Abstract
Background
Bartter's syndrome is a heterogeneous disorder characterized by deficient renal reabsorption of sodium and chloride, and hypokalemic metabolic alkalosis with hyper-reninemia and hyperaldosteronemia. Bartter syndrome type III (BS type III), due to mutations in the CLCNKB gene, is highly variable. The aim of our study was to describe the clinical presentation in a Chinese girl with BS type III and to explore mutations or SNPs of CLCNKB gene in her family.
Case Presentation
The clinic data of the patient was collected. Mutations or SNPs were investigated by sequencing of the exon of CLCNKB gene. The clinic analysis confirmed the diagnosis of BS type III. The coexistence of 13 reported SNPs and 11 novel SNPs of CLCNKB gene were found in the patient and her parent. a novel heterozygous C to G transition at nucleotide 2471 in exon 20 of CLCNKB gene harbored uniquely by the patient were revealed.
Conclusion
A novel heterozygous C to G mutation at nucleotide 2471 of CLCNKB gene and some new SNPs were identified in a Chinese girl with BS type III having persistent hypokalemia. The novel mutation and SNPs make the genetic background of the patient more complicated.
Keywords: Bartter Syndrome, Hypokalemia, Chloride Channel, Metabolic Alkalosis, Mutation
Introduction
Bartter's syndrome (BS) is a heterogeneous disorder characterized by deficient renal reabsorption of sodium and chloride, and hypokalemic metabolic alkalosis with hyper-reninemia and hyperaldosteronemia. The main pathogenesis in BS is the defect of NaCl reabsorption in the thick ascending limb of Henle's loop (TALH). Inherited BS can be divided into five subtypes according to the different inactivating mutations of genes: SLC12A1 mutation encoding the bumetanide-sensitive Na+–K+–2Cl- cotransporter (NKCC2) (type I) and KCNJ1 encoding the inward rectifying K+ channel (ROMK) (type II) for antenatal BS (aBS), CLCNKB encoding the kidney-specific basolateral chloride channel for cBS (type III); BSND encoding the β-subunit of the basolateral chloride channel for aBS (type IV), and gain-of-function mutations in the calcium-sensing receptors for autosomal dominant hypocalcemia associated with hypokalemia (type V)[1].
The features of BS can be shared with Gitelman's syndromes, CLCNKB gene is expressed not only in TALH but also in distal convoluted tubules[2]. BS type III has a group of autosomal-recessive inherited disorders with clinical characteristics such as renal salt wasting, hypokalemic metabolic alkalosis, elevated renin and aldosterone levels, with normal or low blood pressure. BS type III is highly variable and usually presents as a “classic” Bartter variant characterized by an onset in early childhood and less severe or absent hypercalciuria and nephrocalcinosis[3]. In this report, we described a Chinese girl with BS type III who developed persistent hypokalemia and investigated mutations or single nucleotide polymorphisms (SNPs) of CLCNKB gene in her family.
Case Presentation
An 811/12-year-old Chinese girl was brought to the pediatric endocrinology ward because of hypokalemia found occasionally. The girl showed no progressive muscle weakness of the extremities. Her serum potassium was 2.8 mmol/l estimated two weeks ago in a local hospital. Her birth history revealed full-term by normal spontaneous delivery and normal birth weight of 3,400 g without antenatal polyhydramnios.
On admission, her height (129 cm) and body weight (25 kg) were all more than the 40th percentile. Blood pressure was 106/66 mmHg, heart rate 96 beats/min and respiratory rate 22/min. internal jugular vein was flat. There was no diminished muscle strength and no reflex over the lower extremities. Fasciculation, myoclonus and muscular atrophy were not present. Her pure tone audiometry was normal. The remainder of the physical examination was unremarkable.
Complete blood cells counts showed that hemoglobin was 116g/l, white blood cells 5,600/µl and platelets 314,000/µl. Urinalysis revealed pH 7.5, negative protein, glucose, leukocytes, and red blood cells. The data of biochemical studies are shown in Table 1 and Table 2. The most striking findings were hypokalemia (2.5-3.0 mmol/l), mild metabolic alkalosis (HCO3 -32.6 mmol/l), normal renal function (BUN 36.4 mg/dl, Cr 3.52 mg/dl), and normal aldosterone concentration (120 pg/ml).
Table 1.
Serial chemical data of the patient's blood
| Index (Normal range) | Before admission | 1st day after admission | 8th day after admission | 3 months after follow-up | 6 months after follow-up | 9 months after follow-up |
|---|---|---|---|---|---|---|
| pH(7.34–7.45) | 7.45 | 7.410 | 7.40 | 7.367 | 7.37 | 7.43 |
| Na + (135–147 mmol/l) | 131 | 140 | 137 | 141 | 139 | 144 |
| K + (3.5–5.3 mmol/l) | 2.2 | 2.6 | 2.9 | 3.0 | 2.7 | 3.1 |
| Cl - (95–108 mmol/l) | 96 | 109 | 107 | 102 | 99 | 95 |
| HCO 3 - (20–26 mmol/l) | 28 | 27.2 | 29 | 32.6 | 25 | 31 |
| Total Ca 2+ (1.05–1.35 mmol/l) | 1.06 | 1.06 | 1.22 | 1.20 | 1.12 | 1.27 |
| Mg 2+ (2.0–4.0 mg/dl) | 0.42 | 1.82 | 2.2 | 2.01 | 2.4 | 2.8 |
| BUN (2.10–7.90 mmol/l) | 8 | 3.52 | 4.15 | 6.2 | 5.9 | 4.7 |
| Creatinine (22.0–97 mmol/l) | 1.6 | 36.4 | 51.7 | 57.2 | 36.1 | 82.9 |
Table 2.
The data of Aldosterone, Renin, Angiotensin I and Angiotensin II in the patients in different postures
| Parameter | In upright posture | In recumbent posture | ||
|---|---|---|---|---|
| Patient | Normal range | Patient | Normal range | |
| Aldosterone pg/ml | 108.94 | 59–199 | 120.36 | 65–296 |
| Renin ng/ml/h | 10.55 | 1.95–3.99 | 9.2 | 0.05–0.79 |
| Angiotensin I ng/ml | 22.67 | 7.36 | ||
| Angiotensin II pg/ml | 596.75 | 55.3–115.3 | 149.37 | 28.2–52.5 |
The trans-tubular potassium gradient (TTKG) is an index reflecting the conservation of potassium in the cortical collecting ducts of the kidneys. The following is the formula for calculating the TTKG: TTKG =[(urine potassium) × (plasma osmolality)] divided by [(plasma potassium) × (urine osmolality)]. Her TTKG (9.6) was higher, indicative of renal K+ wasting. Serum magnesium (Mg2+) was tested twice and the value was 1.82, 2.0 mg/dl respectively. Serum calcium (Ca2+) levels were normal, but urinary Ca2+ excretion was very low (urine Ca2+/Cr 0.01 mg/mg) (Tables 1 and 2). EKG disclosed a sinus rhythm. Abdominal sonography revealed bilateral normal size kidneys and adrenal glands without nephrocalcinosis or renal stones. With the above-mentioned findings, she was clinically diagnosed as BS type III with the presentation of inactivity, hypokalemia and metabolic alkalosis along with normal renal function.
Administration of intravenous KCl 60 mmol/ day followed by oral KCl 1.5 mEq/kg/day restored and maintained her serum K+ concentration at about 3.0 mmol/l. Fluids were also supplemented. Indomethacin (2 mg/kg/day) and spironolactone (2 mg/kg/day) were also regularly given to control the hypokalemia until now. She did not experience any episodes of Indomethacin overdose or received other potential nephrotoxic medications during therapy periods. The most prominent laboratory data was persistent hypokalemia during one year of follow-up till now, especially after the episodes of acute upper respiratory infection and acute gastroenteritis. Her renal function still persisted in good condition.
Molecular analysis: The human-investigation committee of the Zhejiang University School of Medicine approved the study. The parents were informed about the purpose of this study and gave consent. The polymerase chain reaction (PCR) for amplifying DNA sequences and direct sequencing of all the exons of CLCKB gene was performed using peripheral blood genomic DNA of the patient and her non-consanguineous parent. All the primers were designed according to the sequence of NG_013079.1 (Table 3). Several reported SNPs and some new nucleotide variations were also found in the CLCKB gene of the patient, her mother and father (Table 4). Three of the nucleotide variations are synonymous mutations or silent SNPs, the other nucleotide variations can make the amino acids change, however, these new nucleotide variations were found in the patient and her healthy parents, we concluded that these new nucleotide variations may be SNPs.
Table 3.
The primers for amplifying the CLCNKB gene in the study
| Forward primer | Reverse primer | Length(bp) | Tm(°C) | |
|---|---|---|---|---|
| Exon1 | GGACATTCTAAGTGTTCGCCATAA | CCCAGAAGAAGATCCCACCAG | 269 | 59 |
| Exon2 | ACACCCTCAGTGACGGAAAC | TGCCACCCAGGAGACTTT | 446 | 59 |
| Exon3 | CCACTGTCACCTCCCACAAAT | GCCCAGGATCACAGAGCAAG | 481 | 61 |
| Exon4 | TGGGTGCCTCCCTGATAC | TCCTGGGCCTTTGTCTGT | 265 | 61 |
| Exon5 | ATCTGGCGAGATCGTAATG | GGTAGGGTGGTTGGGATG | 276 | 62 |
| Exon6, 7 | CAAAAGCCATCTGAGGACG | GAGGGGAGGAGCTTGAGG | 424 | 65 |
| Exon8 | TGACTTGATTGGCGGTGCTA | CCCTCCATCCTCCCAGAAG | 646 | 59 |
| Exon9, 10 | TTAGTCTGGGACTTGAGTTTGGG | GGTTGGGAGTACGGTGAGGG | 431 | 59 |
| Exon11 | TTGGGATGTGGGAAAGGG | AAAGGAGGCAACAAATAGGG | 194 | 59 |
| Exon12 | GGGAAGTGGCAGAGGAGGA | CAGGAAATGTGGGTGGAAGG | 393 | 62 |
| Exon13, 14 | TGTCCTGTCCTCCCTTGT | CCCTGACCCACTCACCT | 430 | 62 |
| Exon15 | ACTCCCTCGTGGCTCCTGT | AGCTACGGTGGCGTTTCTTT | 516 | 61 |
| Exon16 | TAGGAGTCCCTCATTCCAG | CACGGTTGGTTGCTAAGTC | 403 | 60 |
| Exon17, 18 | CCCCATAGGAACACCAGAACA | GCAGGAAGCAGCATAAGACG | 552 | 60 |
| Exon19 | ACCTTCTACCCTCCAGTGTTT | TCATCAGTATCCTCTGAATCCC | 259 | 59 |
| Exon20 | CCCTCACAACCTCCTCTACATC | TGACCACGGGAAACTCACTCT | 588 | 60 |
Table 4.
Genotypes in CLCNKB gene from the patient's family (compared to Genebank accession NG_013079.1)
| Reported SNPs | Other nucleotide variations or new SNPS | |
|---|---|---|
| Patient | rs2015352 (exon 2), rs5257 (exon 4), rs2014562(exon 5), rs71493533(exon 8), rs5253, rs5254, rs2275166 and rs2275167 (exon 16), rs79198735 (exon 19), rs112496366, rs1057839, rs79768787 and rs1057854 (exon 20). | a homozygous C to T (gcg to gtg) transition at nucleotide 996(exon 9), homozygous T to C (tgt to tgc; synonymous mutation) transition at nucleotide 1012 (exon 10), heterozygous T to C (ggt to ggc; synonymous mutation) transition at nucleotide 1759, heterozygous GC to AT (cgc to cat) transition at nucleotides 1767-8, and homozygous A to G (aag to gag) transition at nucleotide 1868 (exon 16), homozygous C to A (cac to caa) transition at nucleotide 2071, homozygous G to A (gag to aag) transition at nucleotide 2081, a homozygous T to G (cat to cag) transition at nucleotide 2098 and a homozygous G to A (acg to aca; synonymous mutation) transition at nucleotide 2113(exon 19) |
| Mother | Same as above | Same as above |
| Father | Same as above | Same as above |
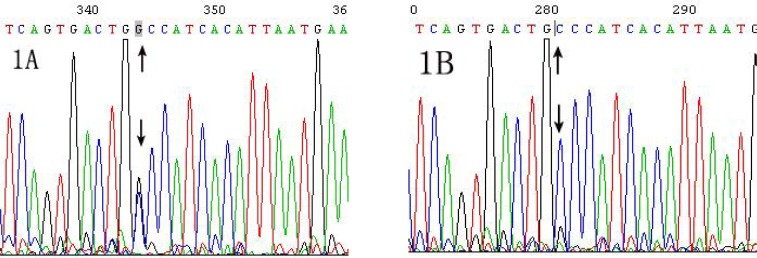
Family tree of new mutation or SNPs in exon 20 of CLCNKB gene was also investigated in this family (Table 5). A novel heterozygous C to G transition at nucleotide 2471 in exon 20 were found in the patient, which has not been detected in her mother or her father (Table 5, Fig. 1). Sequencing for reverse DNA strands confirmed the above result.
Table 5.
Genotypes of exon 20 in CLCNKB gene from the patient's pedigree
| patient's pedigree | Genotype at 2260 bp of exon 20 | Genotype at 2336 bp of exon 20 | Genotype at 2363 bp of exon 20 | Genotype at 2471 bp of exon 20 |
|---|---|---|---|---|
| Father | G/A | C/C | T/T | C/C |
| Mother | G/G | C/C | A/T | C/C |
| Patient | G/G | C/C | A/T | G/C |
Fig. 1.
The new mutation in exon 20 of CLCNKB gene from the patient. Sequencing of the exon 20 in CLCNKB gene was performed in the family. Fig. 1A: A heterozygous C to G transition (black arrow, forward primer) at nucleotide 2471 in exon 20 of CLCNKB gene was shown in the patient, which was also confirmed by sequencing for reverse DNA strands. Fig. 1B: C/C genotype at nucleotide 2471 in exon 20 of CLCNKB gene was shown in her father (black arrow, forward primer). (Genebank accession NG_013079.1).
Discussion
Bartter's syndrome encompasses a group of closely related inherited tubulopathies characterized by markedly reduced NaCl transport[4]. The clinical manifestations of the syndrome varied from lack of symptoms to severe growth retardation[5]. Volume depletion activates the renin–angiotensin–aldosterone system in BS. Renin contributes to renal fibrosis through triggering proliferation of mesangial cell[6].
Angiotensin II plays a crucial role in renal injury by increasing intraglomerular capillary pressure and promoting fibroproliferative effects. Aldosterone directly accelerates renal damage[7]. Racial and environmental differences, genetic background, as well as different types of mutation in CLCNKB gene, should be probably involved in the susceptibility to the deterioration of renal function[8]. Patients with BS are usually associated with high PGE2 production and hypercalciuria[9]. Chronic non-steroid anti-inflammtory drugs (NSAID) administration in aBS and/or BS usually achieved several benefits such as amelioration or correction of hypokalemia, reduction of hypercalciuria, and preservation of renal function. Cyclo-oxygenase inhibitors such as indomethacin have been recommended as the standard treatment for aBS and/or BS because renal prostaglandin is overproduced. Some patients with BS type III had the trend of patholo-gical proteinuria and impaired kidney function after a median of 14 years follow-up[10]. Till now, renal function and histological data were unaffected in our patients after indomethacin treatment for more than one year. Chronic NSAID administration can be considered in this patient if necessary.
In the kidney approximately 30% of NaCl reabsorption takes place in the thick ascending limb of the loop of Henle and this depends upon a chloride channel complex encoded by CLCNKB and BSND[11]. To date, more than 30 CLCNKB mutations have been reported in BS[4, 12]. Wright's fixation index (Fst) increases as the allele frequency difference between populations increases[13]. The Fst values of rs2003943, rs6604910 and rs1414499 in CLCNKB gene were greater than rs2050522 in the Han Chinese, which exhibited strong linkage disequilibrium[14]. A total of 13 reported single nucleotide polymorphisms (SNPs) were found in the patient, her father and mother (Table 4), these SNPs were located close to rs2050522, making it a reasonable proxy for surrounding markers, so it is necessary to analyze linkage disequilibrium among these markers in Chinese Han population. A homozygous G470E mutation in the CLCNKB gene was reported in a Chinese boy[15], however, G470E mutation was not found in our patient.
Some new nucleotide variations were also identified (Tables 4 and 5), among them, three were synonymous mutations or SNPs, the other nucleotide transitions may result in amino acid sequence variations, for example, a homozygous A to G transition at nucleotide 1868 (aag to gag; Lys to Glu). Because these new nucleotide variations were found both in the patient and her healthy parent, we concluded these new nucleotide variations may be new SNPs. The clinical manifestations of BS III were heterogeneous without a genotype-phenotype correlation in a nationwide cohort of 26 Korean children[16]. The molecular diagnostic steps for patients with BS in our population should be designed taking these peculiar genotype distributions into consideration.
Synonymous SNPs do not produce altered coding sequences, and therefore they are not expected to change the function of the protein in which they occur. However, Kimchi-Sarfaty C reported that a synonymous SNP in the multidrug resistance 1 gene encoding P-glycoprotein (P-gp) affects the timing of cotranslational folding and insertion of P-gp into the membrane, thereby altering the structure of substrate and inhibitor interaction sites[17]. It demonstrates for the first time that naturally occurring silent SNPs can lead to the synthesis of protein product with the same amino acid sequence but different structural and functional properties. Synonymous mutations have significant consequences for cellular processes[18]. Thus, silent SNPs in this study should not be neglected in determining the likelihood of development of BS. Direct sequencing also revealed a novel heterozygous C to G mutation at nucleotide 2471 in exon 20 (the last exon) of CLCNKB gene in the patient, which did not exist in her parent (Table 5, Fig. 1).
CLC C-terminal cytoplasmic domains are required for CLC channel function[19]. The location of a novel compound heterozygous mutation in the exon 20 of CLCNKB gene may result in helix instability of mRNA that leads to a loss of translation of protein. It is also possible that the compound heterozygous mutation disrupts a direct interaction with the membrane domain.
Conclusion
We have identified a novel CLCNKB mutation and some new SNPs in a Chinese girl with BS type III having persistent hypokalemia. SNPs and a novel mutation make the genetic background of the patient more complicated. Further functional study of the mutation and/or SNPs should be taken into account.
References
- 1.Watanabe S, Fukumoto S, Chang H, et al. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360(9334):692–4. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 2.Naesens M, Steels P, Verberckmoes R, et al. Bartter's and Gitelman's syndromes: from gene to clinic. Nephron Physiol. 2004;96(3):65–78. doi: 10.1159/000076752. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Soriano J, Vallo A, Pérez de Nanclares G, et al. A founder mutation in the CLCNKB gene causes Bartter syndrome type III in Spain. Pediatr Nephrol. 2005;20(7):891–6. doi: 10.1007/s00467-005-1867-z. [DOI] [PubMed] [Google Scholar]
- 4.Seyberth HW. An improved terminology and classification of Bartter-like syndromes. Nat Clin Pract Nephrol. 2008;4(10):560–7. doi: 10.1038/ncpneph0912. [DOI] [PubMed] [Google Scholar]
- 5.Nozu K, Fu XJ, Nakanishi K, et al. Molecular analysis of patients with type III Bartter syndrome: picking up large heterozygous deletions with semiquantitative PCR. Pediatr Res. 2007;62(3):364–9. doi: 10.1203/PDR.0b013e318123fb90. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Noble NA, Zhang J, et al. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72(1):45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta 1 in renal fibrosis of rats. Hypertension. 2000;35(5):1078–84. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 8.Reinalter SC, Gröne HJ, Konrad M, et al. Evaluation of long-term treatment with indomethacin in hereditary hypokalemic salt-losing tubulopathies. J Pediatr. 2001;139(3):398–406. doi: 10.1067/mpd.2001.117007. [DOI] [PubMed] [Google Scholar]
- 9.Peters M, Jeck N, Reinalter S, et al. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112(3):183–90. doi: 10.1016/s0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 10.Bettinelli A, Borsa N, Bellantuono R, et al. Patients with biallelic mutations in the chloride channel gene CLCNKB: long-term management and outcome. Am J Kidney Dis. 2007;49(1):91–8. doi: 10.1053/j.ajkd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Simon DB, Bindra RS, Mansfield TA, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17(2):171–8. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Xu C, Pan X, et al. Identification and functional analysis of novel mutations of the CLCNKB gene in Chinese patients with classic Bartter syndrome. Clin Genet. 2010;77(2):155–62. doi: 10.1111/j.1399-0004.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- 13.Weir B, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38(6):1358–70. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 14.Sile S, Velez DR, Gillani NB, Alexander CA, George AL, Jr, Williams SM. Haplotype Diversity in Four Genes (CLCNKA, CLCNKB, BSND, NEDD4L) Involved in Renal Salt Reabsorption. Hum Hered. 2008;65(1):33–46. doi: 10.1159/000106060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CM, Tsai JD, Lo YF, et al. Chronic renal failure in a boy with classic Bartter's syndrome due to a novel mutation in CLCNKB coding for the chloride channel. Eur J Pediatr. 2009;168(9):1129–33. doi: 10.1007/s00431-008-0883-y. [DOI] [PubMed] [Google Scholar]
- 16.Lee BH, Cho HY, Lee H, et al. Genetic basis of Bartter syndrome in Korea. Nephrol Dial Transplant. 2012;27(4):1516–21. doi: 10.1093/ndt/gfr475. [DOI] [PubMed] [Google Scholar]
- 17.Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A ’silent‘ polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–8. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 18.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet. 2011;12(1):32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82(2):503–68. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]