Abstract
Objective
Epidermal growth factor receptor (EGFR) and p16 [a surrogate marker of human papillomavirus (HPV) infection] expression are strong prognostic factors in patients with head and neck squamous cell carcinoma (HNSCC).
Study Design
We examined expression levels of total and nuclear EGFR as well as p16 status based on evidence that nuclear EGFR may have a role in DNA damage repair.
Methods
A HPV-negative (SQ20B) and a HPV-positive (UMSCC47) HNSCC cell line were examined for EGFR and γH2AX expression. A tissue microarray (TMA) containing 123 cores obtained from 101 HNSCC tumors was analyzed for EGFR expression by automated quantitative analysis and p16 expression by immunohistochemical staining (IHC) and these results were correlated with available clinical data.
Results
SQ20B had higher EGFR expression than UMSCC47. Nuclear localization of EGFR upon activation with transforming growth factor-alpha was observed in SQ20B, but not in UMSCC47. SQ20B also had increased γH2AX foci compared to UMSCC47 suggesting that SQ20B has more DNA damage compared to UMSCC47. Total and nuclear EGFR was reliably obtained from 80 of 101 patients. p16 levels were determined in 87 of 101 patients. p16 levels were strongly associated with the oropharyngeal subsite and poorly differentiated histology. Expression of total and nuclear EGFR was higher in p16-negative tumors compared to p16-positive tumors (Wilcoxon Rank Test, p=0.038 and p=0.014, respectively).
Conclusions
Further studies are required to determine a mechanistic link between these two prognostic factors, and the significance of EGFR localization to nucleus has in DNA damage repair upon pathway activation.
Keywords: HNSCC, nuclear EGFR, p16
Introduction
Approximately 50,000 new cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed each year in the United States1, and there is an urgent need for understanding therapeutic resistance to the current standard of care. Identifying predictive markers for specific targeted therapies will identify patients for appropriate treatment regimens, and may lead to the identification of novel therapeutic targets. While high expression of epidermal growth factor receptor (EGFR) is associated with poor clinical outcomes2, the presence of human papillomavirus (HPV) infection and/or p16 expression (a surrogate marker of HPV infection) is associated with favorable outcomes.3 Thus, development and utilization of additional biomarkers could allow for deintensification for HPV-positive cancers currently associated with good outcomes. The identification of key molecular pathways that distinguish HPV-positive from HPV-negative HNSCC could shed additional light on the pathogenesis of, and possible paths to targeted therapies for these tumors.
It has been suggested that EGFR and HPV are independent prognostic factors, and that a subgroup of HPV-positive patients with high EGFR expression have worse outcomes compared to HPV-positive patients with low EGFR expression in HNSCC.4 The role of EGFR in proliferative, anti-apoptotic and metastatic signal transduction has been extensively studied.5,6 However, additional data suggesting the unique subcellular roles of EGFR are now emerging.7,8 High levels of nuclear EGFR are detected in many tumors, including adrenocorticoid, breast, bladder, skin, thyroid, glioma, and oropharynx tumors.9–12 While localized in the nucleus, EGFR functions as a transcriptional regulator by binding to promoters that enhance transcription of cyclin D1, inducible nitric oxide synthase (iNOS), B-Myb, cyclooxygenase-2 (COX-2), and aurora A.13–16 Nuclear EGFR also regulates DNA damage repair where it functions as a kinase to phosphorylate and stabilize PCNA, thus increasing the stability of chromatin. Nuclear EGFR has also been shown to interact with DNA-PK and convey resistance to ionizing irradiation and cisplatin.17,18 These postulated functions may have clinical importance by conferring chemoradiation resistance.
Independent of EGFR expression, p16 is an important prognostic marker in HNSCC. p16 is a cyclin-dependent kinase inhibitor that inhibits pRb phosphorylation and blocks cell cycle progression at the G1 to S checkpoint. Loss of p16 expression by deletion, mutation, or hypermethylation is common in HNSCC.19 Patients with transcriptionally active HPV infection express high levels of p16 due to E7-induced inactivation of pRb. It is currently unclear whether p16 has a direct role in the favorable outcome of HPV-positive tumors or whether it is simply a surrogate marker of HPV infection.20 Patients with HPV-positive and/or p16-positive tumors have more favorable outcomes than patients with HPV-negative cancer.3,21,22 These patients are younger, and have less tobacco exposure, better performance status, and smaller primary tumors compared to those with HPV-negative tumors.3 A subset of HPV-positive HNSCC patients with more extensive smoking histories, TP53 mutations and/or high expression of EGFR has worse outcome compared to patients without these factors suggesting that the HPV status alone is not an adequate prognostic marker.3,4,23
To further examine the role of high EGFR expression, we determined the expression levels of EGFR, patterns of EGFR localization upon activation with transforming growth factor-alpha (TGF-α) and the expression of gamma-H2A histone family, member X (γH2AX) as a marker of DNA damage from double strand breaks in a HPV-positive and a HPV-negative HNSCC cell line. We also sought to determine a correlation between expression levels of total EGFR and p16 status to determine whether combining these biomarkers could identify a group of patients with similar prognoses. We further examined the association between nuclear EGFR and p16 status based on the evidence that nuclear EGFR may have a role in DNA damage repair in HNSCC. Identification of such an association may suggest a novel therapeutic strategy in patients with HNSCC who may benefit from molecularly targeted approaches by combining anti-EGFR therapies and DNA damage repair targeted agents.
Materials and Methods
EGFR and γH2AX staining of HNSCC cell lines
SQ20B and UMSCC47 were obtained from Dr. Ralph Weisselbaum at University of Chicago and Dr. Thomas Carey at University of Michigan. SQ20B was grown in DMEM/F12 media with 10% FBS and hydrocortisone (0.4 μg/ml). UMSCC47 was grown in DMEM with 10% FBS. Cells were grown in chamber slides overnight and fixed for staining the following day. Cells were stimulated with TGF-α (R&D Systems, Minneapolis, MN) 10 nM concentration and 30 minutes before the EGFR staining. For EGFR staining, cells were fixed with buffered formalin (10% formalin, 20 min at room temperature) and then permeabilized with PBS + 0.5% Triton X-100 for 20 minutes. For γH2AX staining, cells were fixed with methanol (10 minutes at −20°C). Slides were blocked with Odyssey blocking buffer (15 minutes, room temperature; L I-COR Biosciences, Lincoln NE), then incubated with primary antibodies [EGFR (Cell signaling, Beverly, MA), overnight at 4°C; γH2AX, (Millipore, Billerica, MA), 1 hour at 37°C according to manufacturer's instructions]. Slides were washed and incubated with blocking buffer for 15 minutes and then with secondary antibodies (Alexa Fluor 488 and 568; Invitrogen, Grand Island, NY) 1:2000 in Odyssey buffer for 1 hour at room temperature. Following wash, slides were incubated with 4',6-diamidino-2-phenylindole (DAPI; Roche Applied Science, Indianapolis, IN) in PBS for 10 minutes at room temperature. After DAPI was removed, slides were mounted in ProlonGold (Invitrogen, Grand Island, NY).
Patient materials and tissue microarray (TMA) construction
The TMA was assembled from patients with primary head and neck squamous cell carcinoma treated at Vanderbilt University Medical Center between 1997 and 2003. Following institutional review board approval, the TMA was constructed including 123 cores obtained from 101 HNSCC tumors. Tissue cores were obtained from paraffin-embedded formalin-fixed tissue blocks. Cores were placed on the recipient microarray block using a Tissue Microarrayer (Beecher Instrument, Silver Spring, MD).
Automated quantitative analysis (AQUA)
The TMA was deparaffinized with xylene followed by ethanol. Following rehydration in distilled water, antigen retrieval was accomplished by application of proteinase K for 30 min. Slides were incubated with primary antibody at 4°C overnight. Primary monoclonal antibody to EGFR (clone H11, DAKO, Carpinteria, CA) was used at a 1:50 dilution in 0.3% BSA in TBS. Slides were incubated with goat anti-mouse secondary antibody conjugated to a horseradish peroxidase-dextran polymer backbone (Envision; DAKO) for 1 hour at room temperature. Malignant cells were identified by use of anti-cytokeratin antibody cocktail (rabbit anti-pancytokeratin antibody z0622; DAKO) with subsequent goat anti-rabbit antibody conjugated to Alexa 546 fluorophore (A11035, Molecular Probes, Eugene, OR). 4',6-diamidino-2-phenylindole was added to visualize nuclei. Target (EGFR) molecules were visualized with a fluorescent chromogen (Cy-5 tyramide; Perkin Elmer Corp, Wellseley MA). Monochromatic, high resolution images were obtained from each histospot. Tumor area was isolated from stromal elements by creating a mask from the cytokeratin signal. The DAPI signal within the mask was then used to identify tumor nuclei. The EGFR signal (AQUA score) was scored on a normalized scale of 1 to 255 expressed as pixel intensity divided by target area (tumor mask or tumor nuclei). AQUA scores for duplicate tissues were averaged to obtain a mean AQUA score for each tumor.
Determination of p16 expression
p16 expression levels were determined by immunohistochemical (IHC) staining using p16 monoclonal antibody (clone E6H4; MTM Laboratories Inc., Westbrough, MA). The staining was scored by binary presence and absence of the staining as previously described.3
Statistical Analysis
EGFR and p16 data were correlated with clinical data including gender, TNM stage, histological grade, and primary subsite using Chi-square analysis and the Fisher exact test. 5-year overall survival and recurrence-free survival were also evaluated by Log-rank analyses.
Results
Immunofluorescent staining of EGFR and γH2AX in HNSCC cell lines
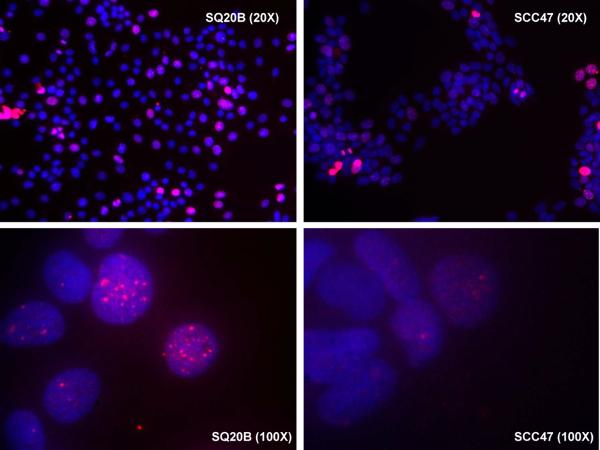
The expression levels of EGFR and γH2AX were determined in HPV-negative SQ20B and HPV-positive UMSCC47. SQ20B had higher EGFR expression compared to UMSCC47. In addition, SQ20B demonstrated a nuclear localization pattern of EGFR upon stimulation by one of its ligands, TGF-α, while EGFR staining remained associated with the cell membrane in UMSCC47 (Figure 1A). γH2AX staining, a marker for DNA double-strand breaks was also higher in SQ20B compared to UMSCC47 (Figure 1B) suggesting that SQ20B has more DNA damage compared to UMSCC47. This finding suggests that there may be differences in the amount of DNA damage and nuclear EGFR expression levels between HPV-positive and HPV-negative HNSCC.
Figure 1.
Immunofluorescent (IF) stainings of human papillomavirus (HPV)-negative head and neck squamous cell carcinoma (HNSCC) cell line, SQ20B, and HPV-positive HNSCC cell line, UMSCC47. A. the IF stainings of epithelial growth factor receptor (EGFR, green) and 4',6-diamidino-2-phenylindole (DAPI, blue) with or without transforming growth factor-alpha (TGF-α) stimulation, B. the IF staining of gamma-H2A histone family, member X (γH2AX, red) and DAPI (blue).
Patient characteristics and biomarker association analysis
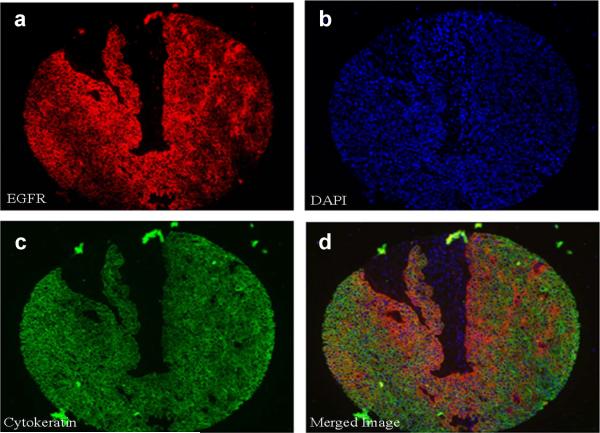
Detailed patient characteristics are summarized in Table 1. Total and nuclear EGFR data could be reliably obtained from 80 of 101 patients without the missing cores and representative staining images are shown in Figure 2A. p16 data could be obtained from 87 of 101 patients [p16 positive, n=29; p16 negative, n=58] and representative images are shown in Figure 2B. p16 staining was strongly associated with the oropharyngeal subsite (Chi2, p=<0.0001) and poorly differentiated histology (Cochran-Armitage Trend Test, p=0.01). No statistically significant differences were seen in p16 status among gender, race, smoking status, alcohol consumption, family history, or clinical stage.
Table 1.
Patient characteristics.
| p16 Negative | p16 Positive | ||
|---|---|---|---|
| Sex | N.S. | ||
|
| |||
| Male | 45 | 26 | |
|
| |||
| Female | 13 | 3 | |
|
| |||
| Race | N.S. | ||
|
| |||
| White | 49 | 26 | |
|
| |||
| Other | 9 | 3 | |
|
| |||
| Primary Site | |||
| p=<0.0001 | |||
|
| |||
| Hypopharynx | 1 | 3 | |
|
| |||
| Larynx | 16 | 4 | |
|
| |||
| Nasopharynx | 3 | 0 | |
|
| |||
| Oral Cavity | 24 | 2 | |
|
| |||
| Oropharynx | 14 | 20 | |
|
| |||
| Smoking | N.S. | ||
|
| |||
| Yes | 44 | 23 | |
|
| |||
| No | 10 | 6 | |
|
| |||
| Differentiation | |||
| p=0.01 | |||
|
| |||
| Well | 7 | 2 | |
|
| |||
| Poor | 36 | 16 | |
|
| |||
| Well-Mod | 6 | 3 | |
|
| |||
| Mod-Poor | 3 | 8 | |
|
| |||
| Stage | N.S. | ||
|
| |||
| T1.N0.M0; I | 8 | 3 | |
|
| |||
| T2, NO, MO; II | 11 | 3 | |
|
| |||
| T1–3, N1, M0; III | 14 | 6 | |
|
| |||
| T1–4, N1–3, M0–1; IV | 25 | 17 | |
Figure 2.

A. Representative images of fluorescent immunohistochemistry for automated analysis. a. Cy5 (red) was used to identify epidermal growth factor receptor (EGFR), b. 4',6-diamidino-2-phenylindole (DAPI, blue) was used to define the nuclear compartment, c. Cytokeratin-Cy3 (green) was used to identify tumor within each histospot, and d. A three-merged color image for each tumor was generated. B. Representative immunohistochemical staining for p16 protein expression. a. p16 positive staining, and b: p16 negative staining.
Analysis of association between EGFR cellular localization and p16 expression
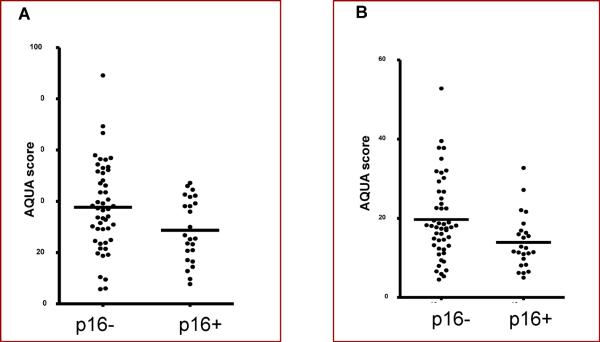
AQUA staining for subcellular EGFR localization has been documented in previous reports24, and a similar methodology was employed in this study. The expression of both total and nuclear EGFR was higher in p16-negative tumors compared to p16-positive tumors by AQUA staining. The AQUA score for total EGFR was 1994 for p16 negative vs. 707 for p16 positive (Wilcoxon Rank Test, p=0.038) and the AQUA score for nuclear EGFR was 2030 vs. 671 (p=0.014), respectively (Figure 3). Higher expression of non-nuclear EGFR (combined cytoplasmic and membranous) staining did not associate significantly with p16 negative tumors (1977 vs. 724, p=0.2), probably due to the small sample size and lack of statistical power.
Figure 3.
A scatter plot depicts nuclear AQUA expression among p16+ and p16-tumors. The p16 negative tumors have higher nuclear EGFR expression than tumors that are p16 positive. A. Total EGFR Expression as a function of p16 status, and B. Nuclear EGFR Expression as a function of p16 status.
Survival analysis based on EGFR and p16 expression
Statistically significant differences in recurrence-free survival (Wilcoxon Rank test p=0.97) or overall survival (Wilcoxon Rank test p=0.4) were not detectable based on EGFR and p16 expression, likely due to the small sample size of p16-positive tumors and heterogeneity in the patient population introduced by varying disease stages, limited smoking exposure history and treatments.
Discussion
Our data suggest an inverse relationship between EGFR expression and p16 status, and that subcellular localization of EGFR to the nucleus may be associated with absence of p16 expression. This observation may be important in understanding why p16-negative tumors with high EGFR expression are associated with resistance to cisplatin and radiation therapy, as they may employ unique DNA damage repair mechanisms via nuclear translocation of EGFR. This study may also help to establish a biologically homogenous population of patients based on the EGFR expression who may benefit from more tailored therapies targeting nuclear EGFR translocation or DNA damage repair pathway. Further studies are necessary to more clearly define the mechanistic role of nuclear EGFR in head and neck cancers.
We have found that an HPV-negative HNSCC cell line (SQ20B) has a greater degree of DNA damage compared to an HPV-positive HNSCC cell line (UMSCC47). In recent whole exome sequencing results of human HNSCC revealed that HPV-negative HNSCC had 5 times more number of mutations per tumor compared to HPV-positive HNSCC (an average number of 20.6 vs. 4.8 mutations, respectively).24 Also, tumors from patients with smoking history had twice the number of mutations per tumor compared to non-smokers (an average number of 21.6 vs. 9.5 mutations, respectively).24 These findings suggest that HPV-negative tumors mostly caused by chronic exposure to tobacco and subsequent DNA damage and genetic abnormalities such as mutations and loss of heterozygosity may have differences in DNA damage repair response compared to HPV-positive tumors. Further studies to understand the DNA damage repair mechanism between these two distinct tumors are necessary.
Nuclear EGFR has a distinct role in DNA damage repair, and this role may be important in conferring chemoradiation resistance in p16-negative tumors. In multivariate analysis, PCNA expression correlated with nuclear EGFR levels.25 Because PCNA has roles in both DNA damage repair and cell proliferation, it would be interesting to examine a potential regulation of PCNA expression through nuclear EGFR. Liccardi, et al. has shown that cells expressing EGFR with mutations that impair nuclear transport showed reduced repair of DNA strand breaks following ionizing radiation and reduced repair of intrastrand crosslinks following treatment with cisplatin.18 In cells with mutated nuclear localization signal sequences in EGFR, it was not associated with DNA dependent protein kinase (DNA-PKcs) which are a central component of the nonhomologous end-joining pathway involved in the repair of DNA strand breaks.18 Furthermore, cells expressing EGFR with decreased nuclear localization showed increased sensitivity to cisplatin.17,18 These data suggest that nuclear translocation of EGFR may play a role in conferring chemoradiation resistance and provides a biologic rationale that may explain why p16-negative tumors in HNSCC are less sensitive to DNA damaging chemotherapy and radiation compare to p16-positive tumors.
The effects of EGFR targeted therapy and other anti-cancer treatments on the extent of EGFR nuclear translocation are still unclear. Ionizing radiation induces EGFR nuclear transport, and this can be inhibited by cetuximab and celecoxib.26–29 In contrast, Laio and Carpenter showed that cetuximab is able to activate EGFR nuclear transport by promoting receptor endocytosis and activating receptor trafficking to the endoplasmic reticulum.30 Nuclear EGFR signal transduction pathway has been proposed as one of the mechanisms of cetuximab resistance.29 Both lapatinib and the Src family kinase inhibitor, dasatinib, block EGFR nuclear entry.30 These observations have provided rationale for selecting novel combination therapies that can overcome nuclear EGFR mediated therapeutic resistance, and clinical trials are currently underway to exploit these pathways. For example, combination of cetuximab and EGFR tyrosine kinase inhibitors might be an effective therapeutic strategy in HNSCC with high nuclear EGFR levels.
An understanding of the current DNA repair targeting pathways including poly-ADP-ribose polymerase (PARP), checkpoint control, and cyclin dependent kinases may also be important for explaining mechanisms of chemotherapy and radiation resistance. Reports have suggested that PARP inhibitors have had some efficacy in radiosensitization in HNSCC.31 The most common agents involved in inhibition of DNA repair are summarized in Table 3, and specific information about potential therapeutic inhibitors for those agents are listed. The higher expression of nuclear EGFR in p16-negative tumors may signify a particular subset of patients in which DNA repair targeting agents may be explored in clinical trials.
Table 3.
Examples of DNA repair targeted agents in development.
| Targeted DNA repair mechanisms | Examples of Targeted Agents | Company Creating Agent | Solid Tumor Malignancies that are being investigated |
|---|---|---|---|
| Checkpoint control Chk1/Chk2 inhibitors | AZD7762 LY2606368 KU-60019 | Astrazeneca Eli Lilly Astrazeneca | NSCLC, brain, colorectal, ovarian |
| Poly(ADP-ribose) PARP inhibitors | Iniparib Olaparib | Sanofi-Aventis Astrazeneca | Breast, ovarian, endometrial, pancreas, NSCLC, GBM |
| Cyclin dependent kinase CDK1–4 | P276-00 | Primal Life Sciences | Melanoma, pancreas, multiple myeloma, head and neck cancer |
Conclusion
In summary, the total and nuclear EGFR expression levels were higher in p16-negative tumors compared to p16-positive tumors. Targeting the subcellular localization of EGFR for therapeutic benefit may be beneficial for treatment of p16-negative HNSCC patients. Further studies are indicated to determine the mechanistic link between these two prognostic factors and the significance of EGFR localization to nucleus upon activation of the pathway in DNA damage repair.
Novelty.
This study demonstrates differential expression of nuclear EGFR between patients with and without p16 expression in HNSCC. p16-negative HNSCC which is largely HPV-negative tumors is more resistant to conventional therapy than p16-positive HNSCC. Our study suggests that p16-negative HNSCC may have increased DNA damage resulting in increased nuclear EGFR expression for DNA damage repair which may confer treatment resistance.
Impact.
Confirmation of our findings will identify a biologically homogeneous group of head and neck cancer patients who may benefit from molecularly targeted approaches combining anti-EGFR agents, DNA damage repair inhibiting agents, and agents that prevent nuclear localization of EGFR.
Table 2.
EGFR expression as a function of p16 status.
| Variable | N | Mean | Minimum | Maximum | Standard Dev | Standard Err | P Value |
|---|---|---|---|---|---|---|---|
| p16 positive, total EGFR | 24 | 28.7 | 7.7 | 47.2 | 12.4 | 2.5 | p = 0.04 |
| p16 positive, nuclear EGFR | 24 | 13.9 | 5.0 | 32.8 | 6.8 | 1.4 | |
| p16 negative, total EGFR | 49 | 37.7 | 5.7 | 89.2 | 17.0 | 2.4 | p = 0.01 |
| p16 negative, nuclear EGFR | 49 | 19.7 | 4.5 | 52.8 | 10.2 | 1.5 |
Acknowledgments
The project was supported in part by Damon Runyon Clinical Investigator Award (CL-28-05) and National Institutes of Health (R01-DE-017982) to CHC and by an endowment to support the Barry Baker Laboratory for Head & Neck Oncology. We thank Applied Genomics, Inc. for construction of the tissue microarray.
Dr. Burtness has received research funding from Genentech and Boehringer Ingelheim (BI), and consulted for Bristol-Myers Squibb (BMS), BI and Genmab. Dr. Chung has received research funding from AstraZeneca, Lilly Oncology, and Bayer, and honoraria from BMS, Amgen, BI and Merck for educational lectures and serving on ad hoc scientific advisory boards.
Abbreviations
- HNSCC
head and neck squamous cell carcinoma
- EGFR
Epidermal Growth Factor Receptor
- HPV
human papillomavirus
- γH2AX
gamma-H2A histone family, member X
- AQUA
automated quantitative analysis
- IHC
immunohistochemical staining
- TMA
tissue microarray
Footnotes
Conflict of interest: The other authors have no potential conflicts of interest to disclose.
Financial disclosure The other authors have no financial disclosure.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chung CH, Zhang Q, Hammond EM, et al. Integrating Epidermal Growth Factor Receptor Assay With Clinical Parameters Improves Risk Classification for Relapse and Survival in Head-and-Neck Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:331–338. doi: 10.1016/j.ijrobp.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 7.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318:124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF, Hung MC. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65:338–348. [PubMed] [Google Scholar]
- 10.Lo HW. EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance. Current molecular pharmacology. 2010;3:37–52. doi: 10.2174/1874467211003010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marti U, Ruchti C, Kampf J, et al. Nuclear localization of epidermal growth factor and epidermal growth factor receptors in human thyroid tissues. Thyroid. 2001;11:137–145. doi: 10.1089/105072501300042785. [DOI] [PubMed] [Google Scholar]
- 12.Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 13.Lo HW, Hsu SC, Ali-Seyed M, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung LY, Tseng JT, Lee YC, et al. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008;36:4337–4351. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One. 6:e19605. doi: 10.1371/journal.pone.0019605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hsu SC, Miller SA, Wang Y, Hung MC. Nuclear EGFR is required for cisplatin resistance and DNA repair. American journal of translational research. 2009;1:249–258. [PMC free article] [PubMed] [Google Scholar]
- 18.Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103–1114. doi: 10.1158/0008-5472.CAN-10-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed AL, Califano J, Cairns P, et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- 20.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 22.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 23.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Psyrri A, Egleston B, Weinberger P, et al. Correlates and determinants of nuclear epidermal growth factor receptor content in an oropharyngeal cancer tissue microarray. Cancer Epidemiol Biomarkers Prev. 2008;17:1486–1492. doi: 10.1158/1055-9965.EPI-07-2684. [DOI] [PubMed] [Google Scholar]
- 26.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 27.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. The radioprotector Bowman-Birk proteinase inhibitor stimulates DNA repair via epidermal growth factor receptor phosphorylation and nuclear transport. Radiother Oncol. 2008;86:375–382. doi: 10.1016/j.radonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Dittmann K, Mayer C, Rodemann HP. Nuclear EGFR as novel therapeutic target: insights into nuclear translocation and function. Strahlenther Onkol. 2010;186:1–6. doi: 10.1007/s00066-009-2026-4. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao HJ, Carpenter G. Cetuximab/C225-induced intracellular trafficking of epidermal growth factor receptor. Cancer Res. 2009;69:6179–6183. doi: 10.1158/0008-5472.CAN-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan K, Araki K, Wang D, et al. Head and neck cancer radiosensitization by the novel poly(ADP-ribose) polymerase inhibitor GPI-15427. Head Neck. 2010;32:381–391. doi: 10.1002/hed.21195. [DOI] [PubMed] [Google Scholar]