intra- and extralamellar compartments and the consequent onset of bright reflectance, before any progressive shift in reflected wavelength (26). Condensation of the reflectins also would reduce the exposure of ionic groups on the protein surfaces, thereby driving an efflux of small ions across the cell membrane invaginations to maintain electro-osmotic equilibrium; this ion efflux, in turn, would drive the expulsion of water in a Gibbs–Donnan equilibration (30). The resulting dehydration, demonstrated here, further increases the intralamellar protein concentration (further increasing the refractive index contrast and brightness, as observed) (26) and simultaneously reduces the platelet thickness, thereby changing the dimensions of the periodic Bragg reflector and—as a result—changing the color of the reflected light. The reversible, diffusional flux of water between the reflectin-containing platelets and the immediately adjacent extracellular space is rapid and facilitated uniformly in all of the platelets by the high surface area membrane invaginations forming the platelets. Thus, the rapid, reversible flux of water across the highly invaginated iridocyte membrane, regulated by neurotransmitter- and signal transduction cascade-mediated modifications of the reflectin proteins, directly controls the optical properties of this biological, tunably adaptive multilayer reflector.
Fig. 4.
Schematic illustration summarizing the proposed mechanism of iridescence activation. Structural and mechanistic details elucidated in this manuscript are diagrammed. (See text for details.)
Materials and Methods
Dermal iridocyte tissue was isolated from freshly euthanized D. opalescens and pinned out into Sylgard 184 (Fisher Scientific) coated dishes. Iridocyte cells were isolated from the connective tissue after treatment with 5 mg/mL Sigma Blend Type H collagenase (Sigma-Aldrich) in artificial seawater (ASW) (7) at 20 °C for 1 h. Isolated cells were collected by aspiration, rinsed in ASW, and fixed with 2.5% (wt/vol) glutaraldehyde in ASW for light and electron microscopy [2% (wt/vol) formaldehyde for fluorescence microscopy]. The fluorescent plasma membrane stain, Cellmask Orange (Molecular Probes) was used with the manufacturer’s protocol and imaged with an Olympus FluoView 500 confocal microscope. Fixed iridocytes were prepared for SEM in hexamethyldisilizane (31), gold-coated, and imaged with a Vega 5130 MM SEM (Tescan) at 20 kV. FIB analyses were performed on a FEI Helios 600 with sample stage rotated 52° and gallium ion beam perpendicular to the cell; rough milling (2.8 nA, 30 kV) to remove outer portion was followed by polishing (0.28 nA, 30 kV).
D2O tracer studies with live iridocyte-rich tissue pinned out in ASW at room temperature containing 1% (vol/vol) D2O. Samples were taken before and after two cycles of stimulation (100 µM ACh) and washing. External D2O was washed away, and the samples were homogenized and the supernatant collected for isotope analysis. In a second type of experiment, iridocyte-rich tissues were first equilibrated in D2O, and ACh-induced release of water was determined from samples taken from the surrounding ASW that was initially free of D2O. D2O concentrations were measured in quadruplicate with a Finnigan Delta Plus XP Isotope Ratio Mass Spectrometer and Thermal Conversion Elemental Analyzer pyrolysis peripheral, with calibration of δ2H measurements relative to the isotope ratio of H2 gas and normalization to the international isotope standards Standard Mean Ocean Water, Greenland Ice Sheet Precipitation, and Standard Light Antarctic Precipitation (32). Each sample was normalized to the protein concentration of the crude reflectin extract (13, 26). Protein concentrations were determined spectrophotometrically with a bicinchoninic acid assay (33).
Acknowledgments
We thank Tim Athens at Outerbanks Fisheries for help in acquiring live and healthy squid; George Paradis at University of California, Santa Barbara (UCSB) Marine Science Institute Analytical Laboratory for assistance with the stable isotope ratio analysis; Mary Raven and UCSB Neuroscience Research Institute Microscopy facility staff for generously sharing their time and equipment; and Dr. Andrea Tao, Dr. Michi Izumi, and Dr. Amitabh Ghoshal for helpful discussions and assistance in this work. This research was supported by the Office of Naval Research via Multidisciplinary University Research Initiative Award N00014-09-1-1053 to Duke University, Army Research Office Grant W911NF-10-1-0139 (to D.E.M.), and we made use of UCSB Materials Research Laboratory central facilities and equipment, which are supported by National Science Foundation Grant DMR-0080034.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Parker AR. 515 million years of structural colour. J Opt A, Pure Appl Opt. 2000;2(6):15–28. [Google Scholar]
- 2.Vukusic P, Sambles JR. Photonic structures in biology. Nature. 2003;424(6950):852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- 3.Land MF. The physics and biology of animal reflectors. Prog Biophys Mol Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- 4.Vignolini S, et al. Pointillist structural color in Pollia fruit. Proc Natl Acad Sci USA. 2012;109(39):15712–15715. doi: 10.1073/pnas.1210105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshioka S, et al. Mechanism of variable structural colour in the neon tetra: Quantitative evaluation of the Venetian blind model. J R Soc Interface. 2011;8(54):56–66. doi: 10.1098/rsif.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mäthger LM, Land MF, Siebeck UE, Marshall NJ. Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus. J Exp Biol. 2003;206(Pt 20):3607–3613. doi: 10.1242/jeb.00599. [DOI] [PubMed] [Google Scholar]
- 7.Cooper KM, Hanlon RT, Budelmann BU. Physiological color change in squid iridophores. II. Ultrastructural mechanisms in Lolliguncula brevis. Cell Tissue Res. 1990;259(1):15–24. doi: 10.1007/BF00571425. [DOI] [PubMed] [Google Scholar]
- 8.Mäthger LM, Denton EJ, Marshall NJ, Hanlon RT. Mechanisms and behavioural functions of structural coloration in cephalopods. J R Soc Interface. 2009;6(Suppl 2):S149–S163. doi: 10.1098/rsif.2008.0366.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boal JG, et al. Behavioral evidence for intraspecific signaling with achromatic and polarized light by cuttlefish (Mollusca: Cephalopoda) Behaviour. 2004;141(7):837–861. [Google Scholar]
- 10.Vaia R, Baur J. Materials science. Adaptive composites. Science. 2008;319(5862):420–421. doi: 10.1126/science.1152931. [DOI] [PubMed] [Google Scholar]
- 11.Walish JJ, Kang Y, Mickiewicz RA, Thomas EL. Bioinspired electrochemically tunable block copolymer full color pixels. Adv Mater (Deerfield Beach Fla) 2009;21(30):1–4. [Google Scholar]
- 12.Kreit E, et al. Biological versus electronic adaptive coloration: How can one inform the other? J R Soc Interface. September 26, 2012;10(78):20120601. doi: 10.1098/rsif.2012.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi M, et al. Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. J R Soc Interface. 2010;7(44):549–560. doi: 10.1098/rsif.2009.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardill TJ, Gonzalez-Bellido PT, Crook RJ, Hanlon RT. Neural control of tuneable skin iridescence in squid. Proc Biol Sci. 2012;279(1745):4243–4252. doi: 10.1098/rspb.2012.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold JM. Organellogenesis of the cephalopod iridophore: Cytomembranes in development. J Ultrastruct Res. 1967;20(5):410–421. doi: 10.1016/s0022-5320(67)80109-6. [DOI] [PubMed] [Google Scholar]
- 16.Brocco SL, Cloney RA. Reflector cells in the skin of Octopus dofleini. Cell Tissue Res. 1980;205(2):167–186. doi: 10.1007/BF00234678. [DOI] [PubMed] [Google Scholar]
- 17.Arnold JM, Young RE, King MV. Ultrastructure of a cephalopod photophore. II. Iridophores as reflectors and transmitters. Biol Bull. 1974;147(3):522–534. doi: 10.2307/1540737. [DOI] [PubMed] [Google Scholar]
- 18.Mirow S. Skin color in the squids Loligo pealii and Loligo opalescens. II. Iridophores. Z Zellforsch Mikrosk Anat. 1972;125(2):176–190. doi: 10.1007/BF00306787. [DOI] [PubMed] [Google Scholar]
- 19.Mäthger LM, Denton EJ. Reflective properties of iridophores and fluorescent ‘eyespots’ in the loliginid squid Alloteuthis subulata and Loligo vulgaris. J Exp Biol. 2001;204(Pt 12):2103–2118. doi: 10.1242/jeb.204.12.2103. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland RL, Mäthger LM, Hanlon RT, Urbas AM, Stone MO. Cephalopod coloration model. I. Squid chromatophores and iridophores. J Opt Soc Am A Opt Image Sci Vis. 2008;25(3):588–599. doi: 10.1364/josaa.25.000588. [DOI] [PubMed] [Google Scholar]
- 21.Mäthger LM, Collins TF, Lima PA. The role of muscarinic receptors and intracellular Ca2+ in the spectral reflectivity changes of squid iridophores. J Exp Biol. 2004;207(Pt 11):1759–1769. doi: 10.1242/jeb.00955. [DOI] [PubMed] [Google Scholar]
- 22.Denton EJ, Land MF. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc R Soc Lond B Biol Sci. 1971;178(50):43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190(3):501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- 24.Hanlon RT, Cooper KM, Budelmann BU, Pappas TC. Physiological color change in squid iridophores. I. Behavior, morphology and pharmacology in Lolliguncula brevis. Cell Tissue Res. 1990;259(1):3–14. doi: 10.1007/BF00571424. [DOI] [PubMed] [Google Scholar]
- 25.Crookes WJ, et al. Reflectins: The unusual proteins of squid reflective tissues. Science. 2004;303(5655):235–238. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- 26.Tao AR, et al. The role of protein assembly in dynamically tunable bio-optical tissues. Biomaterials. 2010;31(5):793–801. doi: 10.1016/j.biomaterials.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Brown PH, Schuck P. On the distribution of protein refractive index increments. Biophys J. 2011;100(9):2309–2317. doi: 10.1016/j.bpj.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaenicke R, Slingsby C. Lens crystallins and their microbial homologs: Structure, stability, and function. Crit Rev Biochem Mol Biol. 2001;36(5):435–499. doi: 10.1080/20014091074237. [DOI] [PubMed] [Google Scholar]
- 29.Stull JT. Ca2+-dependent cell signaling through calmodulin-activated protein phosphatase and protein kinases minireview series. J Biol Chem. 2001;276(4):2311–2312. doi: 10.1074/jbc.R000030200. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89(1):193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 31.Araujo JC, et al. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo) 2003;52(4):429–433. doi: 10.1093/jmicro/52.4.429. [DOI] [PubMed] [Google Scholar]
- 32.Coplen TB. Reporting of stable hydrogen, carbon, and oxygen isotopic abundances. Pure Appl Chem. 1994;66(2):273–276. [Google Scholar]
- 33.Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]