Abstract
Objective
Delayed passage of stool is a result of both gestational immaturity and illness severity. Small for gestational age (SGA) preterm infants are at high risk of gastrointestinal (GI) complications. We aimed to analyse the effects of a strict nutrition and stool protocol on GI problems in SGA compared to appropriate for gestational age (AGA) preterm infants
Methods
Retrospective cohort analysis including all preterm infants with delayed meconium passage hospitalized at the Neonatal Intensive Care Unit of the Medical University of Graz, Austria. Infants were identified by a local data system and by the use of a strict feeding and stool protocol between 2001 and 2009. Main outcome parameters included neonatal morbidity, surgical intervention and mortality.
Findings
Twenty-six SGA (median GA 28.6 weeks, birth weight 825 grams, 46% males) were compared to 101 AGA (median GA 28.4 weeks, birth weight 1168 grams, 55% males) preterm infants. Clinical signs of delayed meconium passage did not differ significantly between groups. Differences regarding percentage of necrotizing enterocolitis, ileus, spontaneous intestinal perforation, and surgical intervention did not differ between groups. Mortality rate was significantly higher in SGA (11.5%) compared to AGA (2.9%) infants (P=0.03).
Conclusion
Despite similar morbidity SGA infants exhibited higher lethal complication rates following delayed meconium passage compared to AGA infants.
Keywords: Preterm Infants, Meconium, Necrotizing Enterocolitis, Neonate, Infant, Small for Gestational Age
Introduction
Delayed meconium passage of prematurity often occurs in very low birth weight infants, and especially Small for Gestational Age (SGA) preterm infants are at high risk of gastrointestinal (GI) complications[1, 2]. Thus, delayed meconium passage in preterm infants is becoming a more prevalent and significant problem. A prompt recognition of this entity with its risk factors resulting in early medical management is essential to avoid early surgical intervention in this vulnerable population[3–5].
Aim of this study was to evaluate the effects of a strict nutrition and stool protocol that early identifies preterm infants at high risk for delayed meconium passage and possibly reduces GI complications by comparison of SGA to appropriate for gestational age (AGA) preterm infants.
Subjects and Methods
Inclusion of all preterm infants with delayed meconium passage hospitalized at the Neonatal Intensive Care Unit of the Medical University of Graz, Austria, a tertiary care center. Infants were identified by a local data system and by the use of a strict feeding and stool protocol between 2001 and 2009. Infants with congenital malformations were excluded. SGA was defined as birth weight below the 10th percentile[6]. The gestational age was defined when the mother's last menstrual period (LMP) began. Perinatal data included gestational age, birth weight and gender.
The study was submitted to the Ethics Committee of the Medical University of Graz and approved.
Clinical signs of delayed meconium passage included gastric residual volumes, abdominal distension, and bilious residua. Delayed meconium passage was defined as absence of first stool following meconium within 48 hours after first feeding with breast milk. Stool was assessed by quality as meconium, first stool and breast milk stool, and by quantity as few, normal or huge. To differentiate NEC from SIP, ultrasonography was used because it may detect signs and complications of NEC before they are evident on radiographs[7].
Feeding and stool protocol: Oral feeding was started as soon as possible with a daily oral intake of 8 ml/kg, assisted by intravenous supplementation. Recent studies have suggested that bolus feeding promotes more “normal” feed-fasting hormonal concentrations that potentially benefit intestinal development and nutrient partitioning[8]. After the first feeding with maltodextrin, infants were fed every 3 hours by nasogastric tube or bottle according to the infant's ability with breast milk or pooled pasteurized human milk. Gastric residuals were assessed by aspiration via nasogastric tube before each feeding. The volume of breast milk was increased alternatively every day. After initiating feedings early, and providing a period of trophic feedings, we increased the volume relative rapidly over a 10-days time period. Full enteral feedings were defined as an oral intake of 160 ml/kg/d[8–11]. In cases of refusal of the mother to breast-feed the newborn or insufficient amounts of breast-milk, hydrolized protein formula for the preterm infant was added.
Abdomen was assessed daily and documented as being normal or abnormal including progressive abdominal distension, rigidity, or tenderness. Additionally vomiting and bilious residual volumes were documented. If meconium was not spontaneously passed during the first 48 h of life, defecation was stimulated by administration of an enema (1ml glycerine - 0.8 g/10mL - added to 9 ml saline solution 0,9% for children >1000g birth weight and 0,5 ml glycerine added to 4,5 ml saline solution 0,9% for children <1000g birth weight) via a disposable gastric tube coated with petrolatum (Vaseline®) for protective insertion into the rectum. Management of meconium obstruction syndrome included repeated enemas and at least oral application of Gastrografin®, an ionic x-ray contrast medium, a mixture of sodium amidotrizoate and meglumine amidotrizoate in a proportion of 10:66.
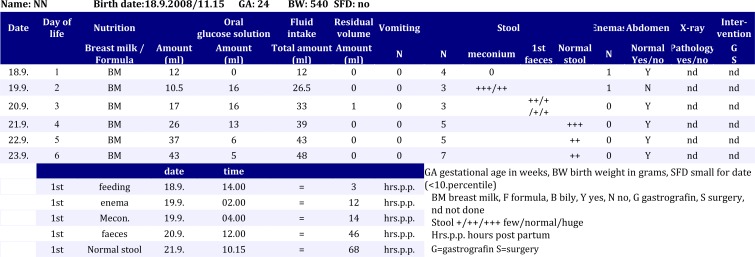
Our stool protocol summarizes the entire nutrition protocol, including also the stool passage, the daily physical examination, and the required conservative and non-surgical treatment. Fig 1 is an example of a normal stool protocol of an AGA preterm infant.
Fig. 1.
An example of a normal stool protocol of an AGA preterm infant
Statistics. Statistical analysis was performed by means of the SPSS package for Windows, version 16.0 (SPSS Inc, Chicago,IL).
Findings
Since 2001, 127 preterm infants were identified prospectively as having delayed meconium passage. Twenty-six SGA (median GA 28 + 4 weeks, birth weight 825 grams, 46% male) were compared to 101 AGA (median GA 28 + 3 weeks, birth weight 1168 grams, 55% male) preterm infants (Table 1). Prenatal risk factors including abruption of placenta, pathological CTG or Doppler flow measurement of the umbilical vessels, and vaginal haemorrhage were observed in SGA compared to AGA infants in 4 vs. 12%, 81 vs. 62% and 15 vs. 26%, respecttively Clinical signs of delayed meconium passage did not differ significantly between groups. Treatment includedenemas (median number 6.5 vs 10.6) and oral gastrografin (92 vs 70%) in SGA compared to AGA infants, respectively. One (3.8%) SGA compared to 9 (8.8%) AGA infants developed necrotizing enterocolitis (P=0.2).
Table 1.
The perinatal characteristics, the risk factors, clinical signs and the conservative therapy of the SGA and AGA preterm infants with delayed meconium passage
| Parameter | SGA-Preterms N= 26 | AGA Preterms N= 101 | |
|---|---|---|---|
| Median Gestational Age (week) | 28 + 4 | 28 + 3 | |
| Birth Weight | Median | 825.1 g | 1186.4 g |
| Range | 415- 2200 g | 512-2812 g | |
| Sex | Male | 12 (46%) | 57 (56.4%) |
| Female | 14 (54%) | 44 (43.6%) | |
| Risk Factor | Abruption of placenta | 1 (4%) | 12 (12%) |
| Pathologic CTG/ Doppler Measurement | 22 (81%) | 62 (62.6%) | |
| Vaginal haemorrhage | 4 (15.8%) | 26 (26.2%) | |
| Clinical Signs | No Residual volume | 3 (11.5%) | 9 (8.9%) |
| Residual volume | 8 (30.7%) | 53 (52.4%) | |
| Bilious residual volume | 15 (57%) | 40 (39.6%) | |
| Pathologic abdomen | 21 (80.7%) | 78 (77.2%) | |
| Delayed meconium > 24h | 7 (26.5%) | 19 (18.8%) | |
| First stool > 48h after breast milk feeding | 21 (80.7%) | 72 (71.2%) | |
| Non-surgical treatment | Enema <24h | 25 (96%) | 84 (83.2%) |
| Median enema‘s | 6.5 | 10.6 | |
| Gastrografin | 24 (92%) | 71 (70.3%) | |
| 2nd Gastrografin | 1 (3.8%) | 4 (3.9%) | |
SGA= Small for Gestational Age; AGA= Appropriate for Gestational Age; GA = Gestational Age
Two (7.7%) SGA compared to five (4.9%) AGA infants had spontaneous distal ileum perforation (P=0.3). Surgery had to be performed in six (23%) SGA compared to 13 (12.8%) AGA infants (P=0.09). Morbidity did not differ between groups, but mortality rate following surgery was significantly higher in SGA (11.5%) compared to AGA (2.9%) infants (P=0.03)(Table 2).
Table 2.
Results and outcome of the SGA and AGA preterm infants with delayed meconium passage
| Outcome | SGA Preterms N = 26 | AGA Preterms N = 101 | P value |
|---|---|---|---|
| Necrotizing Enterocolitis | 1 (3.8%) | 9 (8.8%) | 0.2 |
| Spontanous Intestinal Perforation | 2 (7.7%) | 5 (4.9%) | 0.3 |
| Ileus | 7 (30.0%) | 15 (14.7%) | 0.07 |
| Surgery | 6 (23.0%) | 13 (12.8%) | 0.1 |
| Exitus | 3 (11.5%) | 3 (2.9%) | 0.03 |
SGA: Small for Gestational Age; AGA: Appropriate for Gestational Age; SIP:
Discussion
The immaturity of the intestinal motor mechanisms and associated feeding problems are challenges in the treatment of very low birth weight (VLBW) infants [12].
Timing of the first and last meconium stool is critical for oral feeding tolerance and proper gastrointestinal function[13]. Ninety-five percent of healthy term infants pass their first stool within 24 hours of birth. Preterm infants (<37 weeks gestational age) and low birth weight (<1500g) infants have a delay in passage of the first stool[14] and more than 80% of preterm infants pass their first stool within 48 h[15, 16].
The exact reason for the delay is unclear, but a delay in maturation of the motor mechanisms of the gut has been suggested to play a major role[13, 16]. Additionally pre- and postnatal hemodynamic disturbances have been identified as risk factors for intestinal motility problems [2].
Obstruction of the gastrointestinal tract by tenacious meconium frequently leads to gastric residuals, a distended abdomen, and delayed food passage. Recent data support the concept that rapid evacuation of meconium plays a key role in feeding tolerance[17, 18]. To prevent meconium obstruction and improve feeding tolerance, data suggest major benefits for prophylactic enemas in preterm infants[5, 19–21]. Routine glycerine enema was found to be safe and easy-to-use at the bedside. In any case of resistance the enema was applied under ultrasound observation. No perforation occurred using this maneuver. The procedure was repeated until complete evacuation of meconium was achieved and breast milk stool has passed. If the infant did not pass first stool within 48 hours after first feeding with breast milk, a water soluble x-ray contrast medium, was administered orally (Gastrografin® -x-ray contrast medium for oral and rectal application, Schering, Vienna). As described the contrast medium leads to a propulsive hyperactive gastrointestinal motility. By radiographic views we confirmed the correct placement of the contrast medium 4 and 12 hours after application through the upper gastrointestinal tract and the small bowel. Only in rare cases, if meconium/stool did not pass after the first application of Gastrografin and provided that the clinical condition of the preterm infant did not worsen, Gastrografin was readministered. As reported[5] we found Gastrografin enemas being safe, diagnostic and therapeutic, however, it is not recommended for hemodynamically unstable patients[21]. None of our preterm infants developed symptoms of dehydration as described elsewhere[22]. Rates of surgical interventions in the SGA study patients associated with delayed meconium passage were higher compared to the AGA infants, but did not reach statistical significance. Our results demonstrate that surgical treatment becomes significantly more hazardous in SGA preterm infants[23–26].
The reason therefore is unclear but SGA is often associated with a lack of adequate oxygen supply and a reduction in the fetus’ stores of glycogen and lipids. This often leads to several metabolic problems and circulatory disturbance after birth, predisposing the preterm SGA newborn to further problems caused by poor reserves[27].
A limitation of our study is that is a retrospective analysis of nutrition and stool protocol. So further prospective study to evaluate delayed meconium passage of the preterm and especially SGA preterm infant is needed. Finally, none of the cases was diagnosed as having cystic fibrosis or Hirschsprung’ s disease
Conclusion
In our study we found no differences between SGA and AGA preterm infants regarding short term morbidity following delayed meconium passage using a strict nutrition and stool protocol. In contrast, mortality following surgical intervention was significantly higher in preterm SGA infants.
Acknowledgment
Institute's ethical approval was obtained from the local research ethics committee.
Conflict of Interest
None
References
- 1.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):779–93. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Tsukamoto H, Fukuoka H, Koyasu M, et al. Risk factors for small for gestational age. Pediatr Int. 2007;49(6):985–90. doi: 10.1111/j.1442-200X.2007.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65(2):138–44. doi: 10.1203/PDR.0b013e31818c7920. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Dhanireddy R. Time of first stool in extremely low birth weight infants. J Pediatr. 1993;122(4):626–9. doi: 10.1016/s0022-3476(05)83550-4. [DOI] [PubMed] [Google Scholar]
- 5.Garza-Cox S, Keeney SE, Angel CA, et al. Meconium obstruction in the very low birth weight premature infant. Pediatrics. 2004;114(1):285–90. doi: 10.1542/peds.114.1.285. [DOI] [PubMed] [Google Scholar]
- 6.Haas J, Rosegger H, Haim M. Intrauterine growth-normal curves for gestational age. Z Geburtsh Perinat. 1987;119(3):91–5. [PubMed] [Google Scholar]
- 7.Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):298–304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 8.Xiao-Ming B. Nutritional management of newborn infants: Practical guidelines. World J Gastroenterol. 2008;14(40):6133–9. doi: 10.3748/wjg.14.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Ann Nutr Metab. 2011;58(Suppl 1):8–18. doi: 10.1159/000323381. [DOI] [PubMed] [Google Scholar]
- 10.Tyson JE. A critical perspective on trophic feeding. J Pediatr Gastroenterol Nutr. 2004;38(3):237–8. doi: 10.1097/00005176-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Berseth CL. Feeding methods for the preterm infant. Semin Neonatol. 2000;6(5):417–24. doi: 10.1053/siny.2001.0062. [DOI] [PubMed] [Google Scholar]
- 12.Newell SJ. Enteral feeding of the Micropreemie. Clin Perinatol. 2000;27(1):221–34. doi: 10.1016/s0095-5108(05)70015-4. [DOI] [PubMed] [Google Scholar]
- 13.Meetze WH, Palazzolo VL, Bowling D, et al. Meconium passage in very low birth weight infants. J Parenter Enteral Nutr. 1993;17(6):537–40. doi: 10.1177/0148607193017006537. [DOI] [PubMed] [Google Scholar]
- 14.Weaver LT, Lucas A. Development of bowel habit in preterm infants. Arch Dis Child. 1993;68(3):317–20. doi: 10.1136/adc.68.3_spec_no.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jhaveri MK, Kumar SP. Passage of first stool in very low birth weight infants. J Pediatr. 1987;79(6):1005–7. [PubMed] [Google Scholar]
- 16.Wang PA, Huang FY. Time of the first defecation and urination in very low birth weight infants. Eur J Pediatr. 1994;153(4):279–83. doi: 10.1007/BF01954520. [DOI] [PubMed] [Google Scholar]
- 17.Manning AP, Heaton KW, Harvey RF. Wheat fibre and irritable bowel syndrome. A controlled trial. Lancet. 1977;2(8035):417–8. doi: 10.1016/s0140-6736(77)90605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnoldi R, Leva E, Macchini F, et al. Delayed meconium passage in very low birth weight infants. Eur J Pediatr Surg. 2011;21(6):395–8. doi: 10.1055/s-0031-1291301. [DOI] [PubMed] [Google Scholar]
- 19.Zenk KE, Koeppel RM, Liem LA. Comparative efficacy of glycerine enemas and suppository chips in neonates. Clin Pharm. 1993;12(11):846–8. [PubMed] [Google Scholar]
- 20.Shim SY, Kim HS, Kim DH, et al. Induction of early meconium evacuation promotes feeding tolerance in very low birth weight infants. Neonatology. 2007;92(1):67–72. doi: 10.1159/000100804. [DOI] [PubMed] [Google Scholar]
- 21.Koshinaga T, Inoue M, Ohashi K, et al. Therapeutic strategies of meconium obstruction of the small bowel in very-low-birthweight neonates. Pediatr Int. 2011;53(3):338–44. doi: 10.1111/j.1442-200X.2010.03231.x. [DOI] [PubMed] [Google Scholar]
- 22.Rowe MI, Furst AJ, Altman DH, et al. The neonatal response of gastrografin enema. Pediatrics. 1971;48(1):29–35. [PubMed] [Google Scholar]
- 23.De Backer AI, De Schepper AM, Deprettere A, et al. Radiographic manifestations of intestinal obstruction in the newborn. JBR-BTR. 1999;82(4):159–66. [PubMed] [Google Scholar]
- 24.Emil S, Nguyen T, Sills J, et al. Meconium obstruction in extremely low-birth-weight neonates: guidelines for diagnosis and management. J Pediatr Surg. 2004;39(5):731–7. doi: 10.1016/j.jpedsurg.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara T, Tsuda M, Koyama N. Management of meconium-related ileus in very low-birthweight infants. Pediatr Int. 2007;49(5):641–4. doi: 10.1111/j.1442-200X.2007.02457.x. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui MM, Drewett M, Burge DM. Meconium obstruction of prematurity. Arch Dis Child Fetal Neonatal Ed. 2012;97(2):F147–50. doi: 10.1136/adc.2010.190157. [DOI] [PubMed] [Google Scholar]
- 27.Shah MD, Shah SR. Nutrient deficiencies in the premature infant. Pediatr Clin North Am. 2009;56(5):1069–83. doi: 10.1016/j.pcl.2009.08.001. [DOI] [PubMed] [Google Scholar]