Abstract
The keratin-hemidesmosome interaction is crucial for cell-matrix adhesion and migration in several epithelia including the epidermis. Mutations in constituent proteins cause severe blistering skin disorders by disrupting the adhesion complex. Despite extensive studies, the role of keratins in hemidesmosome assembly and maintenance is only partially understood.
Here, we address this issue in keratinocytes in which all keratins are depleted by genome engineering. Unexpectedly, such keratinocytes maintain many characteristics of normal counterparts. The absence of the entire keratin cytoskeleton, however, leads to loss of plectin from the hemidesmosomal plaque and scattering of the hemidesmosome transmembrane core along the basement membrane zone. To investigate the functional consequences, we performed migration and adhesion assays. These revealed that in the absence of keratins, keratinocytes adhere much faster to ECM substrates and migrate ~2 times faster compared to wildtype cells. Re-expression of the single keratin pair K5 and K14 fully reversed the above phenotype. Our data uncover a novel role of keratins in the maintenance of hemidesmosomes upstream of plectin with implications for epidermal homeostasis and pathogenesis. They support the view that the downregulation of keratins observed during epithelial-mesenchymal transition supports the migratory and invasive behavior of tumor cells.
INTRODUCTION
Cell-matrix adhesion is crucial for a variety of biological processes in skin, including skin development, wound healing, inflammation and malignant progression (Ridley et al., 2003). In the skin, matrix adhesion is maintained by actin-associated focal adhesions and keratin-dependent hemidesmosomes. While the former are well characterized, relatively little is known about mechanisms regulating the interactions of keratins during hemidesmosome formation and maintenance. Disruption of the keratin-hemidesmosome multiprotein complex gives rise to epidermolysis bullosa, a group of severe skin disorders caused by mutations in genes encoding either hemidesmosomal proteins or keratins K5 and K14 (Fine, 2010). The binding of plectin to α6β4 integrin is regarded to be essential for the assembly and stability of hemidesmosomes (de Pereda et al., 2009). Plectin interacts with at least three binding sites to β4 integrin mainly through the N-terminal ABD (actin binding)-domain (Koster et al., 2004). Binding to keratin intermediate filaments involves the C-terminal plakin repeat domain. Owing to its binding sites for actin, intermediate filament proteins and microtubules, plectin qualifies to coordinate the dynamics between hemidesmosomes and focal adhesion dynamics through rearranging the actin and keratin cytoskeletons (Tsuruta et al., 2011;Ozawa et al., 2010;Andra et al., 2003).
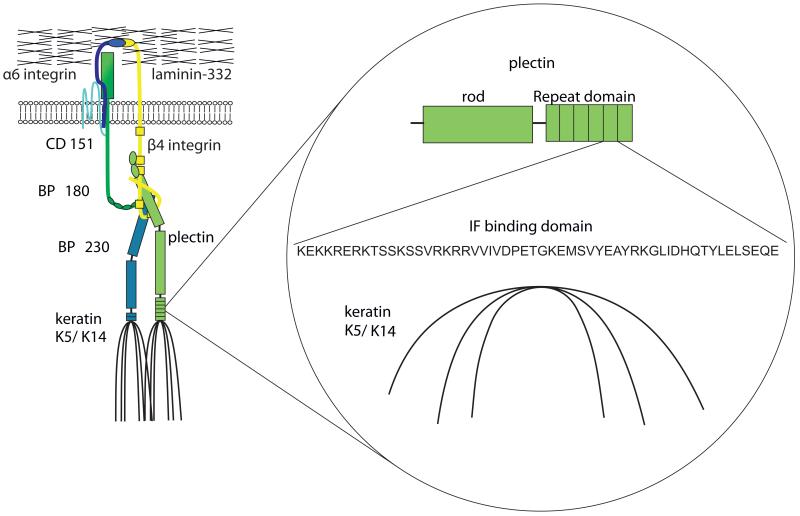
Hemidesmosomes connect the keratin cytoskeleton (K5/K14) with the extracellular matrix (Jones and Green, 1991). The transmembrane proteins BP180, CD 151 and α6β4 integrin directly interact with the extracellular ligand laminin-332 (Jones et al., 1998;Sterk et al., 2000;Borradori and Sonnenberg, 1999). In addition, they contact keratin-associating proteins plectin and BP230 (Green et al., 1992) (Figure 1). The interaction of plectin with α6β4 integrin via the ABD-domain and with keratins via the plakin repeat domains on the C-terminal site is necessary for hemidesmosome assembly (Koster et al., 2004).
Figure 1. Structural organization of hemidesmosomes.
Hemidesmosomes connect the intracellular keratin cytoskeleton (K5/K14) with the extracellular matrix. The participating transmembrane proteins BP180, CD 151 and α6β4-integrin directly interact with the extracellular ligand laminin-332. In addition they stay in contact with keratin-associating proteins plectin and BP230. Thereby the interaction of plectin with α6β4 integrin mainly over the ABD-domain and the association with keratin cytoskeleton by plakin repeat domain are essential for the assembly of hemidesmosomes. Here, we focus on the influence of the keratin cytoskeleton to plectin, which binds on C-terminal site via IF binding domain to keratins.
Keratins form the intermediate filament (IF) cytoskeleton in all epithelia cells and act as supramolecular scaffolds by interacting with cell-matrix and cell-cell contacts (Fuchs and Cleveland, 1998;Simpson et al., 2011). Depending on their primary amino acid sequence, they are grouped in the type I and type II keratin gene families with 28 and 26 members, respectively. Most epithelia express between 6-10 different keratins as pairs of distinct type I and type II proteins (Schweizer et al., 2006;Magin et al., 2007). In the basal epidermis, the keratin pair K5/K14 is expressed, which upon terminal differentiation becomes sequentially replaced by K1/K10 in suprabasal keratinocytes where they support cornified envelope formation (Coulombe and Wong, 2004). In addition to the cytoskeletal function performed by all keratins, certain keratin isotypes act as regulators of cell size, proliferation, protein biosynthesis and organelle distribution (Vijayaraj et al., 2009;Gu and Coulombe, 2007). Wound healing and closure are characterized by profound changes in keratin organization and isotype expression. In stratified epithelia, migration into the wound starts from suprabasal epidermal layers (Coulombe, 1997). During reepithelialization, expression of K6, K16 and K17 is induced, accompanied by the downregulation of differentiation-specific K1 and K10. This correlates with alterations in cell morphology and migratory properties (Paladini et al., 1996;Patel et al., 2006). In addition, it was shown that alterations in K6, K16 and K17 expression also influences wound healing in vivo and keratinocyte migration ex vivo (Wong and Coulombe, 2003;Mazzalupo et al., 2003;Wawersik and Coulombe, 2000). Owing to keratin redundancy, however, isotype-restricted functions of keratins during adhesion and migration, which are critical in wound healing, are not well understood.
During migration and adhesion, hemidesmosomes and focal adhesions are continuously remodeled (Geuijen and Sonnenberg, 2002;Tsuruta et al., 2003). In this context, serine phosphorylation by αPKC of β4 integrin leads to a destabilization and relocalization of hemidesmosomal proteins into lamellipodia (Litjens et al., 2006). Intriguingly, β4 integrin signaling promotes keratinocyte migration by activation of PKB/Akt kinase and members of the mitogen-activated protein kinase family (Kippenberger et al., 2004;Nikolopoulos et al., 2005). Deletion of β4 integrin in mouse keratinocytes enhances migration properties (Raymond et al., 2005). Moreover, focal adhesions can act as mechanosensors and thereby have a major influence on migration (Schwartz, 2010).
Here, we address for the first time the role of the keratin cytoskeleton in localization and regulation of hemidesmosomes during cell adhesion and migration. To that end, we compare murine keratinocyte cell lines lacking the entire set of keratins or re-express the single keratin pair K5/K14 and investigate hemidesmosome maintenance, their adhesive and migratory properties in comparison to normal keratinocytes (Vijayaraj et al., 2009).
RESULTS
Characterization of keratin-free keratinocytes
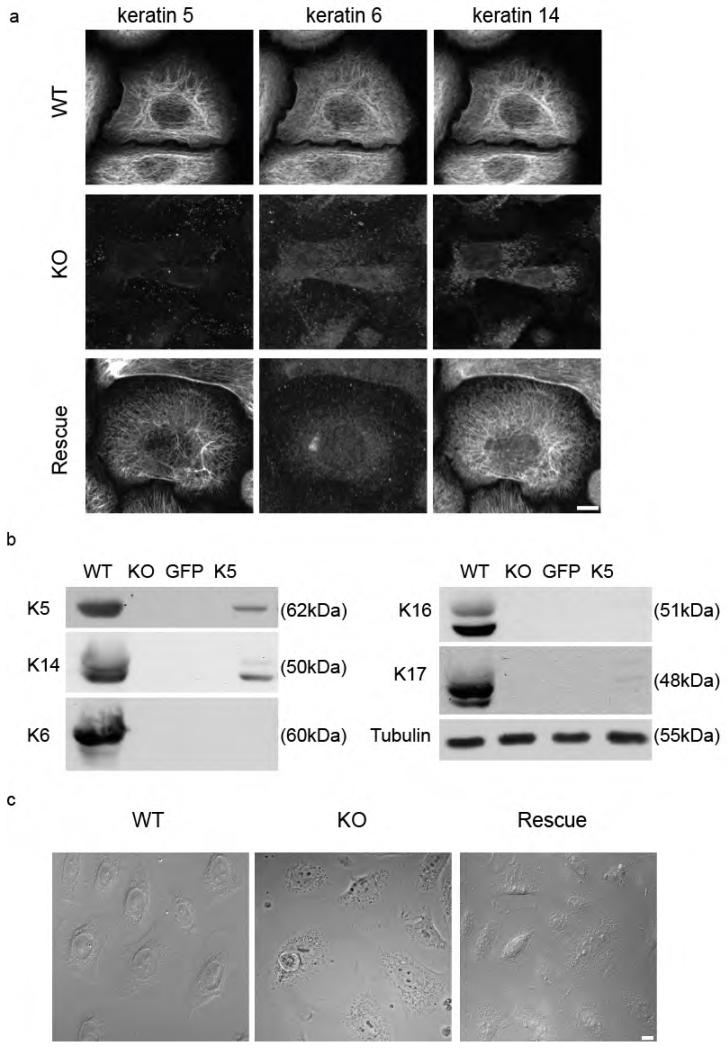
To analyze the functional importance of keratins for keratinocyte morphology, adhesion and migration, we deleted all type II keratin genes which at the protein level led to loss of the entire keratin protein family (Vijayaraj et al., 2009). Here, we report the first characterization of murine keratinocyte lines without keratins (KO cells), one that stably re-expresses the single keratin pair K5/K14 and a wildtype (WT) keratinocyte line (a full description of cells will be presented elsewhere). Absence of all keratins in keratin-free keratinocytes was confirmed by immunofluorescence and western blotting (Figure 2). Rescue cell lines showed co-localization of K5/K14, forming long IF, whereas K6 were absent (Figure 2a). At the biochemical level, the amount of K5 and K14 reached ~ 13 % of corresponding amounts in wildtype cells (Figure 2b). Unexpectedly, loss of keratins had only a slight effect on the morphology of keratinocytes grown under low calcium conditions (Figure 2c).
Figure 2. Characterization of keratin-free keratinocytes.
(a) Immunofluorescence analysis of the cells stained for keratin 5, keratin 6 and keratin 14 were done. (b) Absence of keratins in keratin-free cells and re-expression of keratin 5 in rescued cells were proved by western blotting. (c) Morphology of wildtype, keratin-free and keratin 5 re-expressing keratinocytes are shown by phase contrast images. Bar, 10 μm.
Altered distribution of hemidesmosomal proteins in keratin-free keratinocytes
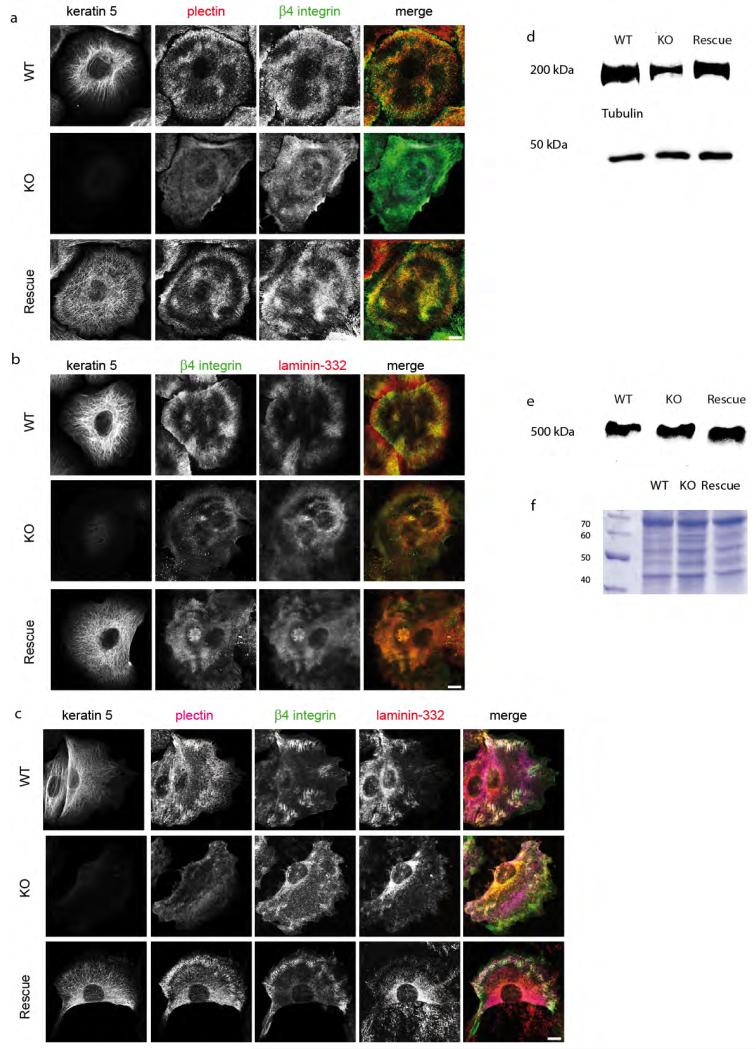
To investigate whether the keratin cytoskeleton affected hemidesmosomes, we analyzed the distribution of plectin, β4 integrin, BP180, BP230 and the extracellular ligand laminin-332 during cell migration, at the migrating front, where hemidesmosomes display a dynamic behaviour and in the middle of the cell monolayer, where static adhesion prevails. Most remarkably, plectin was completely dissociated from β4-integrin in all settings (Figure 3a). At the migrating front of KO cells, staining for β4-integrin, BP180, BP230 and the extracellular ligand laminin-332 revealed that hemidesmosomal proteins were no longer clustered. Further, β4-integrin had lost its patchy localization in migrating KO cells but maintained its patchy localization in static cells (Figure 3c). At the same time, co-localization of β4-integrin and extracellular laminin-332 was maintained in KO cells (Figure 3b). Similar to its β4-integrin binding partner, α6-integrin failed to co-localize with plectin in KO keratinocytes (Supplementary Figure S1a). At the same time, distribution of BP180 and BP230 showed a partial co-localization with β4-integrin in wildtype keratinocytes (Supplementary Figure S1b and c). In contrast, in KO keratinocytes the co-localization of cytosolic BP230 with keratins at HD was reduced compared to wildtype cells. Loss of keratins did not influence the partial co-localization of transmembrane BP180 with β4-integrin.
Figure 3. Altered localization of hemidesmosomes in keratin-free keratinocytes.
Immunofluorescence analysis of wildtype (WT), keratin-free (KO) and keratin 5 re-expressing (Rescue) keratinocytes stained against keratin 5, plectin, β4-integrin and laminin-332 to visualize hemidesmosomes structures. (a) IF analysis of wildtype and keratin 5 re-expressing keratinocytes were characteristic patchy pattern of hemidesmosomal proteins can be seen. Note KO cells have a clustered localization of β4-integrin in the cell layer, but no co-localization with plectin. (b) Staining of extracellular ligand laminin-332 and β4-integrin shows no difference between all three cell types. (c) Keratinocytes immunolabeled for hemidesmosomal proteins on the migrative front. Interestingly, β4-integrin is missing in HD structures of migrating keratin-free cells. (d and e) Total cell lysates analyzed by western blotting using (d) β4-integrin and (e) plectin antibodies. (f) Loading control of protein lysates by a coomassie gel. Bar, 10 μm.
Stable re-expression of K5 and K14 in KO keratinocytes reconstituted the typical localization of plectin to β4- and α6-integrin and clustering of hemidesmosomal proteins, demonstrating keratin dependence of the phenotype (Figure 3). Plectin again co-localized with the keratin cytoskeleton in keratin 5 re-expressing keratinocytes, similar to wildtype cells. In addition, BP 180 and BP230 displayed partial colocalization with hemidesmosomal patches, as seen in wildtype keratinocytes (Supplementary figure S1). By western blotting, presence or absence of keratins did slightly decrease the amount of β4-integrin but did not affect plectin (Figure 3d and e). Together these results suggest that the presence of keratins is necessary for the maintenance of plectin at hemidesmosomes and the proper clustering of hemidesmosomes itself.
Keratins slow down matrix adhesion of keratinocytes
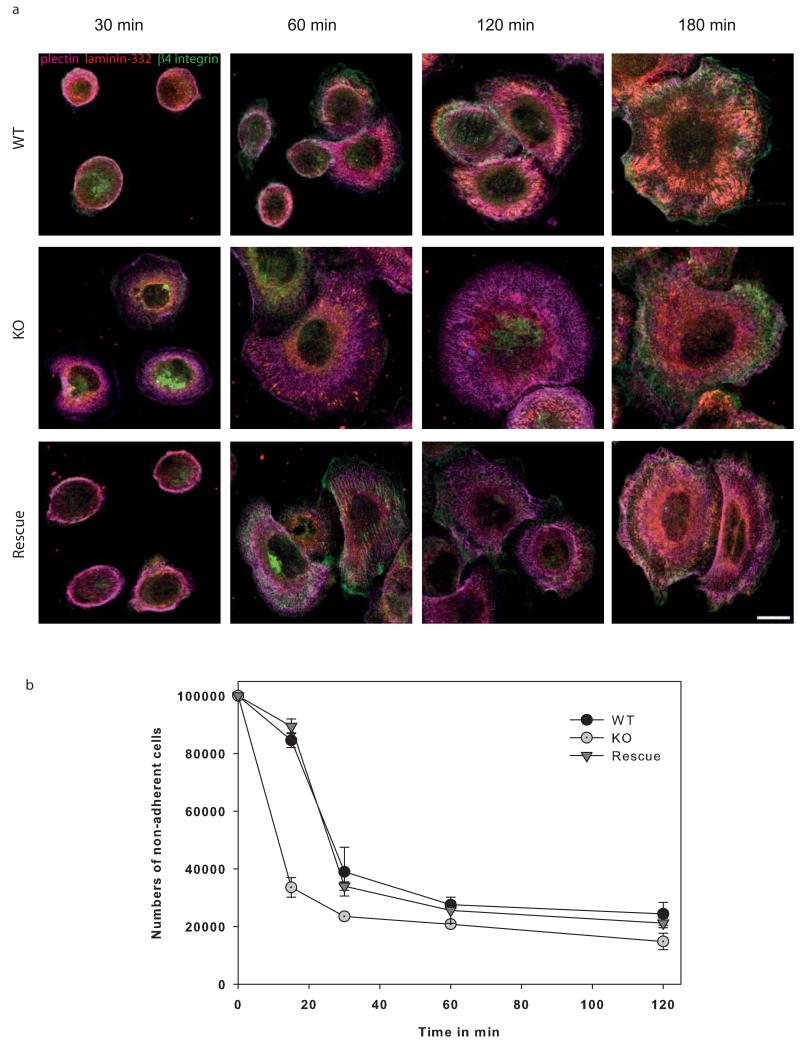
In view of the altered distribution and composition of hemidesmosomes in the absence of keratins, we analyzed keratinocyte adhesion. Wildtype keratinocytes spread and attached completely in ~3h (Figure 4a) and displayed hemidesmosomes, visualized by co-localization of plectin, β4-integrin and laminin-332 (Supplementary Figure S2). In contrast, KO keratinocytes were fully adherent after ~1h but lacked complete hemidesmosomes in which plectin and β4-integrin would co-localize in patches. Both in wildtype and keratin-free keratinocytes, the spreading started with the formation of membrane protrusions. In this setting, the transmembrane β4-integrin is pushed forward first, followed by plectin. Following primary attachment, β4-integrin and laminin-332 became incorporated into nascent hemidesmosomes. Quantification of the adhesion revealed remarkable differences between wildtype and KO keratinocytes, underlining the faster adhesion of the latter (Figure 4b) Similar to wildtype keratinocytes, GFP-K5 re-expressing cells adhered completely in ~3h. Thus, GFP-K5 re-expressing cells have similar adhesion properties like wildtype cells. In summary, absence of keratins supports the faster adhesion of keratinocytes.
Figure 4. Keratin-free cells adhere much faster.
Immunofluorescence analysis of wildtype and keratin-free keratinocytes in the course of adhesion process after 30 min, 1h, 2h and 3h are shown. Therefore, cells were immunolabeled for hemidesmosomal proteins plectin, β4-integrin, the extracellular ligand laminin-332 and keratin 5. (a) Hemidesmosomal structures visualized by plectin and β4-integrin can be seen after 3h in wildtype keratinocytes. In contrast, keratin-free cells are already adherent after 1h. Moreover, plectin and β4-integrin co-localization is missing in keratin-free keratinocytes. (b) Quantitative analysis of non-adherent cells on specific time points. Bar, 10 μm.
Keratins repress cell migration in keratinocytes
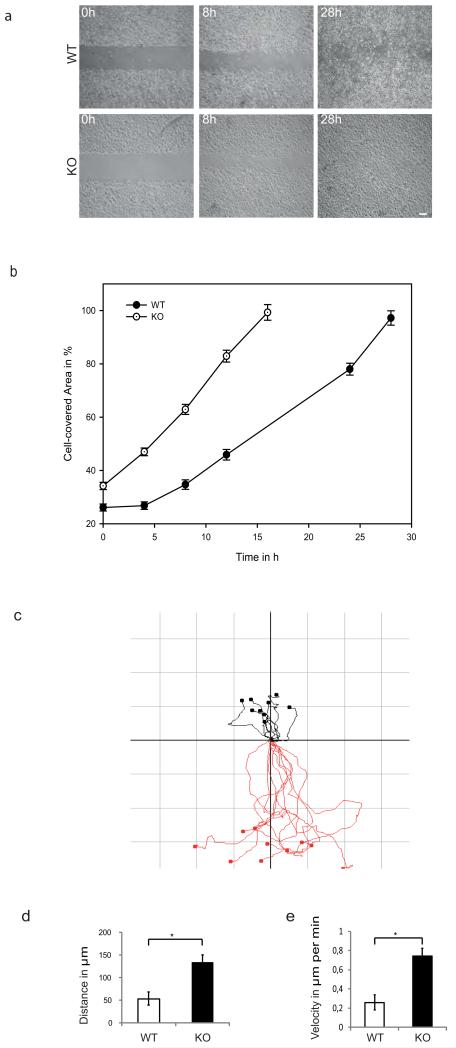
Cell-matrix contacts play a vital role in cell motility processes. Thus, we further analyzed whether the altered composition and distribution of hemidesmosomes affected migration properties of wildtype and KO keratinocytes, applying an established in vitro gap closure assay. KO keratinocytes migrated nearly two-fold faster than wildtype cells, taking ~16 hours to close the gap, whereas wildtype cells required ~28 hours (Figure 5a, 5b). The difference became noticeable already ~8 hours into the assay. Next, we directly compared migration of KO and wildtype keratinocytes using time-lapse video microscopy. Tracking of individual cells to determine directionality of migration revealed differences between wildtype and KO keratinocytes, displayed in vector diagrams (Figure 5c). Further, mean distance and velocity of individual cells was determined (Figure 5d and e). Intriguingly, KO cells migrated faster and displayed an altered directionality compared to wildtype cells.
Figure 5. In vitro wound healing assay of wildtype and keratin-free keratinocytes.
(a) The cell gap was closed after 28h by wildtype keratinocytes, whereas keratin-free keratinocytes close the gap already after 16 h. (b) Wound areas were measured and plotted against the time point. (c) Vector diagram of in vitro wound healing assay of wildtype (black) versus keratin-free keratinocytes (red) depicting migration tracks of 10 individual cells by video analysis. Axes of vector diagrams= 150 μm. (d) Average of the travelled distance of wildtype and keratin-free keratinocytes indicate a longer migrated way of keratin-free cells. (e) Average of velocity of keratin-free cells is enhanced as well. Values are mean ± SEM of three independent experiments.* P < 0.01. Bar, 10 μm.
Genetic rescue confirms keratin dependence
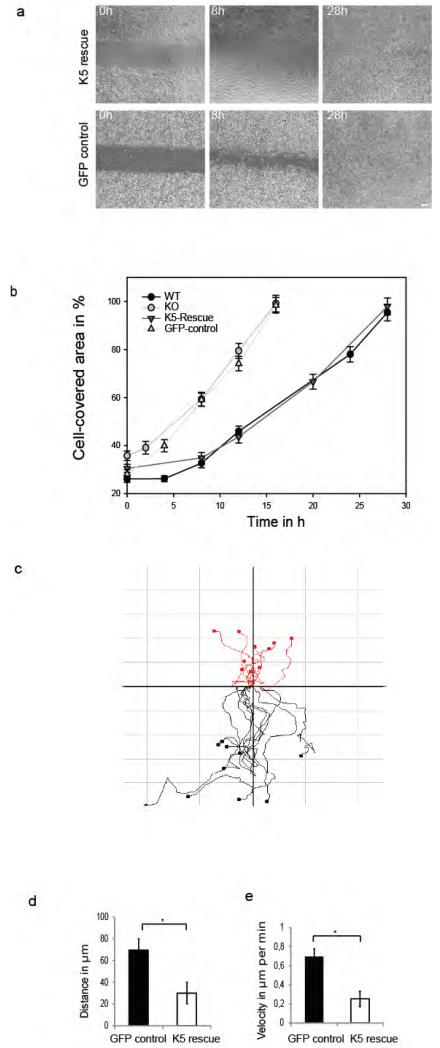
Given the complexity of genetic and epigenetic alterations in KO and control keratinocytes, we were concerned whether changes in plectin distribution, adhesion and migration resulted from the keratin status of both cell lines or from other experimental manipulations. To address this, we performed gain-of-function experiments by stable expression of GFP-K5 in KO keratinocytes, using lentiviral vectors. This leads to a stabilization of endogenous K14 (Figure 2a). As control, KO cells were transfected with the same vector expressing only GFP. Subsequent experiments were performed with cell lines homogenously expressing either GFP-K5 or GFP only. Therefore we investigated migratory properties of K5 re-expressing versus KO keratinocytes using the established in vitro gap closure assay (Figure 6a). Consistent with a major role of keratins in restricting migration, K5 re-expressing KO keratinocytes closed the gap in 28h similar to wildtype cells. In support, KO cells expressing GFP only closed the gap in 16h. Next, video analysis of migrating K5 re-expressing versus GFP control keratinocytes in same approach was performed (Figure 6b). Keratin 5 re-expressing cells are illustrated by red lines and GFP control cells by black lines. This revealed that K5 re-expressing cells displayed an altered directionality, restoring the mode of wildtype cells. Moreover, re-expression of K5 reduced distance and velocity of migrating cells (Figure 6c and 6d). Collectively, our data show that the single keratin pair K5 and K14 is sufficient to maintain keratinocytes adhesion and migration and to localize plectin to β4-integrin.
Figure 6. Functional properties of rescued keratin 5 re-expressing keratinocytes.
Cells are either transfected with a GFP-K5 or a GFP-control vector. (a) In vitro wound healing assay of keratin 5 re-expressing and GFP-control keratinocytes. GFP-control keratinocytes close the cell gap after 16 h, whereas GFP-K5 cells need 26 hours. (b) Wound areas were measured and plotted against the time point. (c) Vector diagram illustrating tracked individual cells of in vitro wound healing assay of keratin 5 re-expressing cells (red) versus GFP control keratinocytes (black) by video analysis. Axes of vector diagrams= 100 μm. d) Average of the travelled distance and velocity (e) of tracked GFP-K5 and GFP control keratinocytes. Values are mean ± SEM of three independent experiments. * P < 0.01. Bar, 10 μm.
In summary, we demonstrate a requirement of the keratin cytoskeleton in the maintenance and distribution of hemidesmosomes upstream of plectin, which has implications for keratinocyte adhesion and migration.
DISCUSSION
The role of keratins in hemidesmosome maintenance, cell adhesion and migration is only partially understood. Comparing keratinocytes that either lack the entire keratin protein family or express the single keratin pair K5 and K14 to normal keratinocytes, we uncovered a new role of keratins in hemidesmosome integrity which is mediated through plectin and β4-integrin.
Keratins are necessary to maintain hemidesmosomes
Our data provide the first evidence for a crucial role of keratins in the localization of hemidesmosomal proteins, based on the observation that loss of keratins caused an altered distribution of plectin and β4-integrin. In line with the established function of plectin in hemidesmosome assembly and maintenance, our data support a novel role of keratins acting through plectin (Koster et al., 2003). In apparent contrast, plectin knockout keratinocytes displayed an altered keratin filament network (Osmanagic-Myers et al., 2006). Possibly, lack of keratins triggers a conformational change of plectin at its C-terminal IF-binding site which might inhibit the N-terminal binding to β4-integrin (de Pereda et al., 2009). At the same time, the interaction between β4-integrin and laminin-332 was unaffected in KO keratinocytes which is consistent with the binding to laminin-332 of a β4-integrin mutant unable to bind plectin (Geuijen and Sonnenberg, 2002). The altered distribution of β4-integrin in KO keratinocytes could be caused by an enhanced endocytosis and recycling of cell-matrix contacts during migration (Ridley et al., 2003). Our data are not at odds with the maintenance of core hemidesmosomes in EBS patients or in K5 and K14 KO mice (Fine, 2010;Lloyd et al., 1995;Peters et al., 2001). In these settings, residual keratins are still expressed, whereas here, keratins are absent. Collectively, our results show that keratins are necessary for the maintenance of intact hemidesmosomes, most likely upstream of plectin. Of note, the single keratin pair K5 and K14 was sufficient for rescue this function. Whether maintenance of hemidesmosomes relies directly or indirectly on keratins requires more detailed investigation.
Keratins negatively regulate adhesion and migration
Hemidesmosomes provide stable attachment and therefore localization of HD is necessary for proper adhesion of keratinocytes (Jones and Green, 1991;Litjens et al., 2006;Borradori and Sonnenberg, 1999). Altered distribution and dynamics of hemidesmosomal proteins can result in a faster adhesion of keratin-free cells, consistent with the reduced, plectin-dependent clustering of β4-integrin that was observed in dynamic, i.e. migrating keratinocytes. In fact, KO keratinocytes adhered faster than wildtype cells, migrated twice as fast and showed a reduced directionality during migration. Mechanistically, this could be due to an increased turnover of hemidesmosomes in KO keratinocytes. Alternatively, altered distribution of HD might affect distribution and functionality of focal adhesions, possibly through plectin (Ozawa et al., 2010;Tsuruta et al., 2011). Further experiments are needed to clarify the mechanism.
The first hemidesmosomal protein pushed forward along the cell membrane by lamellipodia formation is β4-integrin, followed by plectin. In KO keratinocytes, co-localization of β4-integrin and plectin did not take place. However, laminin-332 was established in hemidesmosomal patches. While a previous study suggested that α6β4-integrin forms hemidesmosomal clusters depending on laminin-332 deposition along the cell surface, (Geuijen and Sonnenberg, 2002), our data support a novel role of keratins during this process. by the keratin cytoskeleton.
Increased cell motility in KO keratinocytes can be caused by several mechanisms including altered localization of hemidesmosomal proteins. In support, both plectin and β4-integrin knockout keratinocytes displayed enhanced migration (Raymond et al., 2005;Osmanagic-Myers et al., 2006). Relocalization of bullous pemphigoid proteins BP180 and BP230 can also contribute to a migratory phenotype (Guo et al., 1995;Tasanen et al., 2004). Furthermore, α6β4-integrin was shown to stabilize lamellipodia and therefore affects cell motility (Rabinovitz and Mercurio, 1997). In addition, it was suggested that clustering of β4-integrin and its interaction with laminin-332 determines whether β4-integrin inhibits cell migration (Geuijen and Sonnenberg, 2002). Recent data support a role of hemidesmosomes as signaling platforms in which α6β4-integrin signaling is mediated through plectin. Plectin interacts with RACK1, which is linked to the PKCδ signal pathway (Osmanagic-Myers et al., 2006). Furthermore, a role of hemidesmosomes in upstream signaling of the kinases PKB and Erk was shown (Kippenberger et al., 2010). It is conceivable that loss of the keratin cytoskeleton and therefore decreased maintenance of hemidesmosomes also influences intracellular signaling pathways, which affects the migration of keratinocytes. The fact that plectin, in addition to keratins, additionally interacts with the actin cytoskeleton, provides an alternative hypothesis. Intriguingly, it cannot bind to β4-integrin and actin at the same time as both bind through the ABD domain (de Pereda et al., 2009). Thereby, loss of keratins might affect plectin’s interaction with the actin cytoskeleton, suggesting enhanced adhesion and migration of keratinocytes through additional mechanisms. In support of our data, high expression of keratin 16 in cultured skin explants was accompanied by decreased migration (Wawersik and Coulombe, 2000). In contrast, K17 null embryos showed delayed wound closure (Mazzalupo et al., 2003). Moreover, skin explant cultures of K6α/K6β KO mice migrated faster compared to wildtype counterparts (Wong and Coulombe, 2003). The apparent differences in previous experiments are resolved by our study which precludes compensation by other keratins.
The current study provides the first evidence for a major role of keratins in hemidesmosome maintenance through plectin, with implications for cell adhesion and migration. An attractive hypothesis to be tested is whether the keratin-hemidesmosome complex has a role in mechanotransduction.
METHODS
Cell culture
Isolation of primary mice keratinocytes of knockout and wildtype cells will be described elsewhere (Vijayaraj et al., 2009). Keratinocytes were grown in FAD Medium Chelex-treated (Brennan et al., 1975) (FAD low Ca, Biochrom, Berlin, Germany) supplemented with 10 % FCS Gold (PAA, Pasching, Austria), 0.18 mM Adenine, 0.5 μg/ml Hydrocortison, 5 μg/ml Insulin, 100 pM Choleratoxin (Sigma, St. Louis, USA), 10 ng/ml EGF,100 U/ml and 100 μg/ml Penicillin/Streptomycin and 2 mM Glutamax (Invitrogen, Darmstadt, Germany), 5% CO2 at 32°C. Cells were cultured on collagen I (rat tail, Invitrogen) coated cell culture dishes. Keratin-free keratinocytes stably expressing GFP-K5 and GFP were generated by lentiviral transduction essentially as described (Stöhr et al., 2012 in print).
Antibodies
See Supplementary Methods in the Supplementary Information online.
Immunofluorescence staining
Cells were fixed for 5 min in −20°C methanol, 30s in −20°C acetone. Cells were stained with the primary antibody and incubated for one hour. All antibodies were diluted in TBS containing 1% BSA. Afterwards cells were incubated with the secondary antibody for 30 min and mounted with mounting medium (Dianova, Hamburg, Germany). Images were acquired using an AxioImager Z2 equipped with Zeiss Plan-Apochromat 63×/1.4 oil and recorded with an AxiocamMR camera and with an Axioplan 2 microscope (Carl Zeiss, Goettingen, Germany). Image analysis and processing were performed using the AxioVision 4.8 software Samples were analysed using a fluorescence laser-scanning confocal microscope (LSM 780; Carl Zeiss). Each fluorochrome was scanned individually in single optical sections (“sequential scan”) to avoid cross-talk between channels. For confocal images Pinhole “airy 1” Zeiss standard settings were used to receive signal only from the focal plane. Analysis and processing of acquired images were performed using the Zen-software (Carl Zeiss). Images were cropped and analyzed in Adobe Photoshop CS5 software and Adobe Illustrator CS5 was used for figure design.
In Vitro Wound Healing Assay
For the in vitro wound healing assay an Ibidi Culture insert was used to create a defined 500 μm cell gap (Ibidi, Martinsried, Germany). Briefly, cells were grown in the Ibidi culture insert to confluency on a collagen coated dish. Migration of cells was analyzed on a Nikon Eclipse Ti-S inverted microscope (Nikon, Melville, NY). Images were taken every 2 hours over a total period of 30 h. Images were collected and percentage of wound closure was determined by digital analysis. In addition, wound closure was monitored by time-lapse video microscopy (Nikon Eclipse Ti-microscope). Images were taken in 15-minute intervals over 24-48 hours. 10 single cells per cell type were tracked using Image J and the mean migratory distance and velocity was calculated. Statistical analyses and significance were determined using the SigmaPlot 11 software.
Western blot analysis
See Supplementary Methods in the Supplementary Information online.
Supplementary Material
ACKNOWLEDGMENTS
Work in the Magin lab is supported by the DFG (MA1316-9/3, MA1316-15, INST 268/230-1), the BMBF (network EB) and the Translational Center for Regenerative Medicine, TRM, Leipzig, No 0315883). We thank the Core Facility Imaging for support with live cell imaging and cell migration analyses.
Abbreviations
- ABD
actin binding domain
- BP
bullous pemphigoid
- EBS
epidermolysis bullosa simplex
- HD
hemidesmosome
- K
keratin
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Author Contributions
K.S. conceived the study, designed and performed the experiments, analyzed data, prepared the figures, and wrote the paper. W.R. designed and assisted experiments. F.L. performed immunofluorescence analysis and immunoblots to characterize keratinocytes. M.L. and S.H. generated GFP-K5 and GFP control keratin-free keratinocytes stable cell lines. T.M.M. contributed expertise, designed experiments, and wrote the paper.
REFERENCES
- 1.Andra K, Kornacker I, Jorgl A, Zorer M, Spazierer D, Fuchs P, Fischer I, Wiche G. Plectin-isoform-specific rescue of hemidesmosomal defects in plectin (−/−) keratinocytes. J Invest Dermatol. 2003;120:189–197. doi: 10.1046/j.1523-1747.2003.12027.x. [DOI] [PubMed] [Google Scholar]
- 2.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JK, Mansky J, Roberts G, Lichtman MA. Improved methods for reducing calcium and magnesium concentrations in tissue culture medium: application to studies of lymphoblast proliferation in vitro. In Vitro. 1975;11:354–360. doi: 10.1007/BF02616371. [DOI] [PubMed] [Google Scholar]
- 4.Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- 5.Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol. 2004;6:699–706. doi: 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- 6.de Pereda JM, Lillo MP, Sonnenberg A. Structural basis of the interaction between integrin alpha6beta4 and plectin at the hemidesmosomes. EMBO J. 2009;28:1180–1190. doi: 10.1038/emboj.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine JD. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213–222. doi: 10.1111/j.1749-6632.2010.05463.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 9.Geuijen CA, Sonnenberg A. Dynamics of the alpha6beta4 integrin in keratinocytes. Mol Biol Cell. 2002;13:3845–3858. doi: 10.1091/mbc.02-01-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green KJ, Virata ML, Elgart GW, Stanley JR, Parry DA. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992;14:145–153. doi: 10.1016/s0141-8130(05)80004-2. [DOI] [PubMed] [Google Scholar]
- 11.Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 13.Jones JC, Green KJ. Intermediate filament-plasma membrane interactions. Curr Opin Cell Biol. 1991;3:127–132. doi: 10.1016/0955-0674(91)90175-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Kippenberger S, Hofmann M, Zoller N, Thaci D, Muller J, Kaufmann R, Bernd A. Ligation of beta4 integrins activates PKB/Akt and ERK1/2 by distinct pathways-relevance of the keratin filament. Biochim Biophys Acta. 2010;1803:940–950. doi: 10.1016/j.bbamcr.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Kippenberger S, Loitsch S, Muller J, Guschel M, Kaufmann R, Bernd A. Ligation of the beta4 integrin triggers adhesion behavior of human keratinocytes by an “inside-out” mechanism. J Invest Dermatol. 2004;123:444–451. doi: 10.1111/j.0022-202X.2004.23323.x. [DOI] [PubMed] [Google Scholar]
- 17.Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J Cell Sci. 2003;116:387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- 18.Koster J, van WS, Kuikman I, Litjens SH, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell. 2004;15:1211–1223. doi: 10.1091/mbc.E03-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd C, Yu QC, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E. The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol. 1995;129:1329–1344. doi: 10.1083/jcb.129.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Mazzalupo S, Wong P, Martin P, Coulombe PA. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev Dyn. 2003;226:356–365. doi: 10.1002/dvdy.10245. [DOI] [PubMed] [Google Scholar]
- 23.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Puri C, Tacchetti C, Giancotti FG. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–6102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174:557–568. doi: 10.1083/jcb.200605172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozawa T, Tsuruta D, Jones JC, Ishii M, Ikeda K, Harada T, Aoyama Y, Kawada A, Kobayashi H. Dynamic relationship of focal contacts and hemidesmosome protein complexes in live cells. J Invest Dermatol. 2010;130:1624–1635. doi: 10.1038/jid.2009.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996;132:381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel GK, Wilson CH, Harding KG, Finlay AY, Bowden PE. Numerous keratinocyte subtypes involved in wound re-epithelialization. J Invest Dermatol. 2006;126:497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- 28.Peters B, Kirfel J, Bussow H, Vidal M, Magin TM. Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex. Mol Biol Cell. 2001;12:1775–1789. doi: 10.1091/mbc.12.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional beta4-integrin knockout mice. J Cell Sci. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- 31.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasanen K, Tunggal L, Chometon G, Bruckner-Tuderman L, Aumailley M. Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am J Pathol. 2004;164:2027–2038. doi: 10.1016/S0002-9440(10)63762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuruta D, Hashimoto T, Hamill KJ, Jones JC. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J Dermatol Sci. 2011;62:1–7. doi: 10.1016/j.jdermsci.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuruta D, Hopkinson SB, Jones JC. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton. 2003;54:122–134. doi: 10.1002/cm.10089. [DOI] [PubMed] [Google Scholar]
- 39.Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wawersik M, Coulombe PA. Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes. Mol Biol Cell. 2000;11:3315–3327. doi: 10.1091/mbc.11.10.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.