Abstract
Alkyne-hinged 3-fluorosialyl fluoride (DFSA) containing an alkyne group was shown to be a mechanism-based target-specific irreversible inhibitor of sialidases. The ester-protected analog DFSA (PDFSA) is a membrane-permeable precursor of DFSA designed to be used in living cells, and it was shown to form covalent adducts with virus, bacteria, and human sialidases. The fluorosialyl–enzyme adduct can be ligated with an azide-annexed biotin via click reaction and detected by the streptavidin-specific reporting signals. Liquid chromatography-mass spectrometry/mass spectrometry analysis on the tryptic peptide fragments indicates that the 3-fluorosialyl moiety modifies tyrosine residues of the sialidases. DFSA was used to demonstrate influenza infection and the diagnosis of the viral susceptibility to the anti-influenza drug oseltamivir acid, whereas PDFSA was used for in situ imaging of the changes of sialidase activity in live cells.
Keywords: ABPP probe, click chemistry, imaging agents, proteomics
Sialidase, also called neuraminidase (NA), is an exoglycosidase that catalyzes the hydrolysis of terminal sialic acid residues from the oligosaccharides of glycoconjugates. Sialidases are widely expressed for various functions (1). Many pathogens, such as viruses, bacteria, and protozoa, produce sialidases for invasion, nutrition, detachment, and immunological escape (2). Mammal sialidases also have been implicated in many biological processes, including regulation of cell proliferation/differentiation, modulation of cell adhesion, metabolism, and immunological functions (3, 4). Four types of sialidases have been identified and characterized in mammals. These sialidases are encoded by different genes and expressed at different intracellular localizations as lysosomal (Neu1), cytosolic (Neu2), plasma-membrane (Neu3), and mitochondrial/lysosomal (Neu4) enzymes. Although these enzymes share a common catalytic mechanism, they have little overlapping functions, probably because of differences in subcellular distribution, pH optimum, kinetic properties, and substrate specificities (5). The regulation and detailed functions of these enzymes are largely undefined (6).
Alterations in sialidase activities have been implicated in different diseases. For example, elevated sialidase activities have been reported in BHK-transformed cells and in human breast/colon cancer tissues (7, 8). Animal studies also suggest the roles of sialidases in tumorigenic transformation and tumor invasion. Biochemical characterizations of mammalian sialidases suggest that increases in Neu3 are involved in colon, renal, and prostate cancers. Transfection of the Neu3 gene into cancer cells leads to protection against apoptosis by increased Bcl-2 expression and decreased activity of caspase-3/-9 (9). Furthermore, Neu3 overexpression increases cell motility and invasion by modulation of EGF receptor phosphorylation and Ras activation (10, 11). In contrast to the apparent Neu3 promotion in cancer progressions, other sialidases play roles in cancer reduction through accelerated cell apoptosis, differentiation, and suppression of cell invasion (12). In other aspects, deficiency of the lysosomal sialidase (Neu1) is considered a major cause of sialidosis, an inherited lysosomal storage disease resulting in excessive accumulation of sialylglycoconjugates and development of progressive neurosomatic manifestations (13).
Activity-based protein profiling (ABPP) is a functional proteomic technology that uses chemical probes for specific enzymes (14). An ABPP probe typically is composed of two elements: a reactive group and a tag. The reactive group is designed based on the catalytic mechanism of the target enzyme, and it usually contains an electrophile that can react with a nucleophilic residue in the enzyme active site to form a covalent adduct. The tag may be either a reporter, such as a fluorophore, or an affinity label, such as biotin. The tag may incorporate a moiety, such as an alkyne or azide, for subsequent modification, such as by the Cu(I)-catalyzed azide–alkyne [3+2] cycloaddition (CuAAC), to introduce a reporter (15, 16). ABPP probes have been developed to monitor changes of specific enzymes associated with certain biological states, including serine hydrolases (17, 18), cysteine proteases (19–22), protein phosphatases (23–25), oxidoreductases (26), histone deacetylases (27), kinases (28, 29), metalloproteases (30–32), and glycosidases (33–36).
Two types of sialidase ABPP probes, the quinone methide and the photoaffinity labeling probes, have been reported. These probes often have problems in nonspecific labeling when used in complex protein samples, such as cell lysates (37, 38). In addition, these probes cannot be applied to in situ labeling experiments because they are impermeable to cell membranes. For sialidase profiling under physiological conditions, preparation of a target-specific and cell-permeable ABPP probe is needed to study sialidase changes in living cells.
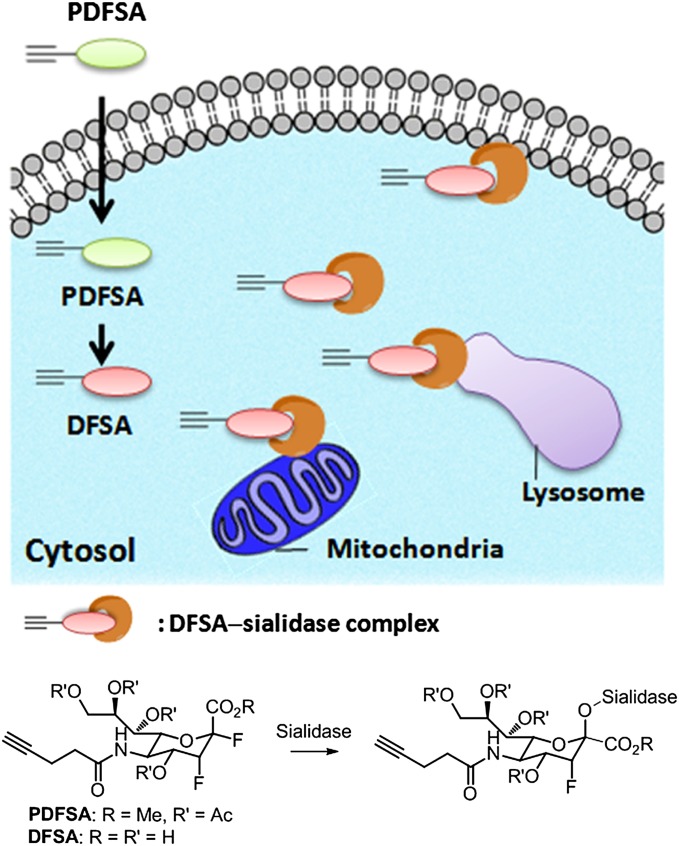
Introducing a fluorine at C-3 of sialic acid has been reported to antagonize sialic acid biosynthesis or modify sialylation (39–47). Because of the strong electron-withdrawing nature, the fluorine can destabilize the formation of positive charge within the carbohydrate ring to inhibit the catalytic activity of sialidases. 3-Fluorosialyl fluoride was used as an effective inhibitor against sialidase (40) or Trypanosoma cruzi transsialidase (TcTs) (46, 47), and these works have prompted us to design an ABPP probe for sialidases by using an alkyne-hinged 3-fluorosialyl fluoride (DFSA) that is expected to form a covalent adduct with sialidases (Fig. 1). In this study, DFSA is shown to be a mechanism-based irreversible inhibitor by trapping the 3-fluorosialyl–enzyme intermediate, which can be ligated with an azide-annexed biotin (azido-biotin) via CuAAC for isolation and identification of sialidases. We also developed an ester-protected DFSA (PDFSA) as the cell-permeable precursor of DFSA to allow cell uptake (48, 49), identification, and in situ imaging of sialidase activities under physiological conditions.
Fig. 1.
Identification and imaging of sialidase with activity changes using these activity-based sialidase probes.
Results
Synthesis of PDFSA and DFSA.
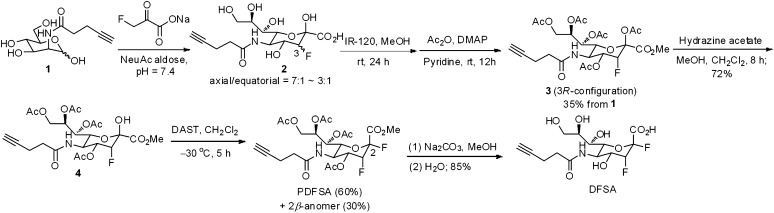
As shown in Scheme 1, the reaction of N-(pent-4-ynoyl)-mannosamine (1) with 3-fluoropyruvic acid (as the sodium salt) was carried out under the catalysis of N-acetylneuraminic acid aldolase (Neu5Ac aldolase, EC 4.1.3.3) to yield adduct 2 as a mixture of C-3 diastereomers (axial/equatorial = 7:1 ∼3:1). The adduct was subjected to esterification, acetylation, and chromatographic isolation to afford ester 3 having the 3R configuration. Selective deacetylation at the anomeric position was achieved by using hydrazine acetate to give 4, which was treated with diethylaminosulfur trifluoride to give PDFSA (α-anomer, 60%) and its β-anomer (30%). Final deprotection of PDFSA under alkaline conditions produced DFSA in 85% yield after purification on a reverse-phase column.
Scheme 1.
Synthesis of PDFSA and DFSA.
DFSA Labeling of Sialidases and Characterization.
To examine the feasibility of DFSA as an activity-based probe, we evaluated the inhibition of various sialidases by DFSA, 3-fluorosialyl fluoride, 2-deoxy-2,3-didehydro-N- acetylneuraminic acid (DANA), and PDFSA using 2-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) as the substrate. The sialidases used in this study are from a variety of species, including influenza virus (NA), bacteria (nanA, nanB, nanC, nanJ, nanI, and nanH), and human (Neu1, Neu2, Neu3, and Neu4) sialidases. Similar to DANA, all sialidases were sensitive to both DFSA and 3-fluorosialyl fluoride with IC50 values at micro- to submicromolar levels (Table S1). In comparison with 3-fluorosialyl fluoride, DFSA slightly attenuated the inhibition for most sialidases. However, enhanced inhibitory effects were observed in Neu1, nanB, and nanC, probably as a result of certain subtle structural differences. The ability to inhibit all these sialidases suggested that DFSA might be a potent activity-based probe for these enzymes. In contrast to the sensitive inhibition by DFSA, the ester-protected analog PDFSA did not inhibit these sialidases, suggesting that esterification of the hydroxy and carboxy groups in PDFSA prevents binding to the sialidase active sites.
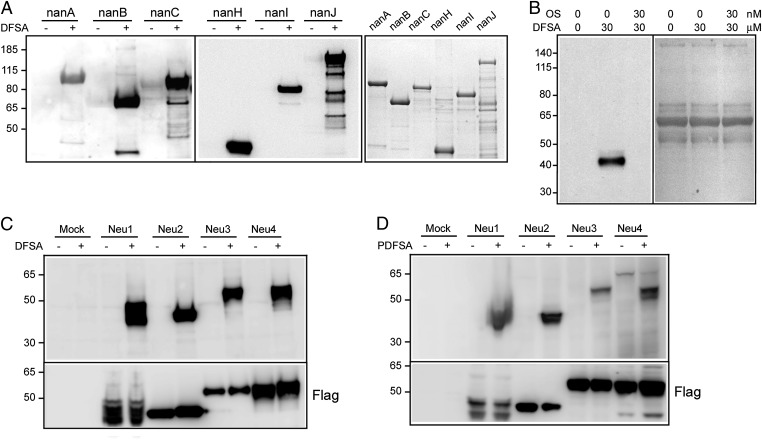
To validate the sialidase labeling by DFSA, we examined the formation of the fluorosialyl–enzyme adducts by SDS/PAGE analyses. All the bacterial sialidases tested in this study formed an adduct that was captured by azido-biotin and detected by the streptavidin-specific reporting signal (Fig. 2A). The influenza NA located at the surface of influenza virus (A/WSN/1933/H1N1) also formed a DFSA adduct that could be outcompeted by the NA inhibitor oseltamivir acid (OS), suggesting that DFSA interacted with NA at the active site (Fig. 2B). To detect the specific DFSA labeling in cells, we overexpressed four human sialidases, Neu1–4 in 293T cells. It has been reported that mature Neu1 and Neu4 proteins can be processed further at N-termini (50–53). To ensure positive anti-FLAG staining, we constructed the expression plasmids with the sequences encoding FLAG tags at both 5′- and 3′-ends of Neu1 and Neu4 genes. Fig. 2C shows that DFSA positively labels all the human sialidases in the protein extracts of transfected 293T cells. We also found that the DFSA labeling of human sialidases was pH sensitive: Neu1 and Neu3 were poorly labeled at neutral to alkaline pH (Fig. S1).
Fig. 2.
Identification of sialidases by DFSA adduct formation. (A) Recombinant sialidases produced in Escherichia coli were treated briefly with DFSA, separated in SDS/PAGE, and transferred to PVDF membranes (Left and Center) that were reacted with the click reaction reagent azido-biotin to ligate the biotin moiety to the alkyne group of the enzyme conjugate. The biotin-modified sialidases present in the washed membrane were detected through the streptavidin-conjugated HRP reporting system. These sialidase adducts also were shown by Coomassie blue staining (Right). (B) Detection of influenza NA was conducted after incubating influenza virus (A/WSN/1933/H1N1) samples with DFSA with or without addition of the specific inhibitor OS to compete with DFSA for binding to the active site (Left). These total lysates also were shown by Coomassie blue staining (Right). (C) Human sialidase samples present in the lysates of 293T transfected or untransfected cells (Mock) were treated with or without DFSA before SDS/PAGE analyses. The sialidases also were detected by immunoblot analyses of the FLAG epitope presented in Neu1, Neu2, Neu3, and Neu4. (D) Labeling of human sialidases also was conducted by incubating PDFSA with sialidase-expressing 293T cells and processed for adduct detection similarly. The sialidases also were detected by immunoblot analyses of the FLAG epitope presented in Neu1, Neu2, Neu3, and Neu4.
To identify DFSA-labeled sites on sialidase, purified sialidases were used for labeling with DFSA and digested with trypsin for liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The identified peptides modified by 3-fluorosialyl moiety are shown in Fig. S2 and Table S2. Except for nanB, there was only one tyrosine residue covalently modified by 3-fluorosialyl moiety in each sialidase. NanB was labeled at an additional adjacent tyrosine on the same peptide. Based on the protein structures of nanA, nanB, and nanI (54–56), it was revealed that DFSA labeled the tyrosine residue located in the activity sites of these sialidases. By sequence alignment, the orthologous tyrosine residue in the catalytic center also was labeled by DFSA in nanC, nanJ, and nanH (Table S3). It was strongly suggested that DFSA specifically labeled the catalytic tyrosine residue in sialidases.
In Situ Labeling of Intracellular Sialidases by PDFSA Treatment of 293T Cells Overexpressing Sialidases.
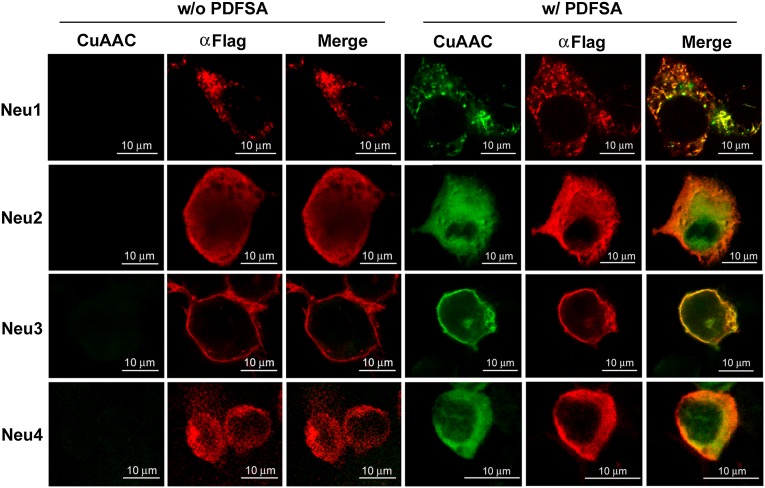
For the profiling of intracellular sialidases, the probe needs to be cell permeable, but DFSA is poorly permeable to cells. To enhance the cellular uptake, we used the ester-protected probe, PDFSA, to test the labeling of intracellular sialidases overexpressed in 293T cells. In comparison with the sialidase labeling of cell extracts with DFSA (Fig. 2C), we observed similar results of PDFSA labeling after incubation of live cells overexpressed with FLAG-tagged Neu1, Neu2, Neu3, or Neu4 (Fig. 2D). The success in sialidase labeling using PDFSA prompted us to determine the cellular localizations of the expressed sialidase activities in live cells with PDFSA and to examine the cellular location of the sialidase adducts in fixed and permeated cells (Fig. 3). The sialidase activities were detected as green signals through the PDFSA-mediated sialidase labeling; the sialidase proteins also were detected as red signals by staining with anti-FLAG antibody. Consistent with previous reports (5), Fig. 3 shows that the sialidase signals are located in lysosomes for Neu1, cytosol for Neu2, and plasma membrane for Neu3. The cytosolic location of Neu4, as detected by both PDFSA labeling and anti-FLAG staining, was different from the lysosomal location reported previously (51–53), which may be a result of the addition of FLAG tags. Here, the analyses of sialidase activity by PDFSA and anti-FLAG staining showed very high colocalization ratios in all the overexpressed human sialidases, suggesting specific PDFSA labeling of the sialidases in live cells.
Fig. 3.
Imaging analyses of sialidase-expressing 293T cells labeled by PDFSA. Live sialidase-expressing 293T cells were treated with PDFSA at 0.2 mM for 15 h. Cells were fixed, permeated, and biotin tagged for confocal microscopic analyses. PDFSA-mediated sialidase labeling is shown in green, and FLAG labeling is shown in red. (Scale bars: 10 mm.)
Profiling of Sialidase Changes Using the DFSA/PDFSA Labeling System.
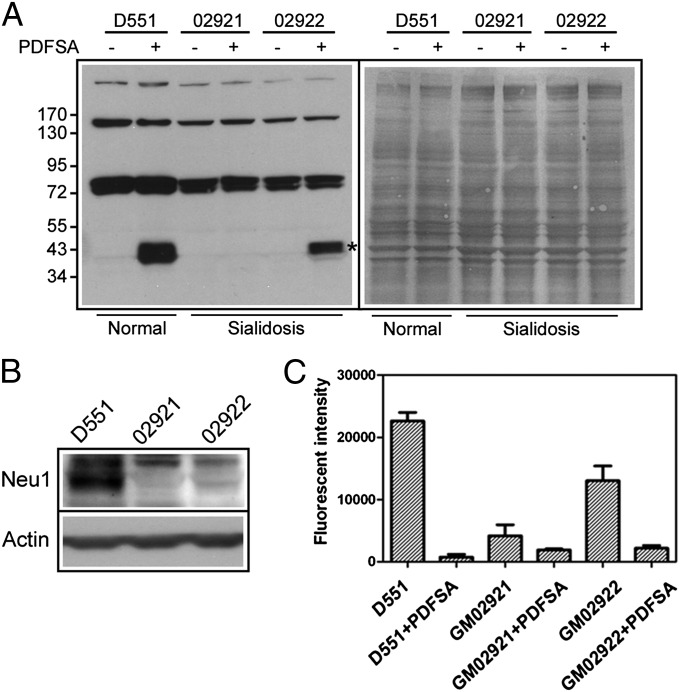
Sialidosis is an inherited lysosomal storage disease usually caused by Neu1 deficiency. We examined Neu1 activity differences in the fibroblasts of a normal person and sialidosis patients by live cell labeling using PDFSA. We found that the sialidase labeling was significantly reduced in the more severe sialidosis (GM02921) fibroblast cells compared with the milder sialidosis (GM02922) cells (Fig. 4A) (57). By blotting with anti-Neu1 antibody (Fig. 4B), results showed a correlation between the expression level of Neu1 and PDFSA-mediated sialidase labeling (Fig. 4A). The differences in sialidase activity observed by PDFSA labeling are consistent with the conventional activity measurement of the cell extracts using MUNANA as the substrate (Fig. 4C). In contrast, no sialidase activities were found in cells treated with PDFSA, suggesting that the intracellular sialidases of treated cells were effectively modified by the adduct formation.
Fig. 4.
Profiling of sialidase changes in the fibroblasts of sialidosis patients. (A) Fibroblast cells derived from normal (D551) or sialidosis patients (GM02921 and GM02922) were cultured for in situ sialidase labeling with PDFSA (10 μM). The relevant sialidase labeling signals are marked with stars (Left). These total lysates also were shown by DB71 staining (Right). (B) Fibroblast cells derived from normal (D551) or sialidosis patients (GM02921 and GM02922) were analyzed by anti-Neu1 antibody. (C) Cellular sialidase activities were measured using MUNANA as the substrate and compared with the sialidase activities in extracts of cells cultured with or without prior incubation with PDFSA. Values are means ± SEM of three independent experiments.
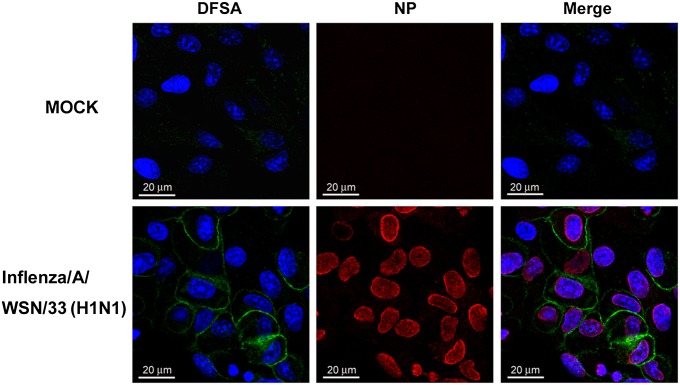
DFSA also successfully detected the NA expressed on influenza virus–infected cells by microscopy (Fig. 5). Furthermore, DFSA was shown to bind at the active site of influenza NA, and the binding can be competitively inhibited by OS (Fig. S3). We expect that OS can inhibit the DFSA labeling to OS-sensitive (OSs) viruses competitively because both compounds bind the active site of NA. However, for OS-resistant (OSr) influenza viruses that have been the prevailing clinical isolates for H1N1 since 2008 (58, 59), OS cannot effectively bind the active site of the mutant NA and should not inhibit the DFSA binding to influenza. Indeed, both OSs and OSr H1N1 influenza viruses were labeled by DFSA in the absence of OS, but only the OSr virus was detectable by DFSA labeling in the presence of OS, suggesting the possibility of using a DFSA probe to detect drug-resistant influenza strains.
Fig. 5.
Visualization of influenza-infected cells using DFSA labeling. Fluorescence image of influenza-infected cells that were treated with 30 μM DFSA, biotin tagged, and stained with FITC-tagged streptavidin. The influenza NA is shown in green, and influenza nucleoprotein (NP) is shown in red after anti-NP monoclonal antibody staining. Cell nuclei are shown in blue by 4′6-diamidino-2-phenylindole (DAPI) staining. MOCK, noninfected cells. (Scale bars: 20 μm.)
Discussion
We have designed an activity-based sialidase probe, DFSA, by using 3-fluorosialyl fluoride as the mechanism-based inhibitor and by incorporating an alkyne group for reporter ligation. DFSA is shown to be an active-site inactivator of all tested sialidases. Biochemical analyses of the DFSA-inactivated sialidases by LC-MS/MS analysis showed that tyrosine residues in the enzyme active site were specifically labeled by DFSA. Our study also demonstrates that the DFSA probe may be used not only for the detection of influenza infections but also for diagnosis of oseltamivir susceptibility. The ability of DFSA to label sialidases from viral, bacterial, and human enzymes suggests that DFSA may be used as a general sialidase probe for various applications.
DFSA is advantageous as a general ABPP probe because of its small size. We further introduce the ester-protected PDFSA to enhance cell-permeable properties and allow the profiling of intracellular sialidases. The ability of PDFSA to probe intracellular sialidases using living cells has an added advantage over the methods using cell lysates containing vulnerable sialidases. The sialidase adducts formed by live cell labeling using PDFSA record the status of sialidase activity under physiological conditions. After the enzyme adducts are formed in live cells, analysis of the sialidase adducts may be applied even in harsh conditions. We also have illustrated the use of these probes to study sialidase changes involved in different biological systems. Because sialidase is known to be involved in various diseases, these probes may be used to study cellular localization changes of sialidases and the differences in sialidase expression in normal and disease states.
Materials and Methods
Membrane Click Reaction.
The PVDF membranes were blocked with blocking buffer, 5% (wt/vol) BSA/Phosphate Buffered saline with Tween 20 (PBST) [0.1% (vol/vol) Tween 20/PBS]. The membranes were washed with PBS for 5 min two times. The protein side of the PVDF membrane was faced down to immerse in click reaction mixture [25 μM azido-biotin (3-azidopropanyl biotin), 0.1 mM Tris-triazoleamine catalyst (60), 1 mM CuSO4, and 2 mM sodium ascorbate, with 1 mL for a blot of mini-gel size] and incubated at room temperature for 1 h. After washing with PBST twice, the membrane was probed with peroxidase-conjugated streptavidin for biotin labels on blots.
In Situ Labeling of Sialidase Expressing Cells with PDFSA.
Sialidase transfectant 293T cells (obtained from ATCC) and normal (D551, obtained from Bioresource Collection and Research Center, Taiwan) and sialidosis fibroblasts (GM02921 and GM02922, obtained from Coriell Cell Repositories) were incubated with PDFSA (10 μM) at 37 °C for 24 h. Cells were lysed by NuPAGE LDS Sample Buffer (Invitrogen, 80 mM DTT) and then heated at 90 °C for 15 min. For each sample, 20 μg total lysate was loaded and separated on 4–12% NuPAGE (Invitrogen). After transferring proteins onto the PVDF membrane (Millipore), membrane click reaction was performed and labeling signal was analyzed by chemiluminescence detector.
For confocal microscopic analysis, sialidase transfectant 293T cells were seeded onto four-well chamber slices (3 × 105/mL per well), and were cultivated in penicillin/ streptomycin-containing 10% FBS/DMEM. Growth medium was supplemented with PDSFA (0.2 mM) and cultured for 15 h. Cells were fixed with 4% paraformaldehyde for 15 min, permeabilized in 0.5% Triton X-100 for 10 min at room temperature, and subjected to the probe labeling reaction consisting of 0.1 mM azide-biotin probe/0.1 mM Tris-triazole ligand/1 mM CuSO4/2 mM sodium ascorbate, in PBS, at room temperature for 1 h. Subsequently, the fixed and labeled cells were rinsed with PBS and stained with DyLight 488–conjugated streptavidin (2.5 μg/mL in 0.5% BSA/PBS) at room temperature for 30 min. Recombinant sialidases were detected by Alexa Fluor 594–conjugated anti-FLAG antibody (5 μg/mL in 0.5% BSA/PBS). Fluorescent images were captured by Leica TCS-SP5-MP-SMD. All the cell lines were obtained with informed consent and approval of institutional review board of Academia Sinica.
Supplementary Material
Acknowledgments
We thank Mr. Chein-Hung Chen for technical support on MS analysis and Ms. Li-Wen Lo for technical support on confocal microscopic analysis. We also thank Academia Sinica for financial support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222183110/-/DCSupplemental.
References
- 1.Saito MW, Yu RK. Biochemistry and Function of Sialidases. New York: Plenum; 1995. pp. 261–313. [Google Scholar]
- 2.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt 9):2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 3.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem Rev. 2002;102(2):439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446(7139):1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 5.Miyagi T, Yamaguchi K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology. 2012;22(7):880–896. doi: 10.1093/glycob/cws057. [DOI] [PubMed] [Google Scholar]
- 6.Monti E, et al. Sialidases in vertebrates: A family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403–479. doi: 10.1016/S0065-2318(10)64007-3. [DOI] [PubMed] [Google Scholar]
- 7.Schengrund CL, Jensen DS, Rosenberg A. Localization of sialidase in the plasma membrane of rat liver cells. J Biol Chem. 1972;247(9):2742–2746. [PubMed] [Google Scholar]
- 8.Bosmann HB, Hall TC. Enzyme activity in invasive tumors of human breast and colon. Proc Natl Acad Sci USA. 1974;71(5):1833–1837. doi: 10.1073/pnas.71.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyagi T. Aberrant expression of sialidase and cancer progression. Proc Jpn Acad, Ser B, Phys Biol Sci. 2008;84(10):407–418. doi: 10.2183/pjab/84.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada T, et al. A crucial role of plasma membrane-associated sialidase in the survival of human cancer cells. Oncogene. 2007;26(17):2483–2490. doi: 10.1038/sj.onc.1210341. [DOI] [PubMed] [Google Scholar]
- 11.Miyagi T, Wada T, Yamaguchi K, Hata K, Shiozaki K. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J Biochem. 2008;144(3):279–285. doi: 10.1093/jb/mvn089. [DOI] [PubMed] [Google Scholar]
- 12.Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: A minireview. Glycoconj J. 2004;20(3):189–198. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- 13.Thomas GH. Disorders of glycoprotein degradation: α-mannosidosis, β-mannosidosis, fucosidosis, and sialidosis. In: Valle D, et al., editors. Scriver’s Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. Available at http://dx.doi.org/10.1036/ommbid.170. [Google Scholar]
- 14.Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006;106(8):3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 15.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8(24):1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: The serine hydrolases. Proc Natl Acad Sci USA. 1999;96(26):14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidd D, Liu Y, Cravatt BF. Profiling serine hydrolase activities in complex proteomes. Biochemistry. 2001;40(13):4005–4015. doi: 10.1021/bi002579j. [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum D, et al. Chemical approaches for functionally probing the proteome. Mol Cell Proteomics. 2002;1(1):60–68. doi: 10.1074/mcp.t100003-mcp200. [DOI] [PubMed] [Google Scholar]
- 20.Kato D, et al. Activity-based probes that target diverse cysteine protease families. Nat Chem Biol. 2005;1(1):33–38. doi: 10.1038/nchembio707. [DOI] [PubMed] [Google Scholar]
- 21.Yuan F, Verhelst SH, Blum G, Coussens LM, Bogyo M. A selective activity-based probe for the papain family cysteine protease dipeptidyl peptidase I/cathepsin C. J Am Chem Soc. 2006;128(17):5616–5617. doi: 10.1021/ja060835v. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Mahesh U, Chen GYJ, Yao SQ. Solid-phase synthesis of peptide vinyl sulfones as potential inhibitors and activity-based probes of cysteine proteases. Org Lett. 2003;5(5):737–740. doi: 10.1021/ol0275567. [DOI] [PubMed] [Google Scholar]
- 23.Walls C, Zhou B, Zhang ZY. Activity-based protein profiling of protein tyrosine phosphatases. Methods Mol Biol. 2009;519:417–429. doi: 10.1007/978-1-59745-281-6_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalesh KA, et al. Peptide-based activity-based probes (ABPs) for target-specific profiling of protein tyrosine phosphatases (PTPs) Chem Commun (Camb) 2010;46(4):589–591. doi: 10.1039/b919744c. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy D, Barrios AM. Profiling protein tyrosine phosphatase activity with mechanistic probes. Curr Opin Chem Biol. 2009;13(4):375–381. doi: 10.1016/j.cbpa.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Adam GC, Sorensen EJ, Cravatt BF. Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat Biotechnol. 2002;20(8):805–809. doi: 10.1038/nbt714. [DOI] [PubMed] [Google Scholar]
- 27.Salisbury CM, Cravatt BF. Activity-based probes for proteomic profiling of histone deacetylase complexes. Proc Natl Acad Sci USA. 2007;104(4):1171–1176. doi: 10.1073/pnas.0608659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patricelli MP, et al. Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry. 2007;46(2):350–358. doi: 10.1021/bi062142x. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Zhang CJ, Chen GY, Yao SQ. Cell-based proteome profiling of potential dasatinib targets by use of affinity-based probes. J Am Chem Soc. 2012;134(6):3001–3014. doi: 10.1021/ja208518u. [DOI] [PubMed] [Google Scholar]
- 30.Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt BF. Activity-based probes for the proteomic profiling of metalloproteases. Proc Natl Acad Sci USA. 2004;101(27):10000–10005. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan EWS, Chattopadhaya S, Panicker RC, Huang X, Yao SQ. Developing photoactive affinity probes for proteomic profiling: Hydroxamate-based probes for metalloproteases. J Am Chem Soc. 2004;126(44):14435–14446. doi: 10.1021/ja047044i. [DOI] [PubMed] [Google Scholar]
- 32.Sieber SA, Niessen S, Hoover HS, Cravatt BF. Proteomic profiling of metalloprotease activities with cocktails of active-site probes. Nat Chem Biol. 2006;2(5):274–281. doi: 10.1038/nchembio781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CS, Li YK, Lo LC. Design and synthesis of activity probes for glycosidases. Org Lett. 2002;4(21):3607–3610. doi: 10.1021/ol0265315. [DOI] [PubMed] [Google Scholar]
- 34.Vocadlo DJ, Bertozzi CR. A strategy for functional proteomic analysis of glycosidase activity from cell lysates. Angew Chem Int Ed Engl. 2004;43(40):5338–5342. doi: 10.1002/anie.200454235. [DOI] [PubMed] [Google Scholar]
- 35.Stubbs KA, et al. Synthesis and use of mechanism-based protein-profiling probes for retaining beta-D-glucosaminidases facilitate identification of Pseudomonas aeruginosa NagZ. J Am Chem Soc. 2008;130(1):327–335. doi: 10.1021/ja0763605. [DOI] [PubMed] [Google Scholar]
- 36.Witte MD, et al. Ultrasensitive in situ visualization of active glucocerebrosidase molecules. Nat Chem Biol. 2010;6(12):907–913. doi: 10.1038/nchembio.466. [DOI] [PubMed] [Google Scholar]
- 37.van der Horst GT, Mancini GM, Brossmer R, Rose U, Verheijen FW. Photoaffinity labeling of a bacterial sialidase with an aryl azide derivative of sialic acid. J Biol Chem. 1990;265(19):10801–10804. [PubMed] [Google Scholar]
- 38.Lu CP, et al. Design of a mechanism-based probe for neuraminidase to capture influenza viruses. Angew Chem Int Ed Engl. 2005;44(42):6888–6892. doi: 10.1002/anie.200501738. [DOI] [PubMed] [Google Scholar]
- 39.Gantt R, Millner S, Binkley SB. Inhibition of N-acetylneuraminic acid aldolase by 3-fluorosialic acid. Biochemistry. 1964;3(12):1952–1960. doi: 10.1021/bi00900a029. [DOI] [PubMed] [Google Scholar]
- 40.Ishiwata K, et al. Tumor uptake study of 18F-labeled N-acetylneuraminic acids. Int J Rad Appl Instrum B. 1990;17(4):363–367. doi: 10.1016/0883-2897(90)90102-7. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara T, Kijima-Suda I, Ido T, Ohrui H, Tomita K. Inhibition of bacterial and viral sialidases by 3-fluoro-N-acetylneuraminic acid. Carbohydr Res. 1994;263(1):167–172. doi: 10.1016/0008-6215(94)00133-2. [DOI] [PubMed] [Google Scholar]
- 42.Burkart MD, Vincent SP, Wong CH. An efficient synthesis of CMP-3-fluoroneuraminic acid. Chem Commun. 1999;(16):1525–1526. [Google Scholar]
- 43.Burkart MD, et al. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: Useful mechanistic probes for glycosyltransferases. Bioorg Med Chem. 2000;8(8):1937–1946. doi: 10.1016/s0968-0896(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 44.Sun XL, et al. Syntheses of C-3-modified sialylglycosides as selective inhibitors of influenza hemagglutinin and neuraminidase. Eur J Org Chem. 2000;2000(14):2643–2653. [Google Scholar]
- 45.Guo CT, et al. An O-glycoside of sialic acid derivative that inhibits both hemagglutinin and sialidase activities of influenza viruses. Glycobiology. 2002;12(3):183–190. doi: 10.1093/glycob/12.3.183. [DOI] [PubMed] [Google Scholar]
- 46.Watts AG, et al. Trypanosoma cruzi trans-sialidase operates through a covalent sialyl-enzyme intermediate: Tyrosine is the catalytic nucleophile. J Am Chem Soc. 2003;125(25):7532–7533. doi: 10.1021/ja0344967. [DOI] [PubMed] [Google Scholar]
- 47.Buchini S, Buschiazzo A, Withers SG. A new generation of specific Trypanosoma cruzi trans-sialidase inhibitors. Angew Chem Int Ed Engl. 2008;47(14):2700–2703. doi: 10.1002/anie.200705435. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar AK, Fritz TA, Taylor WH, Esko JD. Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Gal beta 1—>4GlcNAc beta-O-naphthalenemethanol. Proc Natl Acad Sci USA. 1995;92(8):3323–3327. doi: 10.1073/pnas.92.8.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs CL, et al. Metabolic labeling of glycoproteins with chemical tags through unnatural sialic acid biosynthesis. Methods Enzymol. 2000;327:260–275. doi: 10.1016/s0076-6879(00)27282-0. [DOI] [PubMed] [Google Scholar]
- 50.Vinogradova MV, et al. Molecular mechanism of lysosomal sialidase deficiency in galactosialidosis involves its rapid degradation. Biochem J. 1998;330(Pt 2):641–650. doi: 10.1042/bj3300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seyrantepe V, et al. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J Biol Chem. 2004;279(35):37021–37029. doi: 10.1074/jbc.M404531200. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi K, et al. Evidence for mitochondrial localization of a novel human sialidase (NEU4) Biochem J. 2005;390(Pt 1):85–93. doi: 10.1042/BJ20050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bigi A, et al. Human sialidase NEU4 long and short are extrinsic proteins bound to outer mitochondrial membrane and the endoplasmic reticulum, respectively. Glycobiology. 2010;20(2):148–157. doi: 10.1093/glycob/cwp156. [DOI] [PubMed] [Google Scholar]
- 54.Hsiao YS, Parker D, Ratner AJ, Prince A, Tong L. Crystal structures of respiratory pathogen neuraminidases. Biochem Biophys Res Commun. 2009;380(3):467–471. doi: 10.1016/j.bbrc.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gut H, King SJ, Walsh MA. Structural and functional studies of Streptococcus pneumoniae neuraminidase B: An intramolecular trans-sialidase. FEBS Lett. 2008;582(23-24):3348–3352. doi: 10.1016/j.febslet.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 56.Newstead SL, et al. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J Biol Chem. 2008;283(14):9080–9088. doi: 10.1074/jbc.M710247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pshezhetsky AV, Potier M. Association of N-acetylgalactosamine-6-sulfate sulfatase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J Biol Chem. 1996;271(45):28359–28365. doi: 10.1074/jbc.271.45.28359. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (CDC) Influenza activity—United States and worldwide, 2007-08 season. MMWR Morb Mortal Wkly Rep. 2008;57(25):692–697. [PubMed] [Google Scholar]
- 59.Sheu TG, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008;52(9):3284–3292. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via 3(n,pi)-1(pi,pi) inversion. J Am Chem Soc. 2004;126(29):8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.