Abstract
We recently reported that human T-lymphotropic virus type 1 (HTLV-1) infection is accompanied by a high frequency of CD4+ FoxP3+ cells in the circulation. In asymptomatic carriers of HTLV-1 and in patients with HTLV-1–associated inflammatory and malignant diseases, a high FoxP3+ cell frequency correlated with inefficient cytotoxic T cell-mediated killing of HTLV-1–infected cells. In adult T cell leukemia/lymphoma (ATLL), the FoxP3+ population was distinct from the leukemic T cell clones. However, the cause of the increase in FoxP3+ cell frequency in HTLV-1 infection was unknown. In this study, we report that the plasma concentration of the chemokine CCL22 is abnormally high in HTLV-1–infected subjects and that the concentration is strongly correlated with the frequency of FoxP3+ cells, which express the CCL22 receptor CCR4. Further, we show that CCL22 is produced by cells that express the HTLV-1 transactivator protein Tax, and that the increased CCL22 enhances the migration and survival of FoxP3+ cells in vitro. Finally, we show that FoxP3+ cells inhibit the proliferation of ex vivo, autologous leukemic clones from patients with ATLL. We conclude that HTLV-1–induced CCL22 causes the high frequency of FoxP3+ cells observed in HTLV-1 infection; these FoxP3+ cells may both retard the progression of ATLL and HTLV-1–associated inflammatory diseases and contribute to the immune suppression seen in HTLV-1 infection, especially in ATLL.
Human T-lymphotropic virus type 1 (HTLV-1) is a persistent retrovirus that has been estimated to infect 10–20 million people. The majority of infected individuals remain lifelong asymptomatic carriers (ACs) of the virus. However, 1–3% of infected individuals develop a progressive inflammation of the CNS, called HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) (1). In ~4% of seropositive individuals (2), HTLV-1 also causes a malignant lymphoproliferative disorder called adult T cell leukemia/lymphoma (ATLL) (3–5). The ATLL tumor typically consists of oligoclonal or monoclonal outgrowth of CD4+CD25+ T lymphocytes carrying a complete or defective provirus of HTLV-1. The expression of CD25 on ATLL cells and the severe immune suppression seen in patients with ATLL suggests that the leukemic cells might exert an immunosuppressive or regulatory T (Treg) cell function. However, CD25 expression is also induced by the HTLV-1 transactivator protein Tax (6, 7), and recent studies show that the properties of ATLL cells are inconsistent with Treg cell function (8, 9).
The best currently known single marker of the majority of Treg cells is the transcription factor FoxP3 (10, 11). FoxP3+ cells typically express the chemokine receptor CCR4 and show a chemotactic response to the two CCR4 ligands CCL22 and CCL17 (12–14). It has been suggested that secretion of CCL17 or CCL22 in the environment of certain solid tumors is responsible for accumulation of FoxP3+ Treg cells in the tumor, which might suppress the local immune response (15–18) and favor tumor survival and growth.
A high frequency of FoxP3+ cells has been observed recently in patients with ATLL (9, 19), patients with HAM/TSP (20, 21), and ACs of HTLV-1 (20, 21). However, the reasons for this high FoxP3+ cell frequency have not been identified. It was reported that HTLV-1–infected cell lines secrete large quantities of CCL22, which can attract CCR4-expressing CD4+ T cells (22). Finally, ATLL cells have been shown to express the CCL22 receptor CCR4 (23).
These observations raised the possibility that HTLV-1 infection causes the production of high levels of CCL22, which maintains the high frequency of FoxP3+ T cells. In this study, we show that CCL22 is produced by nonleukemic HTLV-1 Tax-expressing T cells and that the serum concentration of CCL22 is strongly correlated with the frequency of CD4+FoxP3+ T cells, which possess properties characteristic of Treg cells. We also demonstrate that the level of CCL22 produced is sufficient to attract CD4+ FoxP3+ cells and to enhance their viability. We propose that HTLV-1–induced CCL22 maintains the high CD4+FoxP3+ frequency and so contributes to the persistence of HTLV-1 by diminishing the anti–HTLV-1 CTL response. Furthermore, we present evidence that the CD4+FoxP3+ cells can suppress the proliferation of autologous ATLL cells. CD4+FoxP3+ cells may reduce the rate of progression from chronic ATLL to acute ATLL.
Materials and Methods
Subjects and cell sampling
Peripheral venous blood samples, anticoagulated with EDTA, were donated by subjects at the National Centre for Human Retrovirology, St. Mary’s Hospital. Additional ATLL samples were donated by patients attending the Department of Hematology in the University of Kumamoto, Japan. All individuals gave informed written consent, and the study was approved by the local research ethics committee of the appropriate hospital. All patients with ATLL were classified according to the criteria of Shimoyama (24), and all were HTLV-1 seropositive. PBMCs were isolated by density centrifugation on Histopaque (Sigma-Aldrich, Dorset, U.K.) and cryopreserved until use. Cells were cultured in complete medium (RPMI 1640, 10% FCS, penicillin/streptomycin, l-glutamine) at 37°C in 5% CO2 for 18 h.
Plasmids and transfection
Jurkat cells (clone E6.1) were transfected with 5 μg plasmid DNA using a nucleofector device (Amaxa Biosystems, Cologne, Germany) according to the manufacturer’s optimized protocol (program X-05). Jurkat cells were transfected with a control plasmid expressing GFP or the full-length tax gene in the pGFPC1 plasmid (BD Clontech, Palo Alto, CA). We also transfected cells with the full length env gene (pCMV-HT1-Env) and full length gag gene (pCMV-HT1-Gag). The gag- and env-expressing plasmids were supplied by Dr. D. Derse (National Cancer Institute, Frederick, MD). Cells were incubated in medium containing anti-CD3 coated beads (Miltenyi Biotec, Auburn, CA) for 72h before transfection.
Coculture infection
Jurkat cells (clone E6.1) were incubated with the HTLV-1–producing cell line MT2 in a 1:2 ratio for 4 h in a small volume of medium and in the presence or absence of anti-CD3 coated beads (Miltenyi Biotec). The cells were then incubated in RPMI 1640/10% FCS (105 cells/ml) for 5 d. Afterward, the supernatant was analyzed by ELISA for the chemokines CCL17 and CCL22.
Flow cytometry
To detect Tax, FoxP3, and CCL22 proteins in HTLV-1–infected cells, whole PBMCs or CD8+ cell-depleted PBMCs were incubated in vitro for 18 h. The cells were then surface-stained with mAbs to CD4 and CD8 (each at 15 μg/ml; Beckman Coulter, Marseille, France). Cells were then fixed and permeabilized with a commercial kit (Insight Biotechnology, Wembley, U.K.), following the manufacturer’s protocol. Finally, cells were stained intracellularly with the FITC-conjugated anti-Tax protein Ab Lt-4 (25) (diluted 1/100) and anti-human FoxP3-PE Ab (clone 236A/E7; Insight Biotechnology) in permeabilization buffer (Insight Biotechnology) following the manufacturer’s protocol. For CCL22 detection, after 12 h of incubation PBMCs were incubated for 4 h with Brefeldin A (Sigma-Aldrich) and stained with anti-human CCL22 (R&D Systems, Oxfordshire, U.K.). After staining, cells were analyzed on a Coulter Epics XL flow cytometer (Beckamn Coulter, High Wycombe, U.K.). Thirty thousand events were routinely collected. Viable lymphocytes were gated for further analysis using Expo32 analysis software (Beckman Coulter, Fullerton, CA).
Chemokine quantification by ELISA
Culture supernatant was obtained after incubation of unstimulated PBMCs for 18h. Samples of culture supernatant and fresh plasma were subjected to high-speed centrifugation to remove debris and were preserved at −80C. CCL22 and CCL17 were detected by ELISA (Duoset Detection Kit; R&D Systems), according to the manufacturer’s recommendation. Each sample was analyzed in duplicate and at different dilutions; results are shown as the median concentration of chemokine detected.
Chemotaxis
PBMCs from infected patients were placed in the upper chamber of a TransWell plate (3.0-μm–diameter pore) from BD Biosciences (Oxford, U.K.) after 18h of incubation. We placed culture medium containing different concentrations of recombinant CCL22 in the lower chamber (R&D Systems). Cells that migrated were recovered from the lower chamber and analyzed by flow cytometry for expression of Tax, CD4, CD8, and FoxP3. The results were expressed as the fold increase in the number of cells that migrated within 4 h after the addition of CCL22, compared with medium alone.
Proviral load measurement
After extraction of the DNA from PBMCs following the manufacturer’s protocol (DNeasy Tissue Kit; Qiagen, Crawley, U.K.), the HTLV-1 DNA was quantified by quantitative PCR in a Roche (Basel, Switzerland) Light Cycler following the previously described protocol (20, 26, 27).
Cell viability assay
PBMCs from infected individuals were cultured for 18h in the presence of different concentrations of CCL22. Subsequently, cells were labeled with the Live/Dead Fixable Cell Stain Kit (Invitrogen, Paisley, U.K.), Annexin V (BD Bioscience), and FoxP3. Calibrite beads (Beckman Coulter) were again used to quantify the number of viable cells measured.
Proliferation assay
mAbs specific to individual TCRβ-chains were used to identify and to separate dominant T cell clones from the PBMCs of patients with ATLL, as previously described (9). These clones were designated TCRvßn+, according to the TCRvβ-chain expressed. CD4+TCRvßn+ cells were stained with 10 μM CFSE (Invitrogen) for 10 min at 37°C. These cells were coincubated with CD25+ cells isolated by Ab-coupled magnetic microbeads (Miltenyi Biotec) at a ratio of one CD4+TCRvßn+ cell to two CD25+ cells. Proliferation of cells was induced by means of the Treg Suppression Inspector Kit from Miltenyi Biotec (beads labeled with anti-CD3, anti-CD28, and anti-CD2 mAbs) following the manufacturer’s instructions. After 4 d incubation at 37°C in 5% CO2, the cells were labeled as previously described and analyzed using Expo32 analysis software (Beckman Coulter).
Statistical analysis
Nonparametric statistical tests were used as appropriate, taking the null hypothesis and the sample size into account. The Spearman rank-order correlation coefficient was calculated when the significance of observed changes in two parameters across all HTLV-1 infected individuals was tested. The rate of lysis parameter was calculated with the software SPSS 12-0 for Microsoft Windows (Redmond, WA).
Results
CCL22 is present at high concentrations in plasma, and in culture supernatant of PBMCs, from HTLV-1–infected subjects
We quantified the concentration of CCL22 in plasma and culture supernatant of PBMCs from HTLV-1–infected subjects: ACs, patients with HAM/TSP, and patients with ATLL. We observed a significantly higher level of CCL22 in plasma from infected individuals than in plasma from uninfected individuals (Fig. 1A). We also measured the concentration of CCL17 in the plasma of patients. The level of CCL17 was consistently low or below or close to the limit of detection of the ELISA assay, and no difference between controls and infected patients was observed (data not shown). After 18 h of culture, the PBMCs of patients were analyzed for expression of Tax, FoxP3, and CCL22. The results (Fig. 1B) show that the level of CCL22 secretion was significantly higher in ACs and patients with HAM/TSP than in uninfected controls. Among the individuals with ATLL, only those with chronic disease had a level of CCL22 significantly higher than uninfected controls. In contrast, no CCL17 was detected in supernatant of patients’ PBMCs or in the plasma. Furthermore, no significant difference between ACs and patients with HAM/TSP was observed in plasma CCL22 concentration.
FIGURE 1.
CCL22 concentration in plasma and culture supernatant. Concentration of CCL22 in fresh plasma (A) or in PBMC supernatant after 18 h incubation in vitro (B). Median values are shown under each box plot. The value is expressed in picograms per milliliter for plasma samples (A) or in picograms per milliliter per 106 PBMCs (B). The p values were calculated using Student t test.
CCL22 production is associated with spontaneous Tax expression in fresh PBMCs
In four patients with HAM/TSP, we measured the intracellular expression of CCL22. Unstimulated PBMCs were incubated for 14 h in culture medium followed by 4 h incubation in the presence of brefeldin A and then stained with an anti-CCL22 mAb. A representative result is shown in Fig. 2A. The dot plot shows that half of the Tax+ cells expressed CCL22; cells that expressed a higher level of Tax also expressed a higher intensity of CCL22. We also analyzed the expression of CCL22 in FoxP3+ cells. The results of four independent experiments showed that CCL22 was not detected in CD4+FoxP3+ cells (Fig. 2A). In Fig. 2B, we present the frequencies of CCL22+ cells respectively in the CD4+ population and the CD4+Tax+ population. The mean proportion of CD4+Tax+ cells that expressed CCL22 was 48.68%.
FIGURE 2.

Tax expression and CCL22 expression. A, Expression of CCL22 and HTLV-1 Tax protein (left panel) and CCL22 and FoxP3 (right panel) in CD4+ cells from two independent representative HTLV-1–infected patients. B, Frequency in PBMCs of T cells of the following phenotypes: CCL22−CD4+Tax+, CCL22+CD4+Tax+, and CD4+CCL22+. The last column shows the frequency of CCL22+ cells in the CD4+Tax+ population.
We then tested whether the frequency of Tax expression was related to the level of CCL22 produced. We made use of the spontaneous suppression of HTLV-1–expressing (Tax+) cells by autologous HTLV-1–specific CTLs that is observed in fresh PBMCs (20, 28). We compared the quantity of CCL22 secreted per 106 PBMCs in the presence or absence of autologous CD8+ cells. The results (Fig. 3A) show that depletion of CD8+ cells from the infected PBMCs resulted in a significant increase in the level of CCL22 secreted. Furthermore, there was a strong correlation between the CCL22 concentration in plasma and the frequency of CD4+Tax+ cells in patients with HAM/TSP (R2 = 0.769; p = 0.004); this correlation was marginally significant in ACs (R2 = 0.388; p = 0.063; Fig. 3D).
FIGURE 3.
Modulation and induction of CCL22 expression. A, Concentration of CCL22 (picograms per milliliter per 106 PBMCs) in PBMC supernatant after 18 h incubation in vitro of whole PBMCs (left) and PBMCs depleted of CD8+ cells (right). Each line represents the rise in CCL22 concentration for each respective subject after CD8+ cell depletion. B, Concentration of CCL22 (picograms per milliliter per 106 PBMCs) detected in supernatant of Jurkat cells in the presence or absence of anti-CD3 coated beads and in the presence or absence of MT2 cells. C, Concentration of CCL22 (picograms per milliliter per 106 PBMCs) in supernatant of Jurkat cells transfected with different plasmids (5 μg/ml/106 cells). Supernatant was obtained 72 h after transfection. D, Correlation between the plasma concentration of CCL22 (picograms per milliliter) and the frequency of Tax expression in CD4+ T cells. The solid line represents the regression curve for patients with HAM/TSP and the dashed line represents the regression curve for ACs.
HTLV-1 induces CCL22 secretion
To test the hypothesis that HTLV-1 infection itself induces CCL22 secretion, we incubated Jurkat cells in the presence of the HTLV-1–producing cell line MT2. After 5 d of culture, we observed Tax expression in 5% of the Jurkat cell population (data not shown) and measured the level of CCL22 in the supernatant. The results (Fig. 3B) show that Jurkat cells incubated in the presence of MT2 secreted CCL22; this secretion was increased by stimulation with anti-CD3 coated beads. The level of CCL22 measured in Jurkat cells alone and in MT2 cells was low—close to the limit of detection of the ELISA.
In an independent experiment, we transfected Jurkat cells with plasmids expressing the tax, gag, or env genes of HTLV-1. Seventy-two hours after transfection, we measured the level of CCL22 present in the supernatant of the transfected Jurkat cells. The results (Fig. 3C) show that Tax induced CCL22 secretion by Jurkat cells. This result is consistent with what we observed in primary cells (Fig. 2). No secretion of CCL22 was induced by transfection of the gag plasmid. The env-expressing plasmid also induced expression of CCL22 in Jurkat cells: the level was lower than that induced by tax transfection, but this difference was not statistically significant.
CCL22 and proviral load
The proviral load of HTLV-1 has been shown previously to be strongly associated with the frequency of Tax expression. We measured the proviral load in HTLV-1–seropositive individuals without malignant disease and examined the relationship between the proviral load and the plasma concentration of CCL22. The results show (Fig. 4) a significant positive correlation between the proviral load and the CCL22 concentration in plasma.
FIGURE 4.

Proviral load is associated with CCL22 concentration in plasma. There was a significant positive correlation between the proviral load and the plasma concentration of CCL22 in patients with HAM/TSP (n = 10) and ACs (n = 8).
Frequency of FoxP3 expression in PBMCs correlated with plasma CCL22 concentration
In all patients tested for CCL22 secretion, we also measured the frequency of CD4+FoxP3+ cells in the PBMCs. The results show that the frequency of CD4+FoxP3+ cells in the circulation strongly correlated with the concentration of CCL22 in the plasma of the same patients (Fig. 5A). This result was significant in both ACs and patients with HAM/TSP. Furthermore, there was a strong correlation between the frequency of CD4+FoxP3+ cells in the circulation and the concentration of CCL22 in the supernatant after incubation of PBMCs in vitro for 18h (data not shown).
FIGURE 5.

CD4+FoxP3+ frequency in nonleukemic HTLV-1–infected individuals correlates with CCL22 concentration in plasma and supernatant of PBMCs. Correlation between the frequency of circulating CD4+FoxP3+ cells and the concentration of CCL22 in plasma from uninfected controls, ACs, and patients with HAM/TSP (A) and in patients with acute and chronic ATLL (B). The p values were determined by a two-tailed Spearman test.
In patients with ATLL we previously reported that the FoxP3+ Tax− T cell population is the major Treg cell population detected in the circulation (9). The results in Fig. 5B show that the frequency of CD4+FoxP3+Tax− cells in the circulation of patients with ATLL correlated with the CCL22 concentration both in fresh plasma and in the culture supernatant of the patient’s PBMCs after 18 h of incubation (data not shown). The concentration of CCL22 was significantly greater in patients with chronic ATLL than in those with acute ATLL (compare Fig. 1).
CCL22 and chemotaxis of FoxP3+ cells
The chemokine CCL22 is known to attract CCR4+ cells (29). We therefore tested the capacity of CCL22, at the concentration observed in the plasma of HTLV-1–infected subjects, to chemoattract the FoxP3+ population. For this experiment we used a transwell containing PBMCs from a patient with HAM/TSP in the upper chamber and growth medium with CCL22 in the lower chamber. The PBMCs were incubated in vitro for 18 h. The migrated cells were collected and stained with mAbs specific to CD4, FoxP3, and CD8. Calibrite beads were used to quantify the number of cells that migrated in the different conditions. Two concentrations of CCL22 were used: 200 pg/ml (corresponding to the concentration measured in plasma from uninfected individuals) and 1000 pg/ml (corresponding to the mean plasma concentration of CCL22 in patients with a high level of FoxP3+cells). The results of four independent experiments (Fig. 6A) showed that addition of CCL22 resulted in a higher rate of migration of FoxP3+ cells. Furthermore, this migration rate increase was dose-dependent; there was a large and statistically significant increase in the rate of migration of CD4+ and FoxP3+ cells in response to the higher concentration of CCL22. In contrast, no increase in the rate of migration of CD8+ cells was observed.
FIGURE 6.
Functional effects of CCL22 on CD4+FoxP3+ cells of HTLV-1–infected patients. A, Rate of migration of CD4+FoxP3+ cells in a transwell assay. The results are expressed as the fold increase in the number of cells that migrated in response to different concentrations of CCL22, compared with medium alone, during 4 h incubation. The results represent the means of four independent experiments. B, Viability of each respective cell subset following 24h of incubation, according to the level of CCL22 chemokine added. The values represent the absolute number of viable cells, normalized to the control sample. The histogram represents the mean of four independent experiments.
CCL22 promoted viability of FoxP3+ cells
We next measured the capacity of CCL22 to maintain the viability of FoxP3+ cells in vitro. We incubated PBMCs from infected patients for 24h with CCL22 at the normal (200 pg/ml) or high (1000 pg/ml) physiologic concentrations observed. After 24 h, the PBMCs were stained with annexin V, Live/Dead mAbs specific to CD4, and FoxP3, and the viability of different populations was measured by flow cytometry. We labeled live cells as CD4+Foxp3+ Annexin V− Live/Dead− cells. In each sample, we also added Calibrite beads to count the absolute cell numbers and to normalize the results of different experiments. The results of four independent experiments are shown in Fig. 6B. The value represents the number of live cells as a percentage of the cells incubated without chemokine. The data show that CCL22 at both 200 pg/ml and 1000 pg/ml resulted in a significant increase in viability of the CD4+FoxP3+ cells.
Inhibition of proliferation of ex vivo ATLL clones by autologous CD25+FoxP3+ cells
As explained above, it is difficult to separate FoxP3+ cells from ATLL cells because the two populations share characteristic surface markers, in particular CD25 and CCR4. However, leukemic T cell clones can be identified in PBMCs of patients with ATLL by an abnormally high frequency of expression of specific TCRvβ-chains. We recently showed that FoxP3+ cells with immune suppressive (Treg cell) function are distinct from ATLL cells (9): the two populations can be separated using Abs specific to the TCRvβ-chain expressed by the respective dominant ATLL clones, designated TCRvβn+ cells.
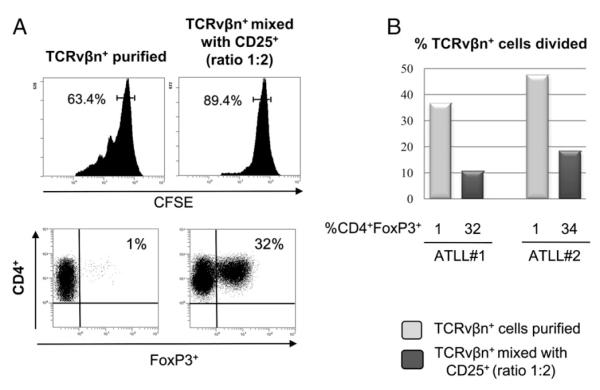
We tested the capacity of CD4+FoxP3+ cells from two patients with ATLL to suppress the proliferation of autologous tumor cells. TCRvβn+ cells were labeled with CFSE and incubated in the presence or absence of the CD25+ population. The results showed that, during 4 d incubation in vitro, the rate of proliferation of TCRvβn+ ATLL cells was significantly reduced in the presence of CD25+FoxP3+ cells (Fig. 6A). This result was reproduced in independent experiments with cells from two patients with chronic ATLL (Fig. 7B).
FIGURE 7.
FoxP3+ cells inhibit the proliferation of autologous ATLL cells in patients with chronic ATLL. A, upper panels, Expression of CFSE in purified TCRvβn+ clones from two patients with ATLL; values represent the percentage of undivided CD4+ cells. Lower panels, CD4 and FoxP3 expression in the CD4+ gated population. B, Percentage of CFSE-labeled leukemic (TCRvβn+) cells undergoing division during 4 d incubation in vitro in the presence of different percentages of autologous CD4+FoxP3+ cells.
Discussion
In this study, we report that the plasma concentration of CCL22 is correlated with the frequency of circulating CD4+FoxP3+ Treg cells in HTLV-1–infected patients. This result is sufficient to explain the origin and maintenance of the high frequency of FoxP3+ cells observed in HTLV-1 infection (20).
We previously reported the presence of an abnormally high frequency of CD4+FoxP3+ cells in both ACs and patients with HAM/TSP (20) and in patients with ATLL (9). In patients with ATLL, we demonstrated that the FoxP3+ population is independent of the leukemia (ATLL) cells (9). However, the origin of this high frequency of FoxP3+ cells in patients with HTLV-1 remained unclear. Hieshima et al. (22) showed that CCL22 was secreted by in vitro cell lines persistently infected with HTLV-1 and that this chemokine could promote the de novo infection of CCR4+ cells. These authors showed an association between Tax expression and CCL22 secretion in these cell lines, and they confirmed that the secreted CCL22 was able to chemoattract CCR4+ cells. In this study, we show that ex vivo CD4+ T cells naturally infected with HTLV-1 produce CCL22. The cell chiefly responsible for this CCL22 production is the CD4+Tax+ T cell (Fig. 2). Four further observations were consistent with this conclusion. First, depletion of CD4+Tax+ T cells by the autologous CTL response led to a reduction in the CCL22 production. Second, patients with acute ATLL, whose circulating leukemic cells typically express low or undetectable levels of Tax, also had low plasma concentrations of CCL22. Third, there was a strong positive correlation between the concentration of CCL22 in plasma and the frequency of circulating CD4+Tax+ cells in patients. Finally, transfection with the tax-expressing plasmid induced CCL22 expression in Jurkat cells. Not all Tax+ cells expressed CCL22 (Fig. 2B), possibly because of limited assay sensitivity or because some cells had not expressed Tax long enough to induce CCL22 production. HTLV-1 env expression also induced CCL22 secretion by Jurkat cells, whereas transfection with the gag-expressing plasmid or the empty plasmid control did not induce detectable CCL22 secretion.
Finally, two observations confirmed the association between the virus infection and the CCL22 expression. First, the proviral load correlated with the CCL22 concentration in plasma (Fig. 4). Second, coculture of Jurkat cells with the HTLV-1 producer cell line MT2 induced CCL22 secretion by Jurkat cells (Fig. 3B).
We next studied the relationship between the frequency of CD4+ FoxP3+ cells in the circulation and the concentration of CCL22, both in plasma and in the supernatant after incubation of PBMCs for 18 h in vitro. Consistent with our previous findings (9, 20), we observed a significantly higher frequency of CD4+FoxP3+ cells in ACs, patients with HAM/TSP, and patients with ATLL than in uninfected subjects. The CD4+FoxP3+ cell frequency showed a strong positive correlation with the concentration of CCL22 in plasma or in PBMC supernatant in ACs, patients with HAM/TSP (Fig. 5A), and patients with chronic ATLL (Fig. 5B). In patients with acute ATLL the correlation was not statistically significant; however, these patients have a low frequency of FoxP3 expression and low CCL22 expression.
In this study, we also confirmed that CCL22, at the physiologic concentrations observed, was able to attract both CD4+ cells and CD4+FoxP3+ cells, but not CD8+ cells, and to increase the viability of the CD4+FoxP3+ cells (Fig. 6B). The frequency of migration of CD4+FoxP3+ cells was not significantly different from that of all CD4+ cells. This finding can be explained by the observation that, whereas FoxP3 is expressed by a low proportion of CD4+ cells, a large fraction of CD4+ cells express CCR4, the receptor for CCL22. However, a large difference was observed in the rate of migration of CD4+ and CD4+FoxP3+ cells in response to the two respective concentrations of CCL22 used—200 pg/ml and 1000 pg/ml. The high concentration of CCL22 (1000 pg/ml), typical of patients with high frequencies of both CD4+ FoxP3+ cells and CD4+Tax+ cells, was associated with strong migration of CD4+FoxP3+ cells.
The question arises: what are the roles of FoxP3+ cells and CCL22 in ATLL? In this study, we tested the ability of CD4+ FoxP3+ cells from a patient with ATLL to suppress proliferation of autologous tumor cells. The results (Fig. 7) showed that CD25+ FoxP3+ cells inhibited the proliferation of autologous TCRvßn+ ATLL clone cells. This result suggests that autologous FoxP3+ cells from a patient with ATLL are able to reduce the proliferation of ATLL cells themselves. This finding may be of particular importance in patients with chronic ATLL, who have a high frequency of FoxP3+ cells and—by definition—slowly progressive disease. Therefore, the FoxP3+ cells could be responsible for the maintenance of the chronicity of the disease by reducing the proliferation of ATLL cells themselves. Simultaneous suppression by FoxP3+ T cells of both the anti–HTLV-1 cellular immune response and proliferation of ATLL cells will lead to complex dynamics in the T cell populations in this disease. This putative combination of protumor and antitumor effects of FoxP3+ cells stands in contrast with the protumor effects of Treg cells reported in solid tumors (30, 31).
It has been shown by others that CCL22 present in the environment of certain solid tumors can attract CD4+FoxP3+ cells (15, 16). Furthermore, certain B-lymphoma cells secrete CCL22 and induce the accumulation of Treg cells (32). Regarding FoxP3, we previously demonstrated that the CD4+FoxP3+ population in patients with ATLL is independent of the leukemic cells, and these FoxP3+ cells were able to inhibit the proliferation of allogeneic CD4+CD25− cells (9). The net effect of Treg cells may indeed differ between solid tumors and ATLL. Treg cells may be confined to the periphery of a solid tumor, where they can inhibit the host immune response but cannot penetrate the tumor itself and retard its growth. In contrast, the greater degree of mixing between different cell types that occurs in leukemia may allow FoxP3+ cells to make frequent contact with ATLL cells and consequently slow the progression of the leukemia.
FoxP3+ cells are also known to influence the activity of the CTL response to viruses (10, 33). Evidence from virus genetics (34), host immunogenetics (35, 36), gene expression microarrays (37), and ex vivo T cell functional assays (38, 39) demonstrates that the activity of the CTL response is a major determinant of the outcome of HTLV-1 infection. In particular, the efficiency or quality of the virus-specific CTL response (40) accounts for much of the variation between individuals in the disease manifestations of HTLV-1 infection. We recently showed a strong inverse correlation between the HTLV-1–specific CTL efficiency and the frequency of FoxP3+CD4+ cells in the circulation (9, 20). This observation suggests that FoxP3+CD4+ cells reduce the efficiency of the anti–HTLV-1 CTL response, although the complex dynamics of the persistent infection in vivo make it difficult to distinguish cause and effect (40). The results reported in this study are consistent with the idea that HTLV-1 induces abnormal CCL22 production to promote the accumulation of CD4+FoxP3+ cells in both asymptomatic HTLV-1 carriers, patients with HAM/TSP, and patients with ATLL. In turn, the CD4+FoxP3+ cells, which retain Treg cell functions, reduce the efficiency of immune surveillance of HTLV-1–infected cells and thereby promote high-level persistence of the infection in the host. Our results do not indicate an association between the frequency of CD4+FoxP3+ cells and the risk of inflammatory diseases, such as HAM/TSP. However, our finding that CD4+FoxP3+ cells inhibit the proliferation of autologous leukemic T cells in patients with ATLL suggests that the CD4+FoxP3+ cells retard the progression of the disease. Therefore, because the CD4+FoxP3+ cells can exert both beneficial (antileukemic) and harmful (immunosuppressive) effects in ATLL, anti-CCR4 (41, 42) Ab treatment of ATLL should be used with caution, especially in patients with chronic ATLL, in whom the net effect of the CD4+FoxP3+ cells might be beneficial.
The abnormally high frequency of CD4+FoxP3+ cells present in HTLV-1 infection could contribute to the susceptibility of HTLV-1–infected individuals to infection with Strongyloides (20, 21), tuberculosis (43), and staphylococcal skin infections (44).
Acknowledgments
We thank Dr. Mohamed Nejmeddine for assistance with transfection of Jurkat and culture of MT2 cells.
This work was supported by the Wellcome Trust and funding under the Sixth Research Framework Programme of the European Union, Project INCA (LSHC-CT-2005-018704).
Abbreviations used in this paper
- AC
asymptomatic carrier
- ATLL
adult T cell leukemia/lymphoma
- HAM/TSP
human T-lymphotropic virus type-1–associated myelopathy/tropical spastic paraparesis
- HTLV-1
human T-lymphotropic virus type 1
- Treg cell
regulatory T cell
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 2.Nagai M, Osame M. Human T-cell lymphotropic virus type I and neurological diseases. J. Neurovirol. 2003;9:228–235. doi: 10.1080/13550280390194028. [DOI] [PubMed] [Google Scholar]
- 3.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 4.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 5.Kalyanaraman VS, Sarngadharan MG, Nakao Y, Ito Y, Aoki T, Gallo RC. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc. Natl. Acad. Sci. USA. 1982;79:1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross SL, Feinberg MB, Wolf JB, Holbrook NJ, Wong-Staal F, Leonard WJ. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 7.Hanon E, Goon P, Taylor GP, Hasegawa H, Tanaka Y, Weber JN, Bangham CR. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood. 2001;98:721–726. doi: 10.1182/blood.v98.3.721. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, Uchihashi K, Kazuto T, Osaka A, Yanagihara K, Tsukasaki K, Hasegawa H, Yamada Y, Kamihira S. Foxp3 expression on normal and leukemic CD4+CD25+ T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur. J. Haematol. 2008;81:209–217. doi: 10.1111/j.1600-0609.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 9.Toulza F, Nosaka K, Takiguchi M, Pagliuca T, Mitsuya H, Tanaka Y, Taylor GP, Bangham CR. FoxP3+ regulatory T cells are distinct from leukemia cells in HTLV-1-associated adult T-cell leukemia. Int. J. Cancer. 2009;125:2375–2382. doi: 10.1002/ijc.24664. [DOI] [PubMed] [Google Scholar]
- 10.Bach JF. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 2003;3:189–198. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 12.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25high-Foxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 13.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 14.Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J, Ye ZJ. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin. Cancer Res. 2009;15:2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 15.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Zhao J, Lei Z, Shen S, Liu C, Li D, Liu J, Shen GX, Zhang GM, Feng ZH, Huang B. Local accumulation of FOXP3+ regulatory T cells: evidence for an immune evasion mechanism in patients with large condylomata acuminata. J. Immunol. 2008;180:7681–7686. doi: 10.4049/jimmunol.180.11.7681. [DOI] [PubMed] [Google Scholar]
- 17.Ohara M, Yamaguchi Y, Matsuura K, Murakami S, Arihiro K, Okada M. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol. Immunother. 2009;58:441–447. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohno T, Yamada Y, Akamatsu N, Kamihira S, Imaizumi Y, Tomonaga M, Matsuyama T. Possible origin of adult T-cell leukemia/lymphoma cells from human T lymphotropic virus type-1-infected regulatory T cells. Cancer Sci. 2005;96:527–533. doi: 10.1111/j.1349-7006.2005.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111:5047–5053. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, White AC., Jr. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl. Trop. Dis. 2009;3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hieshima K, Nagakubo D, Nakayama T, Shirakawa AK, Jin Z, Yoshie O. Tax-inducible production of CC chemokine ligand 22 by human T cell leukemia virus type 1 (HTLV-1)-infected T cells promotes preferential transmission of HTLV-1 to CCR4-expressing CD4+ T cells. J. Immunol. 2008;180:931–939. doi: 10.4049/jimmunol.180.2.931. [DOI] [PubMed] [Google Scholar]
- 23.Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, Izawa D, Hieshima K, Tatsumi Y, Matsushima K, Hasegawa H, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 24.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br. J. Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Tanaka Y, Tozawa H. Monoclonal antibody defining tax protein of human T-cell leukemia virus type-I. Tohoku J. Exp. Med. 1989;157:1–11. doi: 10.1620/tjem.157.1. [DOI] [PubMed] [Google Scholar]
- 26.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 27.Tosswill JH, Taylor GP, Clewley JP, Weber JN. Quantification of proviral DNA load in human T-cell leukaemia virus type I infections. J. Virol. Methods. 1998;75:21–26. doi: 10.1016/s0166-0934(98)00093-7. [DOI] [PubMed] [Google Scholar]
- 28.Hanon E, Hall S, Taylor GP, Saito M, Davis R, Tanaka Y, Usuku K, Osame M, Weber JN, Bangham CR. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–1392. [PubMed] [Google Scholar]
- 29.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 30.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 31.Gallimore A, Godkin A. Regulatory T cells and tumour immunity - observations in mice and men. Immunology. 2008;123:157–163. doi: 10.1111/j.1365-2567.2007.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Niewiesk S, Daenke S, Parker CE, Taylor G, Weber J, Nightingale S, Bangham CR. The transactivator gene of human T-cell leukemia virus type I is more variable within and between healthy carriers than patients with tropical spastic paraparesis. J. Virol. 1994;68:6778–6781. doi: 10.1128/jvi.68.10.6778-6781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vine AM, Witkover AD, Lloyd AL, Jeffery KJ, Siddiqui A, Marshall SE, Bunce M, Eiraku N, Izumo S, Usuku K, et al. Polygenic control of human T lymphotropic virus type I (HTLV-I) provirus load and the risk of HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Infect. Dis. 2002;186:932–939. doi: 10.1086/342953. [DOI] [PubMed] [Google Scholar]
- 37.Vine AM, Heaps AG, Kaftantzi L, Mosley A, Asquith B, Witkover A, Thompson G, Saito M, Goon PK, Carr L, et al. The role of CTLs in persistent viral infection: cytolytic gene expression in CD8+ lymphocytes distinguishes between individuals with a high or low proviral load of human T cell lymphotropic virus type 1. J. Immunol. 2004;173:5121–5129. doi: 10.4049/jimmunol.173.8.5121. [DOI] [PubMed] [Google Scholar]
- 38.Asquith B, Mosley AJ, Barfield A, Marshall SE, Heaps A, Goon P, Hanon E, Tanaka Y, Taylor GP, Bangham CR. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J. Gen. Virol. 2005;86:1515–1523. doi: 10.1099/vir.0.80766-0. [DOI] [PubMed] [Google Scholar]
- 39.Kattan T, MacNamara A, Rowan AG, Nose H, Mosley AJ, Tanaka Y, Taylor GP, Asquith B, Bangham CR. The avidity and lytic efficiency of the CTL response to HTLV-1. J. Immunol. 2009;182:5723–5729. doi: 10.4049/jimmunol.0900069. [DOI] [PubMed] [Google Scholar]
- 40.Bangham CR. CTL quality and the control of human retroviral infections. Eur. J. Immunol. 2009;39:1700–1712. doi: 10.1002/eji.200939451. [DOI] [PubMed] [Google Scholar]
- 41.Ishida T, Ishii T, Inagaki A, Yano H, Kusumoto S, Ri M, Komatsu H, Iida S, Inagaki H, Ueda R. The CCR4 as a novel-specific molecular target for immunotherapy in Hodgkin lymphoma. Leukemia. 2006;20:2162–2168. doi: 10.1038/sj.leu.2404415. [DOI] [PubMed] [Google Scholar]
- 42.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norrgren HR, Bamba S, Larsen O, Da Silva Z, Aaby P, Koivula T, Andersson S. Increased prevalence of HTLV-1 in patients with pulmonary tuberculosis coinfected with HIV, but not in HIV-negative patients with tuberculosis. J. Acquir. Immune Defic. Syndr. 2008;48:607–610. doi: 10.1097/QAI.0b013e31817efb83. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira, Mde F, Brites C, Ferraz N, Magalhaes P, Almeida F, Bittencourt AL. Infective dermatitis associated with the human T cell lymphotropic virus type I in Salvador, Bahia, Brazil. Clin. Infect. Dis. 2005;40:e90–e96. doi: 10.1086/430064. [DOI] [PubMed] [Google Scholar]