Abstract
Cellular immune responses directed against protozoan parasites are key for controlling pathogen replication and disease resolution. However, an uncontrolled, or improperly controlled, response can be deleterious to the host in terms of both allowing for the establishment of pathology, as well as less effective establishment of memory responses. Human cutaneous leishmaniasis is a disease caused by the infection with Leishmania spp. following a bite from the sandfly, the natural vector of this disease. Tens of millions worldwide are currently infected with Leishmania and no effective vaccines have been developed to date. In the face of the complexity presented by the interaction between a host (humans) with the parasite, Leishmania, and the fact that this parasite is inoculated by another complex, biologically active, vector, the sandfly, it is clearly important to study the immunoregulatory mechanisms that are induced in humans naturally infected by this parasite if we hope to develop effective vaccines and immunotherapeutic treatments in the future. Our laboratory has focused over the years on the study of the local and systemic T cell response during the first episode of cutaneous leishmaniasis suffered by individuals before they undergo antimony treatment. The goal of this review is to briefly outline our findings with hopes of putting our most recent studies concerning the dichotomy between alpha/beta TCR and gamma/delta TCR expressing, CD4- CD8- (double negative-DN) T cells in the context of a balanced immune response against Leishmania and to discuss the implications of these findings toward our understanding of human leishmaniasis.
Keywords: cutaneous leishmaniasis, human, cytokines, T lymphocytes, Th1, Th2, NK T cells, double negative T cells
Human leishmaniasis- a complex disease resulting from the host-parasite-vector interaction
Human leishmaniasis is caused by the infection with the obligate intracellular protozoan parasite, Leishmania, and is expressed as one or more of several possible clinical forms including principally the visceral and tegumentary diseases (localized cutaneous, mucosal or disseminated) (1). Several factors influence the progression of an infected individual into these clinical forms of disease including, but not limited to, host genetics (protective and susceptibility factors), host environment and previous immunological experience, parasite genetics (unique species and sub-strain characteristics leading to tropism differences, different virulence factors and immunogenicity differences), and the influence of the vector, phlebotomine sandflies. Obviously, the elucidation of the role that all of these factors have on the progression of disease is a daunting task requiring the work of specialists in many fields of research, and here we will focus our discussion on the immune response of individuals infected with Leishmania braziliensis and presenting with cutaneous disease. The majority of the results we discuss were obtained by studying peripheral blood mononuclear cells (PBMC) or lesions from individuals during their first manifestations of cutaneous disease as identified by the presence of an ulcerated lesion. The choice to study this clinical form is several fold, based on both practical considerations and on scientific grounds. Practically, it is the most prevalent clinical form in Brazil and scientifically, it is the clinical form that, amongst the clinical forms caused by L. braziliensis infection, is the most benign and leads to a fairly high rate of cure and subsequent immunologic protection amongst the population (2, 3) (4). Thus, by studying individuals with this clinical form, we can have an idea as to the nature of the immune response that leads to the formation of a lesion, followed by control of the infection, and long lived immunity in a high percentage of individuals.
Protection vs. Pathology vs. Memory…a three-way balancing act
The study of the cellular immune response and determination of cytokine profiles in both murine models and in humans has been determined over the years through the application of a number of quantitative and semi-quantitative methodologies. These methods have ranged from single-cell cytoplasmic staining of cytokines within specific cell populations to ELISA from culture supernatants or body fluids, and quantitative PCR. In tissues investigators have used in situ detection of cytokines by confocal or fluorescent microscopy and cytokine specific probes. For quite some time it has been known that a biased Th1 response in animal models of Leishmania major infection lead to control of parasite replication and cure of the animal, while a biased Th2 response leads to an exacerbated infection (5) (6) (7). This picture has grown in complexity over the years and we now appreciate nuances of the model including the important role of IL-10 in regulating the response (8, 9). Over the years, dozens of studies in animal models have helped to elucidate the cellular and molecular mechanisms involved during the immune response against Leishmania spp, and have pointed to the importance of many factors that influence the progression of disease following infection with Leishmania (10–14). These factors vary from the strain of the infecting parasite, to the method of inoculation, and host genetics, to name a few. Thus, these models continue to provide insights towards understanding basic mechanisms of immunity and memory, as well as clarify factors involved in complex interactions between host and pathogen. However, interpretation and extrapolations of results obtained from animal models to the human realm should be performed carefully due to complex differences between the systems.
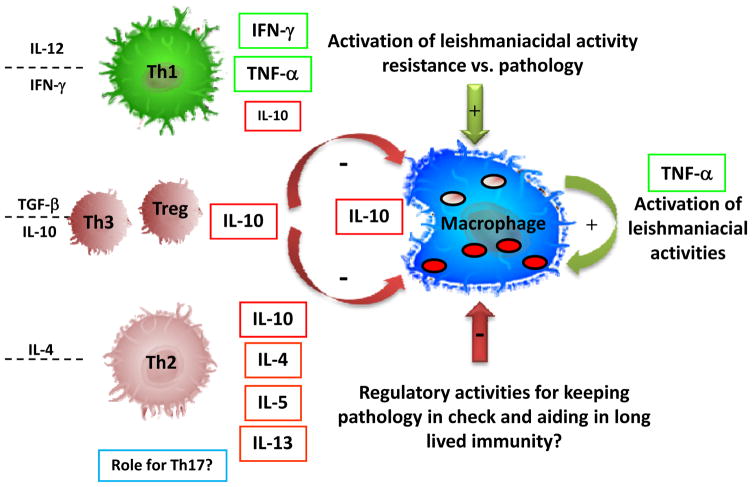
It has recently been demonstrated in a number of animal models that cellular immune responses resulting in a sterile cure of Leishmania major infection, lead to poor long term protection or memory responses (11, 15, 16). Moreover, in human cutaneous disease caused by L. braziliensis infection, we have found that an exacerbated, Th1 response (determined using flow cytometry, ELISA, and confocal microscopy) is associated with severe pathology in mucosal disease (17–19), and even in relatively controlled responses in cutaneous disease, higher frequencies of IFN-gamma producing CD4+ T cells, as determined using flow cytometry, are associated with larger lesions (20). Thus, it seems likely that a balance between responses that induce leishmaniacidal activities, those that could induce pathology, and those that maintain persistence, may be the most desirable response in the natural human response directed against L. braziliensis infection (Figure 1).
Figure 1. CD4+ T lymphocyte balance and activation of macrophages for control of Leishmania.
Classically, Th1 and Th2 CD4+ T cell subsets act to control the leishmaniacidal activity of host monocytes and macrophages. While this model still explains many aspects of the cellular immune response in human and mouse models of leishmaniasis, several other subpopulations are also involved in the regulation of effective leishmaniacidal immune responses. They cytokines on the left are important for differentiation of the given cell populations and the cytokines to the right of each T cell subset are key immunoregulatory cytokines that can be produced by the given subset. Importantly, IL-10, a down modulatory cytokine, can be produced by several T cell subpopulations (Th2, Th1, Treg and Th3), as well as by infected or activated macrophages. The same is true for TNF-alpha which can be produced by both T cells and activated macrophages. In addition, it is clear that these responses must be controlled for the limitation of pathology and likely to allow persistence and thus, maintenance of an effective memory response. The possible role of Th17 cells in the induction of pathology or protection in human leishmaniasis has not been determined, but represents another possibly important population in the dynamics of the response following Leishmania infection. Several cellular sources of important immunoregulatory cytokines exist, including not only subpopulations of CD4+ T cell, but also the macrophage itself and other leukocytes and granulocyte populations not highlighted in this figure.
Using single cell cytokine staining to identify directly subpopulations of lymphocytes producing immunoregulatory cytokines in human cutaneous disease caused by L. braziliensis, we demonstrated that indeed, CD4+ Th1 cells are the major source of IFN-gamma with very little to undetectable CD4+ T cells producing IL-4 or IL-5. In this same study we also identified CD4+ T cells and monocytes as important sources of IL-10 (21) (Figure 1). Interestingly, however, upon further study we found that IL-10 production by lymphocytes was not associated with lower frequencies of TNF-alpha or IFN-gamma producing T cells, but rather, the two were positively correlated, indicating a coordinate regulation in the frequency of CD4+ T cells producing cytokines capable of activating leishmaniacidal activities (IFN-gamma and TNF-alpha) and those producing the down regulatory cytokine, IL-10 (22). This finding points to an active immunoregulation of cells with protective and potentially pathogenic potentials, together with IL-10 producing T cells. The immediate biological activity of Leishmania antigen specific T cells to produce IL-10 with down-modulatory activities on host monocytes was also demonstrated, and thus, while there is not a direct correlation between higher frequencies of IL-10 producing lymphocytes and lower frequencies of IFN-gamma producing T cells, there is a greater decrease in monocyte activation (as measured by TNF-alpha production) in individuals with higher frequencies of antigen specific, IL-10 producing lymphocytes (22). Interestingly, it was recently suggested that a lower IFN-gamma/IL-10 ratio could be associated with a more favorable prognosis amongst individuals infected with L. braziliensis (23). Thus, co-production of IL-10 could be critical for both controlling pathology, as well as regulation of an “over-active” Th1 response. As mentioned above, the failure to regulate the Th1 response could lead to the poor formation of long lived, effective memory. Whether in the human disease these cells producing IFN-gamma and IL-10 are the same cell population, or different cells, has not yet been determined. As pointed out in our study, it has been recognized for many years that human Th1 cells can also produce IL-10 as described by Sornasse et al. (24), and it now appears that mouse T cells can do the same as recently shown following L. major infection (25) (26). Thus, it seems clear that active human cutaneous leishmaniasis is associated with a multifaceted regulated Th1 type response with components that will aid in the activation of host macrophages for activation of leishmaniacidal activities, as well as cytokines that aid in the control of this response (Figure 1). Many other cell types and cytokines not discussed in this review could clearly play an important role in the immunoregulation of human cutaneous disease including Th17 cells, Treg and Th3 populations, some of which have been investigated in human and animal models of disease (10, 27). The future elucidation of the role that these populations play in protection and pathology is clearly important to understanding the overall regulatory mechanisms of human leishmaniasis.
While CD4+ T cells are clearly an important source of cytokines for activation of leishmaniacidal activities, it is equally clear that several other cell types play an important role in establishment of the overall cytokine microenvironments important for both initiation and differentiation of effective T cell immune responses, as well as for local immune responses at the site of infection (18). On this note, when studying the cellular sources of immunoregulatory cytokines from cutaneous leishmaniasis patients, we found that the second most prevalent cell type responsible for IFN-gamma production were lymphocytes negative for both CD4 and CD8 (21). Based on this finding, further studies were carried out to determine the possible role of these cells in immunity and pathology (28).
CD4- CD8- (double negative-DN) T cells in human cutaneous leishmaniasis: alpha/beta DN T cells likely have a role in protection and pathology, while gamma/delta DN T cells could play a more important role in negative regulation
In addition to classic CD4+ and CD8+ T cells, there is a minority subpopulation of T cells that express neither CD4 nor CD8, thus termed double negative (DN) T cells. Within this DN T cell population several subpopulations can be found. In the broad sense, they contain T cells expressing either the gamma/delta or alpha/beta TCR, and within each of these populations, several subpopulations can be defined. Amungst the alpha/beta TCR DN T cells in humans, studies have identified cells of both negative-regulatory nature (29) (30), as well as associated with several autoimmune disorders (31–33). Of further interest are the alpha/beta TCR+ DN T cells that are restricted to CD1 presented antigens, of which some express a restricted TCR and often recognize lipid antigens presented by one of the CD1 family of molecules. These T cells often express a restricted, or invariant, TCR, and have been classified by many as invariant NK T cells. These are of particular interest due to their potent cytokine producing activity and highly activated profile as reviewed by others (34–37). In particular, CD1 restricted NK T cells were recently implicated in the host immune response to Leishmania (38) and a MHC class II restricted subset of DN T cells in protection to mycobacteria infection in mice (39).
In our work by Bottrel et al., we determined that DN lymphocytes were the second most prevalent cell type producing IFN-gamma in human cutaneous leishmaniasis, and that this IFN-gamma production was seen after short term cultures with media alone, as well as after stimulation with soluble Leishmania antigen (SLA) (21). Given the fact that we identified these cells solely on their positioning within the lymphocyte gate and their lack of expression of CD4 and CD8, further studies were required to determine if they were in fact T cells, and if so, determine what TCR they expressed, either alpha/beta or gamma/delta. In non-infected individuals, earlier studies had demonstrated that over 80% of peripheral blood DN T cells expressed the gamma/delta TCR. Moreover, several studies in human leishmaniasis had been performed that demonstrated a possible role for gamma/delta T cells (40–42). Strikingly, our findings published by Antonelli et al. (28) first identified that the great majority of DN T cells in the circulation from cutaneous leishmaniasis patients express the alpha/beta TCR. In fact, this population makes up approximately 75% of the DN T cells in cutaneous leishmaniasis patients, with the remainder expressing the gamma/delta TCR. In contrast, as had been shown earlier, we also observed that non-infected controls, have an inverted ratio with alpha/beta TCR expressing DN T cells making up at most 20% of the DN T cells in the circulation, and the great majority express the gamma/delta TCR. Upon studying the activation state of the DN T cells, it became clear that they express a hyper-activated ex vivo profile based on the expression of CD69 and the expression of IFN-gamma and TNF-alpha after 20 hour culture with media alone. This profile of hyper-activation ex vivo, coupled with cytokine production in the absence of exogenously added antigen, adds to the concept that these cells could be highly involved in an active response following infection by Leishmania, and may play an important role in the innate response as well. Moreover, this profile is different from what is seen for both CD4 or CD8 cells, where we see antigen induced activation and cytokine production, but very little production ex vivo or after media alone cultures (21). Thus, the DN T cell subpopulation, and in particular, the alpha/beta DN T cell subpopulation seems to fit into a highly activated T cell subpopulation producing biologically relevant cytokines for the activation of monocytes and macrophages.
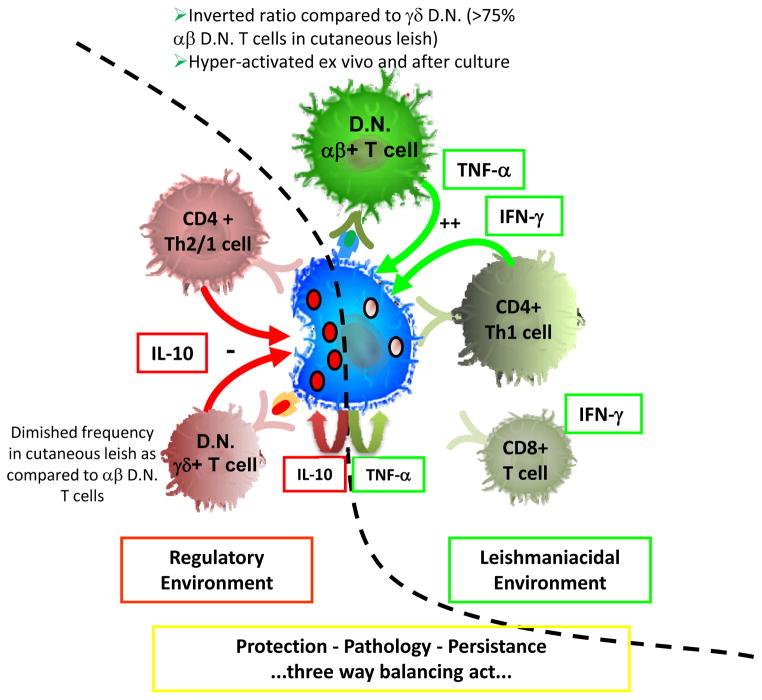
As a means of determining their physiologic role we further characterized the cytokine profiles of the alpha/beta and the gamma/delta DN T cells and demonstrated that the alpha/beta DN T cell subpopulation expresses high IFN-gamma or TNF-alpha to IL-10 ratios (approximately 20 and 10, respectively) after stimulation with SLA. Interestingly, these ratios are approximately twelve to fifty times higher than that seen for the gamma/delta DN T cell subpopulation for IFN-gamma/IL-10 and TNF-alpha/IL-10, respectively. Thus, the role of the alpha/beta DN T cells is likely skewed toward activation of leishmaniacidal activity and possibly in pathology (if not regulated), while the gamma/delta DN T cells would be more active as negative regulators of this activity (Figure 2). Finally, it is noteworthy that in media alone, the alpha/beta DN T cells display a more balanced cytokine profile with a diminished IFN-gamma/IL-10 ratio. Thus, it is possible that different subpopulations of alpha/beta DN T cells are being activated in the conditions of ex vivo and media alone as compared to after stimulation with SLA. It is clear that DN NKT cells will be contained within the alpha/beta DN T cell population and further studies are being carried out to determine their role in the overall profile we have identified.
Figure 2. αβ+ D.N. T cells contribute to a leishmaniacidal immune environment while, γδ+ D.N. T cells appear to contribute more towards a down regulatory environment in human cutaneous leishmaniasis caused by L. braziliensis.
Several cellular sources contribute to the overall immune environment in human cutaneous leishmaniasis. Depending on the dynamics of the interaction between these cell populations, their relative frequency, and the timing of their appearance and activation, each cell type could not only influence the effector cellular immune response, but also shape the cytokine microenvironment necessary for subsequent T cell differentiation. IFN-gamma and TNF-alpha are both key for optimal macrophage activation and for creating a microenvironment beneficial for driving Th1 cell development. Both CD4+ Th1 cells and αβ+ D.N. T cells are important sources of these cytokines in cutaneous disease. IL-10 is an important cytokine for down regulation of inflammatory responses and controlling macrophage activation. It comes from several sources in human cutaneous disease including CD4+ T cells, D.N. T cells (the balance in γδ+ D.N. T cells is skewed toward IL-10 as compared to the αβ+ D.N. T cells) and monocytes/macrophages. For several years it has been known that IL-10 is a poor indicator of Th1 and Th2 subsets in humans, and in cutaneous disease we see a co-regulation of IL-10 production along with IFN-gamma or TNF-alpha. The overall balance of these cell types and the cytokines they produce will determine if an immune response is effective at controlling the parasite and limiting pathology, while allowing for some degree of persistence which may be important for long lived and effective memory. The diagram is based on work by our group and represents the balance between subpopulations taken from PBMC of infected cutaneous leishmaniasis patients.
Clearly, the identification of what antigen presenting molecule(s) and antigen(s) are responsible for activation of the DN T cells in human leishmaniasis is an important step for understanding the role these cells play in induction of protective or pathogenic immune responses, as well as their possible role in the maintenance and generation of memory responses.
Acknowledgments
We would like to thank the collaboration over the past ten years of Dr. Edgar Carvalho and his group at Hospital Edgard Santos, UFBA, in Salvador, Bahia, Brazil, without which the studies described in this overview of our work would not have been possible. We would also like to thank the following funding agencies for support of our work over the years: WHO/TDR, CNPq, FINEP, FAPEMIG, and NIH-NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herwaldt BL. Leishmaniasis. Lancet. 1999 Oct 2;354(9185):1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002 Apr;96( Suppl 1):S173–8. doi: 10.1016/s0035-9203(02)90072-6. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira-Neto MP, Mattos MS, Perez MA, Da-Cruz AM, Fernandes O, Moreira J, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. International journal of dermatology. 2000 Jul;39(7):506–14. doi: 10.1046/j.1365-4362.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 4.Barral A, Pedral-Sampaio D, Grimaldi Junior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. The American journal of tropical medicine and hygiene. 1991 May;44(5):536–46. doi: 10.4269/ajtmh.1991.44.536. [DOI] [PubMed] [Google Scholar]
- 5.Coffman RL, Chatelain R, Leal LM, Varkila K. Leishmania major infection in mice: a model system for the study of CD4+ T-cell subset differentiation. Research in immunology. 1991 Jan;142(1):36–40. doi: 10.1016/0923-2494(91)90009-8. [DOI] [PubMed] [Google Scholar]
- 6.Scott P. T-cell subsets and T-cell antigens in protective immunity against experimental leishmaniasis. Current topics in microbiology and immunology. 1990;155:35–52. doi: 10.1007/978-3-642-74983-4_3. [DOI] [PubMed] [Google Scholar]
- 7.Locksley RM, Wakil AE, Corry DB, Pingel S, Bix M, Fowell DJ. The development of effector T cell subsets in murine Leishmania major infection. Ciba Foundation symposium. 1995;195:110–7. doi: 10.1002/9780470514849.ch8. discussion 7–22. [DOI] [PubMed] [Google Scholar]
- 8.Chatelain R, Mauze S, Coffman RL. Experimental Leishmania major infection in mice: role of IL-10. Parasite immunology. 1999 Apr;21(4):211–8. doi: 10.1046/j.1365-3024.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. The Journal of experimental medicine. 2001 Nov 19;194(10):1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunological reviews. 2006 Oct;213:159–79. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunological reviews. 2004 Oct;201:225–38. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott P. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunologic research. 2003;27(2–3):489–98. doi: 10.1385/IR:27:2-3:489. [DOI] [PubMed] [Google Scholar]
- 13.Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, et al. The immunopathology of experimental visceral leishmaniasis. Immunological reviews. 2004 Oct;201:239–53. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 14.Belkaid Y. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert opinion on biological therapy. 2003 Sep;3(6):875–85. doi: 10.1517/14712598.3.6.875. [DOI] [PubMed] [Google Scholar]
- 15.Scott P. Immunologic memory in cutaneous leishmaniasis. Cellular microbiology. 2005 Dec;7(12):1707–13. doi: 10.1111/j.1462-5822.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 16.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine. 2004 Oct;10(10):1104–10. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 17.Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006 Jan;63(1):70–8. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 18.Faria DR, Gollob KJ, Barbosa J, Jr, Schriefer A, Machado PR, Lessa H, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005 Dec;73(12):7853–9. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002 Dec;70(12):6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005 Nov 15;101(2):226–30. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Bottrel RL, Dutra WO, Martins FA, Gontijo B, Carvalho E, Barral-Netto M, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001 May;69(5):3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004 May;136(2):341–8. doi: 10.1111/j.1365-2249.2004.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes-Silva A, de Cassia Bittar R, Dos Santos Nogueira R, Amato VS, da Silva Mattos M, Oliveira-Neto MP, et al. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007 Sep;149(3):440–4. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. The Journal of experimental medicine. 1996 Aug 1;184(2):473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. The Journal of experimental medicine. 2007 Feb 19;204(2):285–97. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nature reviews. 2007 Jun;7(6):425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 27.Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006 May 1;193(9):1313–22. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- 28.Antonelli LR, Dutra WO, Oliveira RR, Torres KC, Guimaraes LH, Bacellar O, et al. Disparate immunoregulatory potentials for double-negative (CD4- CD8-) alphabeta and gammadelta T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006 Nov;74(11):6317–23. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson CW, Lee BP, Zhang L. Double-negative regulatory T cells: non-conventional regulators. Immunologic research. 2006;35(1–2):163–78. doi: 10.1385/IR:35:1:163. [DOI] [PubMed] [Google Scholar]
- 30.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Blood. 2005 Apr 1;105(7):2828–35. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 31.Sieling PA, Porcelli SA, Duong BT, Spada F, Bloom BR, Diamond B, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000 Nov 1;165(9):5338–44. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- 32.Liu MF, Yang CY, Chao SC, Li JS, Weng TH, Lei HY. Distribution of double-negative (CD4-CD8-, DN) T subsets in blood and synovial fluid from patients with rheumatoid arthritis. Clinical rheumatology. 1999;18(3):227–31. doi: 10.1007/s100670050089. [DOI] [PubMed] [Google Scholar]
- 33.Liu MF, Li JS, Weng TH, Lei HY. Double-negative (CD4-CD8-) TCRalphabeta+ cells in patients with systemic lupus erythematosus. Scandinavian journal of rheumatology. 1998;27(2):130–4. doi: 10.1080/030097498441001. [DOI] [PubMed] [Google Scholar]
- 34.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunology today. 2000 Nov;21(11):573–83. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nature immunology. 2003 Dec;4(12):1164–5. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- 36.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nature reviews. 2007 Jul;7(7):505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 37.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Current opinion in immunology. 2007 Jun;19(3):354–64. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. The Journal of experimental medicine. 2004 Oct 4;200(7):895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derrick SC, Evering TH, Sambandamurthy VK, Jalapathy KV, Hsu T, Chen B, et al. Characterization of the protective T-cell response generated in CD4-deficient mice by a live attenuated Mycobacterium tuberculosis vaccine. Immunology. 2007 Feb;120(2):192–206. doi: 10.1111/j.1365-2567.2006.02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alaibac M, Harms G, Zwingenberger K, Morris J, Yu R, Chu AC. Gamma delta T lymphocytes in oriental cutaneous leishmaniasis: occurrence and variable delta gene expression. The British journal of dermatology. 1993 Apr;128(4):388–92. doi: 10.1111/j.1365-2133.1993.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 41.Raziuddin S, Telmasani AW, el-Hag el-Awad M, al-Amari O, al-Janadi M. Gamma delta T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992 May;22(5):1143–8. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 42.Russo DM, Armitage RJ, Barral-Netto M, Barral A, Grabstein KH, Reed SG. Antigen-reactive gamma delta T cells in human leishmaniasis. J Immunol. 1993 Oct 1;151(7):3712–8. [PubMed] [Google Scholar]