Abstract
Background
Neonatal Marfan syndrome is a rare and severe phenotype of this disease. A poor prognosis is anticipated due to the high probability of congestive heart failure, and mitral and tricuspid regurgitations with suboptimal response to medical therapy and difficulties in surgical management at an early age.
Case Presentation
We present two consecutive patients with this disease who are the first reported cases from Iran to the best of our knowledge. Unfortunately both of them died shortly after diagnosis.
Conclusion
Neonatal Marfan syndrome is reported from Iran and has a poor prognosis like the patients reported from elsewhere.
Keywords: Marfan Syndrome, Ghent Criteria, Congestive Heart Failure, Neonate
Introduction
Marfan syndrome (MFS) is an autosomal dominant genetic disorder with skeletal, cardiac and ocular involvement. Mutations in the fibrillin-1 gene (FBN1) on chromosome 15 are responsible for the development of MFS. Complete picture of the syndrome is age-dependant and seldom occurs in younger ages. However, MFS is rarely presented in the neonatal period (Neonatal Marfan syndrome, NMFS). NMFS is the severest phenotype of this disease and has a poor prognosis [1]. The main reason for death is congestive heart failure (CHF) in contrast to the classic MFS. We report on two patients with NMFS who are the first reported from Iran to the best of our knowledge.
Case Presentations
Case 1
A 47-day old male infant was referred due to respiratory distress, food intolerance and weight loss. He was born after 40 weeks of gestation through cesarian section due to fetal bradycardia. He weighed 2800 grams at birth, and had Apgar scores of 7 and 9 at the first and 5th minute, respectively. He was the second child of the family. His mother had an uneventful pregnancy. Ultrasonography reported no abnormality in the fetus. His parents were first cousins. The father had normal body habitus (85 kg, 182 cm), normal echocardiographic results (aortic root 2.53 cm, no mitral abnormality) and normal eye examination. The mother (75 kg, 163 cm, aortic root 1.9 cm) and the only sibling (16.5 kg, 112 cm, aortic root 1.66 cm) were normal as well.
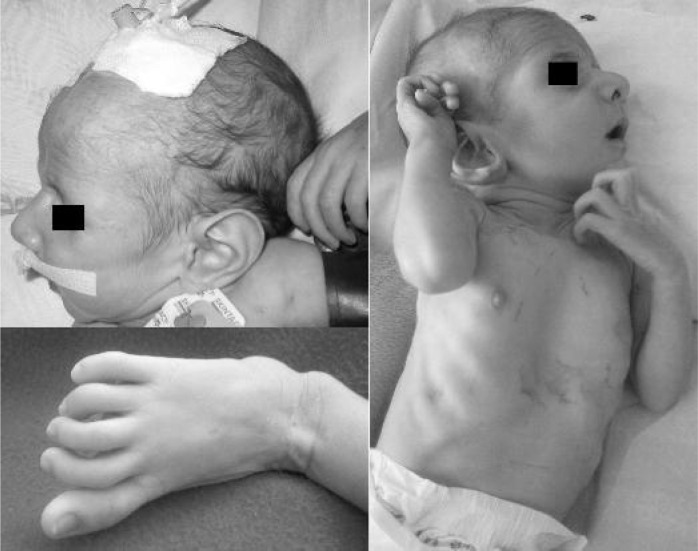
On physical examination, his total height, upper and lower segments, and arm span were 58, 33, 25, and 64 cm, respectively (upper to lower segment ratio 1.32, arm span to height ratio 1.1). His actual and maximum weights were 3.3 and 4 kg, respectively. The anterior fontanel was open (2.5 cm × 3 cm). Ophthalmologic examination revealed mild right strabismus and no lens dislocation. Large ear lobes, enophthalmos, micrognathia and high palate were evident (Fig. 1). The skull was dolichocephalic. Forehead skin was redundant.
Fig. 1.
Photographs of the first patient
The fingers and toes were abnormally long (Fig. 1). Both thumb and wrist signs were positive. The knee and elbow joints had limitation of motion. Mild pectus excavatum was evident (Fig. 1) as well as slight scoliosis. Rales were auscultable on both lungs especially on the bases. Cardiac examination revealed a grade III/VI holosystolic murmur over the left sternal border and gallop rhythm. The abdomen was soft and the liver was palpable 2 cm below the costal margin. There was bilateral inguinal hernia (more prominent on the right).
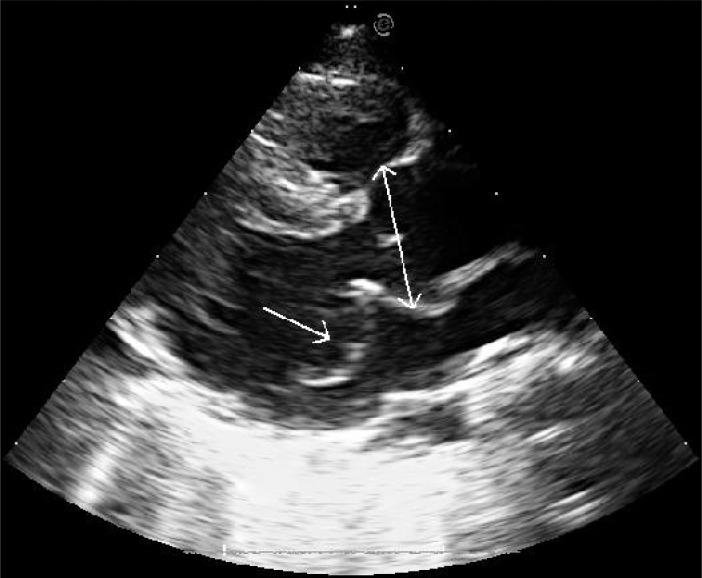
Blood methionine level was normal (13.4 micromol/l, normal range 6-49), as well as the karyotype (46XY). Chest X-ray showed right diaphragm eventration. Fluoroscopy confirmed this diagnosis. Brain sonography yielded no abnormality. Electrocardiography showed left atrial enlargement. Echocardiography revealed dilated aortic root (23 mm, Z value ≥+3) and prolaptic mitral valve with severe regurgitation (Fig. 2). Tricuspid valve was prolaptic and severely regurgitant as well. There was moderate pulmonary hypertension (pressure gradient of tricuspid regurgitation = 43 mmHg).
Fig. 2.
Echocardiogram of the first patient at long-axis parasternal view. Dilated aortic root at sinuses of Valsalva (double-arrow line) and mitral valve prolapse (single-arrow line) are evident.
The patient was hospitalized in our intensive care unit for 24 days. He had respiratory distress needing oxygen, intravenous inotropic agents (dopamine, dobutamine, milrinone), angiotensin-converting enzyme inhibitor (captopril), and diuretic (furosemide). He first received parenteral nutrition and after improvement of the respiratory condition, fortified formula and medium-chain triglycerides to overcome his weight loss. As he had gastroesophageal reflux, relevant medications (omeperazole, ranitidine) were initiated. After improvement of the respiratory distress, he was discharged in a controlled general condition. One week later, he was visited at our outpatient clinic. His general condition showed no change. Another one week later, he died at a local hospital due to fever and severe respiratory distress as reported by his parents.
Case 2
A 90-day old male infant was referred due to respiratory distress. He was born preterm through cesarian section due to maternal oligohydramnios with a weight of 2060 grams. He was the first offspring of the family. His mother had an uneventful pregnancy. Ultrasonographic examinations reported no abnormality in the fetus but oligohydramnios at 35th week of gestation.
His non-relative parents had normal body habitus (father 75 kg and 175 cm, mother 81 kg and 174 cm). They refused ophthalmologic and cardiac examinations. On physical examination, his total height, upper and lower segments, and arm span were 58, 24.5, 23.5, and 62 cm, respectively (upper to lower segment ratio 1.04, arm span to height ratio 1.07). His maximum and actual weights were 4.4 and 3.9 kg, respectively.
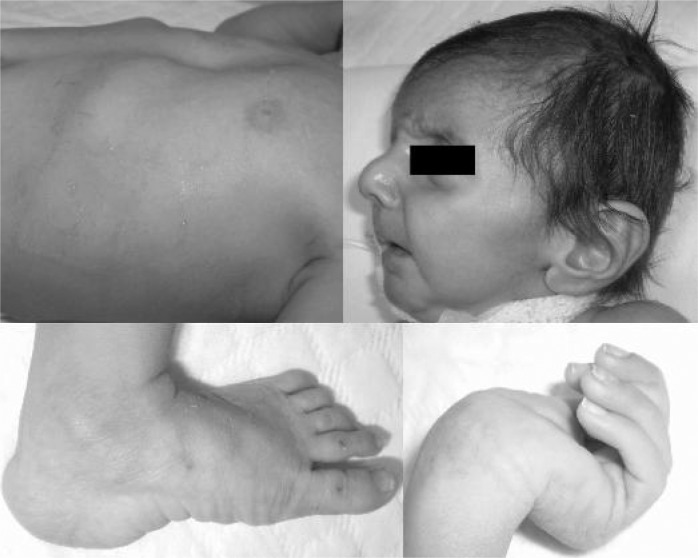
The anterior fontanel was open (0.5 cm × 0.5 cm). Ophthalmologic examination revealed no lens dislocation. Large ear lobes, micrognathia, dolichocephaly, redundant forehead skin and enophthalmos were evident (Fig. 3).
Fig. 3.
Photographs of our second patient.
The fingers were abnormally long but the toes appeared normal (Fig. 3). Both thumb and wrist signs were positive. The elbow joints had limitation of motion. A deep pectus excavatum was evident (Fig. 2). Cardiac examination revealed a grade III/VI holosystolic murmur over the left sternal border and gallop rhythm. The abdomen was soft. His karyotype was normal 46XY.
Chest X-ray showed cardiomegaly. Brain, abdomen and pelvic sonographic examinations yielded no abnormality. Electrocardiography showed right ventricular hypertrophy. Echocardiography revealed dilated aortic root (23.8 mm, Z value ≥+3) and prolaptic mitral valve with severe regurgitation. Tricuspid valve was prolaptic and severely regurgitant. There was severe pulmonary hypertension (pressure gradient of tricuspid regurgitation = 70 mmHg), pulmonary artery dilation (27.9 mm) and an atrial septal defect (7.7 mm in diameter) through an aneurismal interatrial septum with left-to-right shunt.
Despite receiving high-dose intravenous inotropic agents (dopamine, dobutamine, milrinone), furosemide, and captopril for 6 weeks, CHF did not improve and he finally succumbed to the disease.
Discussion
The revised Ghent criteria is the most recently proposed nosology for the diagnosis of MFS [2]. According to this nosology, any patient fulfilling certain combinations of aortic dilation, ectopia lentis, systemic features, family history and FBN1 mutation can be diagnosed as MFS (Table 1). Aortic dilation is defined as aortic root Z value ≥2, except for the combination of family history and aortic dilation in which Z value ≥3 should be applied. Systemic features and their scores consist of wrist and thumb signs (each 1 point, together 3), pectus carinatum (2), pectus excavatum (1), chest asymmetry (1), hindfoot deformity (2), plain pes planus (1), pneumothorax (2), dural ectasia (2), protrusio acetabuli (2), reduced upper to lower ratio and increased arm/height and no severe scoliosis (1), scoliosis or thoracolumbar kyphosis (1), reduced elbow extension (1), facial features (at least 3 of dolichocephaly, enophthalmos, downslanting palpebral fissures, malar hypoplasia, and retrognathia, 1 point), skin striae (1), myopia >3 diopters (1), and mitral valve prolapse (all types, 1 point). At least 7 points are necessary for the fulfillment of systemic features [2]. Our patients met these criteria (Aortic Z score more than 2 and at least 7 scores of the systemic manifestations) [2], therefore a diagnosis of MFS was made.
Table 1.
A simplified table for the diagnosis of Marfan syndrome based on the revised Ghent criteria*
| Criteria | Aortic dilation | Ectopia lentis | Systemic features | Family history | FBN1 mutation |
|---|---|---|---|---|---|
| Aortic dilation | - | MFS | MFS | MFS | MFS |
| Ectopia lentis | MFS | - | - | MFS | MFS*; |
| Systemic features | MFS | - | - | MFS | - |
| Family history | MFS | MFS | MFS | - | - |
| FBN1 mutation | MFS | MFS ‡ | - | - | - |
Some combinations of at least two features out of 5 confirm the diagnosis (marked by MFS). See text for more explanation
Only FBN1 mutations with known risk for aortic dissection are acceptable
There are both genotypic and phenotypic differences between NMFS and classic MFS. Faivre et al showed that mutations in exons 24-31 are present in around 90% of NMFS patients but in only 20% of the classic cases[3]. Family history is negative in 70-100% of classic MFS; however, it is negative in only 20-30% of NMFS patients [4]. Joint contractures, mitral valve prolapse, and mitral, tricuspid, and pulmonary regurgitations were more prevalent in NMFS. However, aortic regurgitation is less frequent. The prognosis of NMFS is poor; mean age at death was 16.3 months in a review of 86 cases while classic MFS patients live several decades[1]. In contrast to the classic syndrome in which main cause of death is aortic dissection or rupture, NMFS patients die mostly from CHF associated with mitral and tricuspid regurgitations[4]. Stheneur et al showed that valvular insufficiencies, diaphragmatic hernia, and mutations in exons 25 and 26 can be associated with worse prognosis in infants with MFS [5].
The main pattern of MFS inheritance is autosomal dominant [6]. However, autosomal recessive inheritance was also reported [7]. This pattern was suspected for our first patient as their parents were first cousins but it could not be confirmed. Patent dutus arteriosus may be seen rarely in infantile MFS and is especially important due to the risks of aneurysm formation, rupture, and acceleration of aortic dilation [8].
Medical therapy of CHF and mitral regurgitations often is unsuccessful to control the symptoms [1]. Mitral valve repair may be difficult due to severe pathology of this valve. Therefore, valve replacement may be necessary for these patients. However, this operation may be difficult in infancy due to the high probability of mortality and morbidity including complete heart block, thrombosis, and stroke [1]. There is a report of mitral valve replacement during infancy: Strigl et al successfully replaced all cardiac valves in a female with MFS [1]. Her mitral valve was replaced first, at 9 months of age. Finally, heart transplantation was reported as a life-saving approach in infantile MFS [9].
Conclusion
The features of both reported patients were classic for NMFS, except the diaphragmatic eventration of the first. Their prognosis was poor, like those reported from elsewhere.
References
- 1.Strigl S, Quagebeur JM, Gersony WM. Quadrivalvar replacement in infantile Marfan syndrome. Pediatr Cardiol. 2007;28(5):403–5. doi: 10.1007/s00246-006-0066-4. [DOI] [PubMed] [Google Scholar]
- 2.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 3.Faivre L, Masurel-Paulet A, Collod-Beroud G, et al. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics. 2009;123(1):391–8. doi: 10.1542/peds.2008-0703. [DOI] [PubMed] [Google Scholar]
- 4.Shih H, Liu W, Chen T. Neonatal Marfan syndrome - A case report. Acta Cardiol Sin. 2004;20:171–5. [Google Scholar]
- 5.Stheneur C, Faivre L, Collod-Beroud G, et al. Prognosis factors in probands with an FBN1 mutation diagnosed before the age of 1 year. Pediatr Res. 2011;69(3):265–70. doi: 10.1203/PDR.0b013e3182097219. [DOI] [PubMed] [Google Scholar]
- 6.Davari MH, Kazemi T, Alimirzaei H, et al. Cardiovascular manifestation of a family with Marfan's syndrome. J Teh Univ Heart Ctr. 2010;6(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- 7.Tayebi N, Tashakor M. A case of Marfan syndrome with severe kyphoscoliosis in recessive autosomal form of inheritance. Rafsanjan Univ Med Sci J. 2008;7(3):207–14. [In Persian] [Google Scholar]
- 8.Zanjani KS, Wong AR, Sadiq M, et al. Device closure of patent ductus arteriosus in Marfan patients: safety and effect on the aortic root diameter. Congenit Heart Dis. 2010;5(5):439–43. doi: 10.1111/j.1747-0803.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 9.Krasemann T, Kotthoff S, Kehl HG, et al. Cardiac transplantation in neonatal Marfan syndrome - a life-saving approach. Thorac Cardiovasc Surg. 2005;53(Suppl 2):S146–8. doi: 10.1055/s-2004-830455. [DOI] [PubMed] [Google Scholar]