Abstract
Genetic and environmental factors are both likely to contribute to neurodevelopmental disorders, including ASDs (autism spectrum disorders). In this study, we examined the combinatorial effect of two factors thought to be involved in autism – reduction in the expression of the extracellular matrix protein reelin and prenatal exposure to an organophosphate pesticide, CPO (chlorpyrifos oxon). Mice with reduced reelin expression or prenatal exposure to CPO exhibited subtle changes in ultrasound vocalization, open field behaviour, social interaction and repetitive behaviour. Paradoxically, mice exposed to both variables often exhibited a mitigation of abnormal behaviours, rather than increased behavioural abnormalities as expected. We identified specific differences in males and females in response to both of these variables. In addition to behavioural abnormalities, we identified anatomical alterations in the olfactory bulb, piriform cortex, hippocampus and cerebellum. As with our behavioural studies, anatomical alterations appeared to be ameliorated in the presence of both variables. While these observations support an interaction between loss of reelin expression and CPO exposure, our results suggest a complexity to this interaction beyond an additive effect of individual phenotypes.
Keywords: autism, cerebellum, hippocampus, olfactory bulb, reeler, social interaction, ultrasonic communication
Abbreviations: ACHE, acetylcholinesterase; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; CPO, chlorpyrifos oxon; ECL, enhanced chemiluminescence; WT, wild-type
INTRODUCTION
The heritability of neurodevelopmental disorders including ASDs (autism spectrum disorders) suggests a strong genetic component to these diseases and a number of contributing genes have been identified (Persico and Bourgeron, 2006; Abrahams and Geschwind, 2008). However, a variety of environmental factors may also predispose genetically vulnerable individuals to develop ASDs and other neurodevelopmental disorders (Herbert, 2010). From the first report of maternal infection contributing to schizophrenia (Murray et al., 1992), environmental perturbations including infection and immune dysfunction, altered levels of neurotransmitters, neuropeptides and growth factors, hormonal alterations, obstetric complications and exposure to exogenous agents including medications and illicit drugs, metals and environmental pollutants have been suggested as predisposing factors in neurodevelopmental disorders including schizophrenia, autism and ADHD (attention deficit hyperactivity disorder) (reviewed in Newschaffer et al., 2007). Changes in exposure to these environmental factors may also contribute to the increased prevalence of these disorders (Herbert, 2010).
In this study, we have examined the behavioural and anatomic effects of combining genetic and environmental factors thought to be potential candidates for involvement in ASDs. Specifically, we have examined the interactions between the reelin gene and prenatal exposure to an organophosphate pesticide, an environmental risk factor (Persico and Bourgeron, 2006). Several lines of evidence implicate reelin as a candidate gene for ASDs. First, on average, patients with autism have significantly decreased serum levels of reelin protein (Persico et al., 2001; Fatemi et al., 2002). Additional studies demonstrate decreases in reelin protein and mRNA levels in frontal and cerebellar areas of autistic brains as well as alterations in mRNA expression of VLDLR (very-low-density lipoprotein receptor) and Dab1, downstream mediators of reelin signalling (Fatemi et al., 2005). Secondly, reelin is located on chromosome 7q22 and this locus has been associated with ASDs in a variety of linkage studies (Bacchelli and Maestrini, 2006; Abrahams and Geschwind, 2008). Thirdly, there are distinct similarities between neuroanatomical alterations observed in reeler mutant mice, which carry a naturally occurring deletion mutation in the reelin gene (D’Arcangelo et al., 1995) and in some autistic patients (Persico and Bourgeron, 2006). The heterogeneity of autism precludes establishing a concrete set of neuroanatomical phenotypes; however, autistic patients show pathological changes including increased cell density, neuronal disorganization and poor lamination in the cerebral cortex, decreased Purkinjie cell number in the cerebellum, increased cell density and reduced neuronal size in the entorhinal cortex, hippocampus and amygdala, and decreased dendritic branching in the hippocampus (Bailey et al., 1998; Pappas et al., 2001; Bauman and Kemper, 2005). Neuroanatomical alterations in reeler mutant mice are more pervasive and extreme than those seen in autistic patients but have a number of similarities including lamination defects and neuronal disorganization in the cortex, decreased and disorganized Purkinje cells, cytoarchitectonic disturbances in the hippocampus, and reduced dendritic branching (Rice and Curran, 2001; Niu et al., 2008).
Several lines of evidence suggest pesticide exposure as a contributing environmental factor in neurodevelopmental disorders. A retrospective case-control study in humans suggested a link between increased exposure to organochlorine pesticides and ASDs (Roberts et al., 2007) and organophosphate exposure, as measured by the presence of urinary metabolites, may contribute to ADHD prevalence (Bouchard et al., 2010). Mutations that reduce or inactivate PON1, which encodes an organophosphate inactivator, show significant association with autism in Caucasian–North American families, but not in Italian families (D’Amelio et al., 2005). There is less organophosphate usage in European households compared with North American households, suggesting region-specific gene–environment interactions. Finally, organophosphate exposure specifically affects the behaviour exhibited by reeler mutant mice (Laviola et al., 2006), suggesting a direct interaction between pesticide exposure and the activity of the reelin gene. Together, these pieces of evidence have led to the hypothesis that prenatal organophosphate exposure can affect cell migration by modulating reelin activity, thus potentially contributing to the neurodevelopmental basis of autism (Keller and Persico, 2003).
To evaluate the effects of possible gene–environment interactions in the development of behaviours and anatomical phenotypes relevant to ASDs, we have exposed heterozygous reeler (Rl+/−) mice to the active organophosphate CPO (chlorpyrifos oxon) during embryonic development. Rl+/− mice have reduced levels of reelin protein and show subtle but discernible differences in neuroanatomy including decreased density of NOS (nitric oxide synthase)-expressing cells in the cortex (Tueting et al., 1999), selective reduction in forebrain cholinergic neurons (Sigala et al., 2007), alterations in dendritic spine morphology in the hippocampus (Pappas et al., 2001; Niu et al., 2008) and altered synaptic activity in the brain including decreased GAD67 expression and changes in NMDA (N-methyl-d-aspartate) receptor expression and activity (Liu et al., 2001; Ventruti et al., 2011; van den Buuse et al., 2012). Behavioural testing suggests minor deficits in reversal learning, but no differences in anxiety or social behaviour (Salinger et al., 2003; Podhorna and Didriksen, 2004; Brigman et al., 2006; Laviola et al., 2006; Teixeira et al., 2011). In particular, we sought to determine whether reelin haploinsufficiency coupled with CPO exposure would affect behaviours specifically relevant to ASDs, such as communication and social interaction. We also examined brain morphology to determine if interactions between reelin and CPO would produce discernible changes in brain structure. Our findings suggest that both behaviour and brain anatomy are affected individually by reelin haploinsufficiency or by CPO exposure, but that exposure to both variables does not appear to be additive in effect. Exposure to both variables resulted in mitigation of some behavioural and anatomical phenotypes, producing behaviours and anatomy similar to those seen in control animals. This counterbalancing effect suggests that while genetic and environmental factors both affect brain development, interactions between these variables are not necessarily additive, and in some cases, may mask symptoms caused by one or the other variable. In this regard, fine discrimination may be needed to discern the effects of genetic and environmental variables in neurodevelopmental disorders.
MATERIALS AND METHODS
Mouse lines and minipump implantation
Reeler mice (B6C3Fe-a/a-Relnrl/+, referred to in this study as Rl mice) were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.) and Dab1exKIneo mice, in which a lacZ reporter gene replaces exon 1 of Dab1, were provided by Dr Brian Howell (Pramatarova et al., 2008). Both lines of mice are maintained on a C57Bl/6 background. For behavioural studies, heterozygous reeler (Rl+/−) female mice were crossed with WT (wild-type) males. The initiation of pregnancies was determined by visual inspection for vaginal plugs; the first appearance of a plug was denoted as gestation day (G) 0.5. At G13.5, pregnant females were anaesthetized with isofluorane and implanted with an osmotic minipump (Alzet 1003D, 100 μl reservoir) loaded with 6 mg/ml CPO (the active metabolite of the organophosphate pesticide chlorpyrifos, Chem Service) dissolved in 1:1 DMSO/PBS or with a vehicle-loaded pump. Females delivered their litters on average on G20. Pups were raised by their mothers to 28 days, then weaned and group housed for the remainder of their testing period. For anatomical studies, Rl+/− females were mated with Dab1exKIneo+/− males and minipumps were implanted as above. Offspring from all matings were genotyped by PCR as described (D’Arcangelo et al., 1995; Howell et al., 1997; Khialeeva et al., 2011). Animals of each sex were divided into four groups – vehicle-treated Rl+/+ and Rl+/− mice, and CPO-treated Rl+/+and Rl+/− mice, with a minimum of six animals per group used for behavioural testing and a minimum of three animals per group used for anatomical and biochemical analysis. All animal studies were performed in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, and approved by the UCLA Chancellor's Animal Research Committee.
ACHE (acetylcholinesterase) activity
ACHE activity was measured using a modified Ellman reaction. Heads were harvested from E16.5 embryos, 3 days after minipump implantation, flash-frozen and stored at −80°C. Frozen heads were homogenized in 0.1M phosphate buffer, centrifuged at 14 000 rev./min for 5 min and supernatant collected for the assay. Total protein concentration was determined using a Bradford assay and ACHE activity was determined by colorimetric analysis utilizing the QuantiChrom™ Acetylcholinesterase Assay Kit (BioAssay Systems) following the manufacturer's instructions. The protein quantification and ACHE assays were repeated in triplicate, then the samples were averaged to determine the final results.
Western blotting for reelin protein quantification
Embryonic heads were harvested at E16.5, 3 days after minipump implantation, flash-frozen and stored at −80°C. Heads were homogenized in lysis buffer containing 50 mM Tris/HCl, 0.25% (w/v) sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1% (w/v) Nonidet P40 (Igepal), with phosphatase inhibitor cocktail (ZmTech Scientific) containing 1–2% Na3VO4 (sodium orthovanadate), 1–3% AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride], 1–3% aprotinin and 1–3% leupeptin. Then 50 μg samples were resolved on 4–12% (w/v) mini-SDS polyacrylamide gels (Invitrogen) and transferred to Immobilon PVDF membranes. Blots were blocked with 5% (w/v) non-fat dried skimmed milk in PBS-T [17 mM KH2PO4, 50 mM Na2HPO4, 1.5 mM NaCl, pH 7.4, and 0.05% (v/v) Tween 20] for 1 h at room temperature and then incubated overnight at 4°C in PBS-T plus 5% (w/v) non-fat dried skimmed milk containing primary mouse anti-reelin clone 142 (1:750 dilution) antibodies (Millipore). Relative reelin protein levels in each sample were determined by comparison with β-actin levels, which were determined by Western blotting as above using mouse anti-β-actin antibodies (1:10000, Sigma, 1 h incubation at room temperature). Protein bands were detected using an ECL (enhanced chemiluminescence) 2 kit for reelin and ECL kit for β-actin (Amersham Pharmacia Biotech), with HRP (horseradish peroxidase)-conjugated secondary goat anti-mouse antibodies (1:3000, Cell Signaling Technologies). Relative intensities of the protein bands were quantified by scanning densitometry using ImageJ.
Behavioural testing
Ultrasound vocalization
Mice at postnatal day (P) 7 were separated from their dams and placed into warm soundproof chambers equipped with ultrasonic detectors connected to recording and analysis software (UltraVox, VisualSonics). The number and duration of vocalizations at 70 Hz were recorded during a 10-min testing period. Male and female mice were assessed separately, with four groups of animals (vehicle-treated Rl+/+, CPO-treated Rl+/+, vehicle-treated Rl+/− and CPO-treated Rl+/−) tested for each sex. The collected data were scrubbed for outliers and statistical significance across the four test groups in each sex was determined using individual t-test comparisons and aggregate ANOVA analysis.
Open-field behaviour
Mice at P30 were introduced into a 22.5 cm×22.5 cm clear plexiglass arena and video-recorded for 20 min using TopScan (CleverSys Inc.). A central rectangle, occupying 62.6% of the total area was superimposed over the arena profile and animals were scored for the amount of time spent in the centre against the periphery of the chamber. The number of sniffing events, an exploratory behaviour, was also determined for the centre of the chamber and the periphery during the recording.
Social interaction
Mice were tested at P30 for social interactions using a three-chambered social interaction apparatus (Crawley, 2004). Prior to testing, animals were habituated for 5 min in the apparatus; testing was then conducted in two phases. In the first phase, an age- and sex-matched stranger mouse was introduced under a wire mesh cup in one of the two-side chambers, along with an empty mesh wire cup in the other side chamber. The amount of time spent in each chamber was recorded for the test mouse in a 10-min trial using TopScan (CleverSys Inc.). In the second phase, a novel stranger (Stranger #2) was introduced into the second side chamber under a mesh cup, whereas the original stranger mouse (Stranger #1) remained in its original position. Again, the amount of time spent in each of the three chambers was recorded for the test mouse. The number of sniffing events in each of the three chambers was also scored.
Marble burying
Marble burying was used as a test of repetitive and perseverative behaviour (Thomas et al., 2009). Standard shoebox mouse cages were filled with 4 cm of SaniChip bedding and an array of 20 black glass marbles in a 4×5 arrangement was laid on top of the bedding. Male mice at P60 were introduced to the arena for a 20-min testing period; at the end of the testing period, the array was photographed and the buried marbles were counted. Marbles were scored as buried if >50% of the marble was covered by bedding. In addition to scoring the number of marbles buried, the activity of the animals was also assessed by scoring for digging behaviour for 2 s intervals every 20 s throughout the testing period.
Neuroanatomical characterization
In order to visualize cells potentially responsive to reelin signalling, we utilized a Dab1lacZ reporter mouse (Dab1exKIneo; Pramatarova et al., 2008; Khialeeva et al., 2011). In this animal, Dab1 coding sequences are replaced with a lacZ gene. Dab1 is a downstream mediator of reelin signalling; thus those cells that express the lacZ reporter are potentially responsive to reelin signalling. Dab1exKIneo male mice were intercrossed with heterozygous reeler female mice (Rl+/−), which were then used for osmotic minipump implantation as described above. CPO- and vehicle-treated Rl+/+ and Rl+/− mice that were also heterozygous for the Dab1exKIneo allele were collected at P14, perfused with 4% (v/v) PFA (paraformaldehyde) and the brains dissected and fixed by immersion for 4 h. After fixation, the brains were washed, infiltrated overnight with 30% (w/v) sucrose, then embedded and frozen in the OCT mounting medium (Sukura Finetek). Sections were cut at 30 μm, air-dried, then reacted with X-Gal reaction buffer as previously described (Choe et al., 2006). Selected sections were photographed using a Zeiss Axioskop equipped with a cooled CCD camera; photographs were minimally processed for colour balancing using Adobe Photoshop. Densitometry was performed using NIH ImageJ by creating a column average plot for each section using profile plot on a weighted greyscale value (RGB 0.30, 0.59, 0.11) assigned to each pixel.
RESULTS
To evaluate the effects of possible gene–environment interactions in the development of behaviours and anatomical phenotypes relevant to ASDs, we have examined mice that express reduced levels of reelin protein and were exposed to organophosphate pesticides during a period of active cortical development. We used a combination of biochemical analysis, to assess ACHE activity and the levels of reelin protein, behavioural analysis, to assess phenotypes relevant to ASD and anatomical analysis, to assess changes in the organization and anatomy of olfactory, cortical and cerebellar regions. A schematic outline of the experimental design is provided in Figure 1. All mice were provided with exposure to either CPO or to a vehicle control at E13.5–E16.5. This period corresponds to a time of robust cortical development in mice when reelin signalling is known to be active, and has also been used in prior studies (e.g. Laviola et al., 2006), thus allowing comparison of results across different laboratories. Behavioural testing was performed following a set schedule and in the same order for each cohort of mice, to control for testing or age-related artefacts. Anatomical analysis was performed on mice collected at P14, when cortical and subcortical development is largely complete, eliminating possible artefacts due to immaturity of the brain. In addition, this age was chosen to ensure strong expression of the Dab1lacZ reporter. Dab1 activity is incrementally decreased postnatally (Keshvara et al., 2002), thus, to ensure accurate detection of cells that are likely to be responsive to reelin signalling, anatomical analysis was performed while reporter expression was still strong.
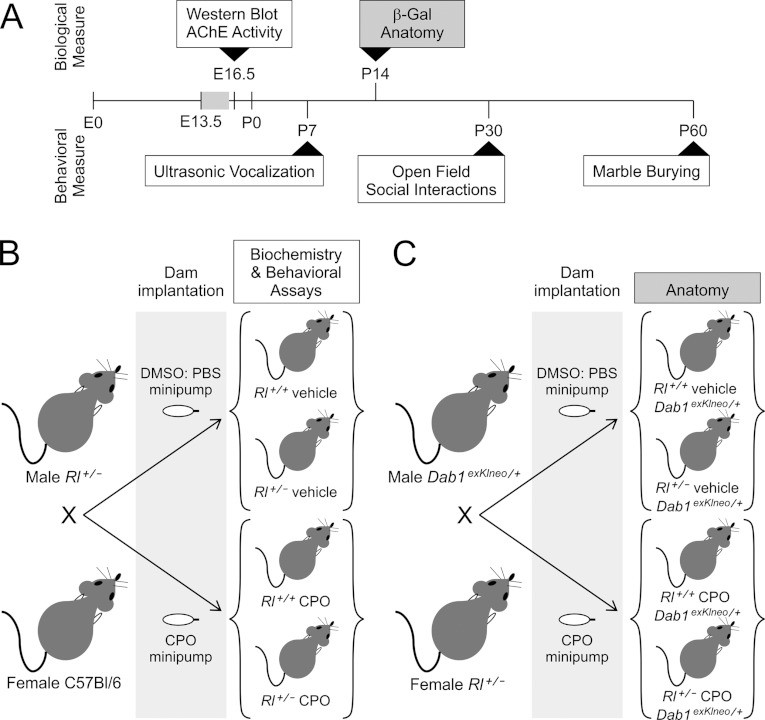
Figure 1. Timeline (A) and mouse-breeding scheme (B, C) for data presented in this study.
Minipumps were implanted at E13.5 and were active for 3 days (grey box in A). Embryos were collected at E16.5 for ACHE activity analysis and for Western blotting. Behavioural testing was conducted from P7 to P60, with ultrasound vocalization tested at P7, open field behaviour and social interactions tested at P30 and marble burying tested at P60. Mice used for behavioural testing were generated by intercrossing Rl+/−− males and C57Bl/6 females (B). Mice used for anatomical analysis were generated by intercrossing male Dab1exKIne°/+ mice with Rl+/− females (C). In both breeding schemes, females were implanted with either CPO- or vehicle-loaded minipumps. The genotypes of offspring used for anatomical and behavioural analysis are illustrated in the brackets in (B) and (C).
Survival, growth, ACHE activity and reelin protein levels
To determine the prenatal effects of CPO exposure, we first examined the average litters of CPO-treated pups compared with vehicle-treated pups. Prenatal CPO exposure did not have significant effects on litter size, with CPOtreated litters averaging 7.4 pups (n=9 litters) and vehicle-treated litters averaging 7.5 pups (n=13 litters). We were curious whether prenatal organophosphate exposure might differentially affect Rl+/− mice, which have reduced levels of reelin protein, as compared with Rl+/+ mice. However, we found an approximately equal distribution of Rl+/+ and Rl+/− mice in our litters, with 52% of pups being Rl+/+ and 48% of pups being Rl+/− after CPO treatment and 48% of pups being Rl+/+ and 52% being Rl+/− after vehicle treatment (Table 1). This suggests that prenatal CPO exposure did not differentially affect the survival of Rl+/+ or Rl+/− embryos. We did observe an increased percentage of males in CPO-treated litters (66% male against 34% female), however, only 67 pups were obtained in total, so this distribution may reflect normal variation in litter composition.
Table 1. Number of litters and pups derived from vehicle-implanted or CPO-implanted dams.
| Group | Total pups | Number of litters | Average litter size | Males | Females | +/+ | +/− |
|---|---|---|---|---|---|---|---|
| Vehicle-treated | 98 | 13 | 7.5 | 51 (52%) | 47 (48%) | 47 (48%) | 51 (52%) |
| CPO-treated | 67 | 9 | 7.4 | 44 (66%) | 23 (34%) | 35 (52%) | 32 (48%) |
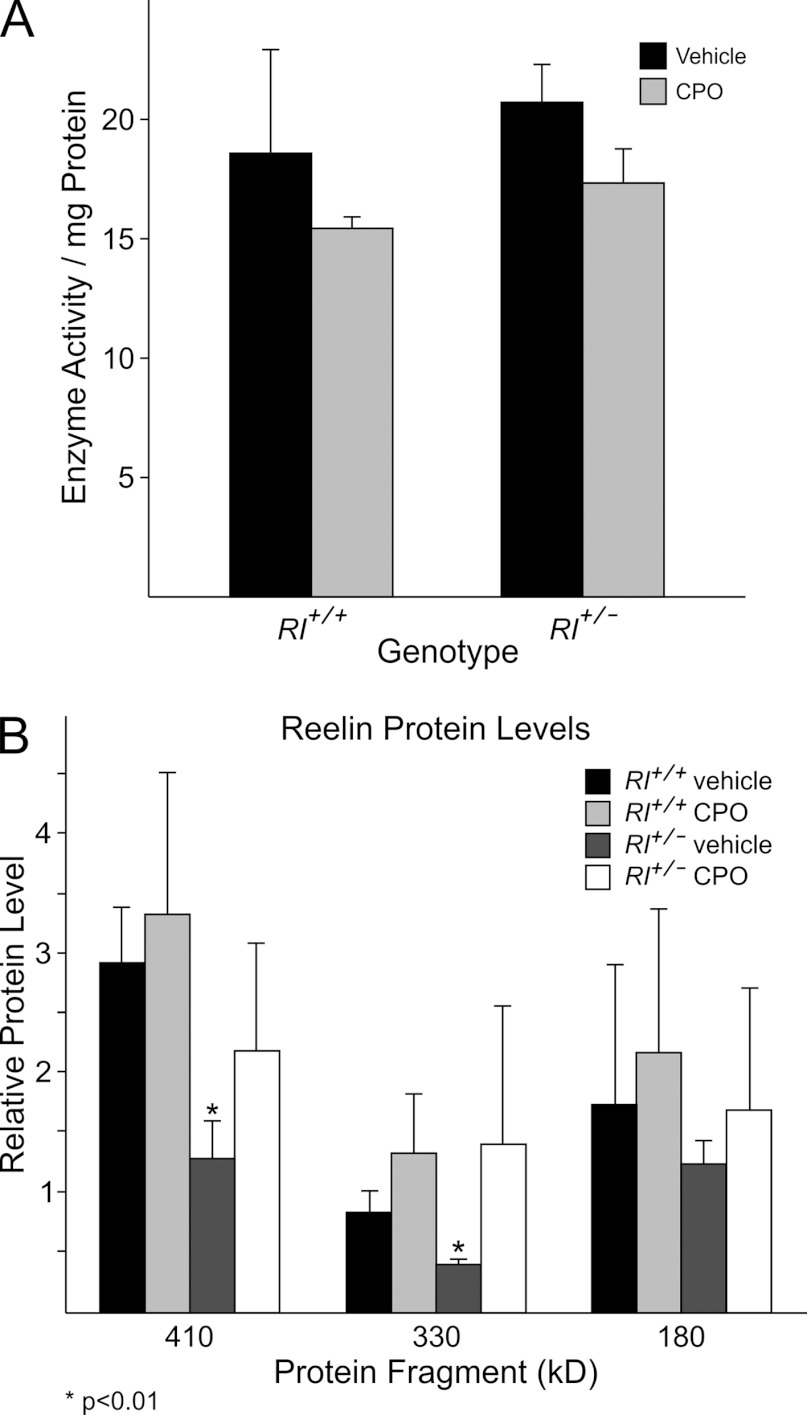
To assess the efficacy of prenatal CPO exposure, we measured ACHE activity in embryos. ACHE is a serine protease and serine protease activity is specifically inhibited by organophosphate pesticides. CPO was administered via an osmotic minipump for 3 days beginning at E13.5. This period corresponds to the peak period of neurogenesis and cell migration in the mouse cerebral cortex (Caviness, 1982); thus this window of application is the most likely time to affect the development of anatomical alterations relevant to autism. Three days after minipump implantation, we found that baseline ACHE activity was slightly higher in Rl+/− animals compared with Rl+/+ animals (Figure 2), similar to results reported for postnatal mice (Laviola et al., 2006). CPO treatment reduced ACHE activity in both Rl+/+ and Rl+/− mice to approximately 85% of the starting value, demonstrating that CPO derived from the osmotic minipumps can affect embryonic brain biochemistry.
Figure 2. ACHE activity (A) and relative levels of reelin protein normalized to β-actin (B) in E16 mouse brains following 3 days of prenatal vehicle or CPO treatment.
To directly assess the effects of prenatal CPO exposure on reelin protein, we performed Western blotting. Full-length reelin protein is approximately 410 kDa; cleavage of the protein yields two major fragments at 330 and 180 kDa. As expected, vehicle-treated Rl+/− embryos showed approximately 50% less full-length reelin protein than vehicle-treated Rl+/+ embryos (Figure 2). The 330 kDa fragment was also reduced approximately 50%, whereas the 180 kDa fragment was reduced, but not to the same extent. CPO treatment of Rl+/+ embryos did not significantly change the levels of either full-length reelin or the cleavage fragments. Surprisingly, CPO treatment restored the levels of reelin protein to near WT levels in Rl+/− embryos, both for full-length protein and for the cleavage fragments. This supports our hypothesis that CPO exerts a protective effect on reelin protein.
Behavioural alterations in response to loss of reelin and to CPO exposure
Three different behavioural measures were used to assess phenotypes relevant to ASD. First, we recorded ultrasound vocalization on mice at P7. Ultrasound vocalization has been reported to peak at P7–P10 in most strains of mice. Empirical observations in our laboratory suggested that the most robust vocalizations were produced at P7 in our mice; therefore we used this age for all additional testing. Mice were then screened for open field behaviour and social interaction at P30 and for marble burying and digging behaviour at P60. Each cohort of animals was tested at the same ages and following the same schedule.
Ultrasound vocalization
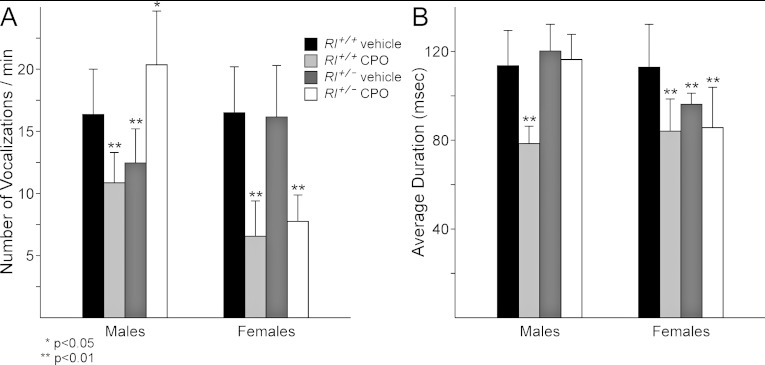
Infant mice emit ultrasonic vocalizations when separated from their dams to elicit pup retrieval, suggesting that these vocalizations play an important role in communication between mothers and their pups. Changes in ultrasound vocalization have also been used to characterize possible mouse models for autism (e.g. Scattoni et al., 2009; Young et al., 2010), although unlike human language, mouse ultrasound vocalizations are innate behaviours suggesting that these types of communication are not equivalent (Fischer and Hammerschmidt, 2011). Rl+/+ and Rl+/− pups with and without CPO exposure were assessed for ultrasound vocalization during a brief separation from their dams at P7 (Figure 3).
Figure 3. Number (A) and duration (B) of ultrasound vocalizations in mice at P7.
Statistical significance as determined by t-test comparisons with vehicle-treated Rl+/+ animals is indicated by asterisks over the appropriate bars.
The number of vocalizations varied significantly across all four groups in both male and female mice by ANOVA [males: F(3,36)=16.1954, P=7.9229×10−7; females: F(3,36)=26.4314, P=3.2636×10−9]. In male mice, both CPO-treated Rl+/+ mice and vehicle-treated Rl+/− mice produced fewer vocalizations than vehicle-treated Rl+/+ animals (Figure 3A). In contrast, CPO-treated Rl+/− mice made more cries than vehicle-treated Rl+/+ animals. This suggests both individual effects of reduced reelin and CPO exposure and combinatorial effects of the two variables together. In female mice, both CPO-treated Rl+/+ and Rl+/− animals showed significantly reduced vocalizations, whereas vehicle-treated animals showed similar numbers of vocalizations (Figure 3A). The number of female vocalizations thus seems specifically sensitive to CPO exposure, with no additional effect derived from reduced reelin expression.
Examination of the average duration of vocalizations also showed significance across the four groups by ANOVA [males: F(3,36)=15.0805, P=1.6148×10−6; females: F(3,36)=33.9508, P=1.3528×10−10]. In males, duration of vocalizations was significantly reduced only in CPO-treated Rl+/+ males (Figure 3B), with no apparent reductions in Rl+/− mice. However, duration of vocalization was restored to control levels in CPO-treated Rl+/− mice, again suggesting a combinatorial interaction between variables in male mice. In contrast, in female mice, both CPO treatment and loss of reelin expression appeared to reduce the number of vocalizations to levels below that exhibited by vehicle-treated Rl+/+ animals. In females, combinatorial interactions between reduced reelin and CPO exposure were not apparent, as there was no change in vocal duration between vehicle-treated Rl+/− mice and CPO-treated Rl+/− mice.
Open field behaviour
Rl+/+ and Rl+/− pups with and without CPO exposure were assessed for open field behaviour at P30 (Figure 4). For these experiments, mice were introduced to an open field enclosure and allowed to explore for 20 min. Mice were scored for the amount of time spent in the centre of the chamber against the periphery, as well as for sniffing, an exploratory behaviour, in both the centre and the periphery of the arena. All groups of male mice spent approximately 60% of their time in the periphery and 40% in the centre, with no significant differences between any of the groups (Figure 4A). Most female mice showed a similar spatial distribution, with the exception of CPO-treated Rl+/− mice, which showed a small but significant skewing toward more time spent in the centre and less time spent in the periphery, when compared with vehicle-treated Rl+/+ mice (Figure 4D). This suggests a possible decrease in anxiety in CPO-treated Rl+/− female mice. Similar decreases in anxiety with CPO treatment have been observed previously in mice using an elevated plus maze (Riccieri et al., 2006).
Figure 4. Open field behaviour.
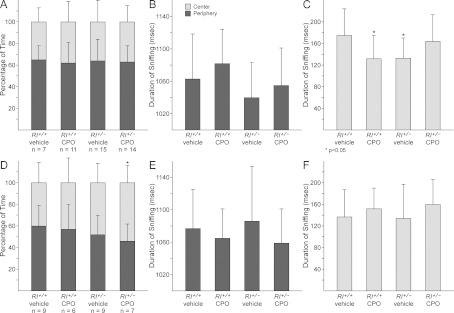
Male (A–C) and female (D–F) mice were scored for time spent in the centre against the periphery (A, D) and for duration of sniffing in both the periphery (B, E) and the centre (C, F). Statistical significance as determined by t-test comparisons with vehicle-treated Rl+/+ animals is indicated by asterisks over the appropriate bars. ANOVA analysis of the time distribution across all four groups did not indicate significance [males: F(3,45)=0.1251, P=0.9448; females: F(3,44)=1.2901, P=0.2896].
Sniffing behaviour in the centre and periphery of the arena was quantified by measuring the amount of time engaged in sniffing in each of these areas. Female mice did not show any significant alterations in sniffing in either of the two areas regardless of genotype or treatment, but significant decreases in sniffing in the centre of the arena were seen in both CPO-treated Rl+/+ and vehicle-treated Rl+/− male mice; both of these alterations can be interpreted as slight increases in anxiety (Figure 4C). However, CPO-treated male Rl+/− mice showed sniffing durations that were the same as vehicle-treated Rl+/+ male mice. This again suggests a combinatorial interaction between reduced reelin and CPO exposure, similar to that observed in male ultrasound vocalization behaviour. In summary, both male and female mice showed slight changes in open field behaviour in response to either decreasing levels of reelin or to CPO exposure, although by different measures. Females showed a combinatorial effect of both treatments on the distribution of time spent in the centre against periphery of the arena, whereas males showed a combinatorial effect of both treatments on sniffing behaviour in the centre. These results also demonstrate sex-specific changes, with males showing slightly increased anxiety and females showing decreased anxiety.
Social interaction
Social interactions were tested in a three-chamber social interaction apparatus (Crawley, 2004). Two testing paradigms were used. In the first (phase I, Figures 5A–5D), the test mouse was introduced to a single stranger mouse, contained under a wire mesh cup in one of the two side chambers in the apparatus, whereas the other side chamber contained an empty cup. In this paradigm, the amount of time spent in each of the three chambers, along with the amount of sniffing, was measured. As expected, vehicle-treated Rl+/+ males and females both showed an increased preference for spending time in the chamber with the stranger mouse and this preference was not significantly altered by either genotype or CPO treatment (Figure 5). Sniffing behaviour was also assessed by quantifying the amount of time spent sniffing at the cup containing the stranger mouse and at the empty cup. Males and females both showed an increased preference for sniffing at the stranger mouse. In males, sniffing behaviour was not significantly affected by either genotype or CPO treatment, but in females, CPO treatment significantly increased the amount of time spent sniffing at the stranger mouse for both Rl+/+ and Rl+/− mice.
Figure 5. Social interaction behaviour.
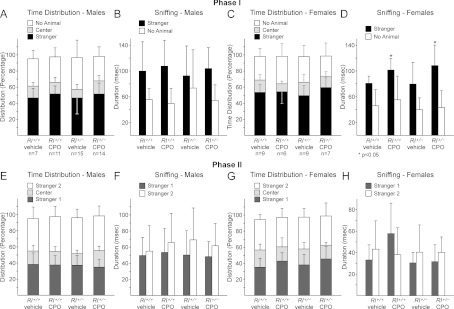
Male and female mice were assessed in phase I (A–D, stranger against no stranger) and phase II (E–H, novel stranger against familiar stranger) paradigms. Statistical significance as determined by t-test comparisons with vehicle-treated Rl+/+ animals is indicated by asterisks over the appropriate bars. ANOVA analysis showed no significant change in the time spent with the novel stranger for either phase I or phase II [phase I males: F(3,43)=0.4010, P=0.7530; phase I females: F(3,27)=0.9031, P=0.4525; phase II males: F(3,43)=0.2048, P=0.8925; phase II females: F(3,27)=0.1341, P=0.9389].
In the second testing paradigm (phase II, Figures 5E–5H), a novel stranger mouse was introduced under the previously empty cup and time in each of the chambers and sniffing behaviour was recorded for all animals. Both males and females showed a decrease in the amount of time spent with a familiar stranger (Stranger 1) and a concomitant increase in the time spent with a novel stranger (Stranger 2); neither genotype nor CPO treatment affected this distribution. Sniffing at the novel stranger was moderately increased for all males and for most females. However, CPO-treated Rl+/+ female mice showed the opposite response and sniffed more at the familiar stranger than at the novel stranger (Figure 5H), although the differences were not statistically significant. These observations suggest that genotype and CPO treatment had moderate effects on social behaviour, with only significant effects on sniffing behaviour in female mice. CPO treatment appears to slightly increase sniffing behaviour in female mice in the phase I trials, and to slightly alter sniffing only in Rl+/+ females in phase II trials.
Marble burying
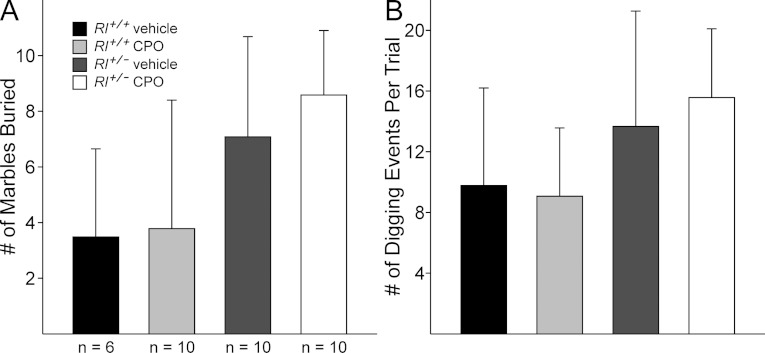
Marble burying is often used as a measure of anxiety in mice, but recent studies suggest it may be more accurately used as a measure for repetitive and perseverative behaviours (Thomas et al., 2009; Gould et al., 2012), which are some of the core behaviours in ASDs. We examined two behaviours using this assay – the total number of marbles buried at the end of the 20-min test period and the number of digging events occurring throughout each trial. The total number of marbles buried was affected by genotype, but not by CPO treatment, as elevated levels of marble burying were observed in Rl+/− animals regardless of CPO exposure (Figure 6). t-test comparisons did not identify significant differences between vehicle-treated Rl+/+ mice and any other group, but the distribution of marbles buried across all four groups was significant by one-way ANOVA [F(3,35)=4.263, P=0.012]. A moderate increase in digging was also observed in Rl+/− animals, but this increase was not statistically significant [ANOVA: F(3,35)=2.607, P=0.069].
Figure 6. Marble burying (A) and digging behaviour (B).
The marble burying assay was scored for the total number of marbles buried (A) and the number of digging events throughout the trial (B).
In summary, our behavioural testing yielded some specific behavioural alterations attributable either to prenatal CPO exposure or to decreased reelin expression. Although our model would have predicted an increase in autistic-like behaviours in animals with decreased reelin expression and CPO exposure, the changes we saw were not entirely consistent with this model. Some behaviours seemed sensitive to CPO exposure, whereas others were somewhat more sensitive to loss of reelin expression. Interestingly, combining CPO exposure and loss of reelin expression seemed to have more of an effect in female mice rather than in male mice. Given the highly increased prevalence of autism in human males, this is surprising. Although decreased social interactions were evident in both males and females, they appeared to be differentially induced, with females showing decreased social interactions in response to CPO exposure, whereas males showed decreased social interactions with decreased reelin expression.
Anatomical alterations in response to loss of reelin and to CPO exposure
Reelin signalling is known to be critically important for the development of layered structures in the brain including the cortex, hippocampus and cerebellum. We investigated the development of several layered structures that have a particular relevance to autism, including the olfactory bulb, the piriform cortex, the neocortex, the hippocampus and the cerebellum. To identify those cells that are potentially responsive to reelin signalling, and would thus be the cells likely to be affected by changes in reelin expression or activity, we used a Dab1lacZ reporter mouse. Dab1 is an intracellular adaptor protein that is phosphorylated in response to reelin signalling; thus its presence is an indicator of those cells that are capable of responding to reelin signalling.
The olfactory system
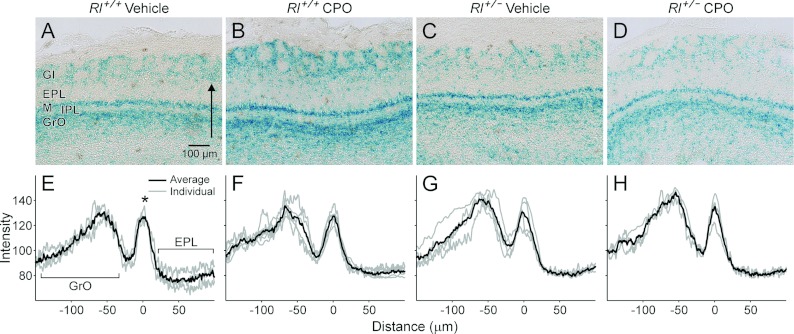
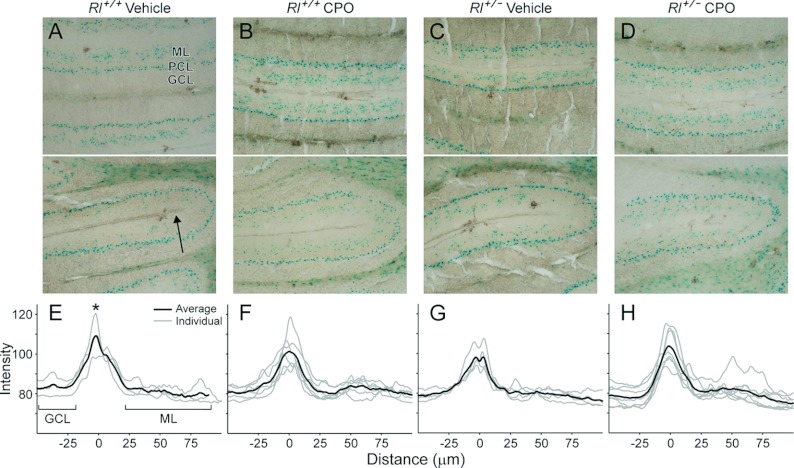
Olfactory identification has been reported to be impaired in several studies of ASD patients (Suzuki et al., 2003; Bennetto et al., 2007) and impaired olfactory discrimination may provide a categorical tool for identifying sensory subtypes of autism (Lane et al., 2011). We examined the organization of two structures implicated in olfactory reception and information processing, the olfactory bulb (Figure 7) and the piriform cortex (Figure 8). In vehicle-treated Rl+/+ mice, three distinct regions clearly express the Dab1lacZ marker in the olfactory bulb – the glomerular layer, the mitral cell layer and the granule cell layer (Figure 7A). LacZ expression outlines the glomeruli, and is found at high levels in both the mitral cell layer and the granule cell layer, with lower lacZ expression in the external and internal plexiform layers. Vehicle-treated Rl+/− mice and CPO-treated Rl+/+ and Rl+/− mice all retain Dab1lacZ expression in these layers, although with some distinct differences in the distribution of lacZ expression. No differences were noted in the glomerular layer, but Rl+/− animals, both with and without CPO treatment showed variability in labelling in the granule cell layer, often with an apparent decrease in the intensity of labelling, along with an increase in the thickness of the internal plexiform layer (Figures 7C and 7D). The mitral cell layer also appears thinner in these animals.
Figure 7. β-Galactosidase expression (A–D) and intensity plots (E–H) for the olfactory bulb.
The arrow in (A) indicates the span and direction of intensity values plotted in (E–H). The asterisk in (E) indicates the position of the mitral cell layer, and the granule cell layer and external plexiform layer are indicated by the brackets. Gl, glomerular layer; EPL, external plexiform layer; M, mitral cell layer; IPL, internal plexiform layer; GrO, graule cell layer.
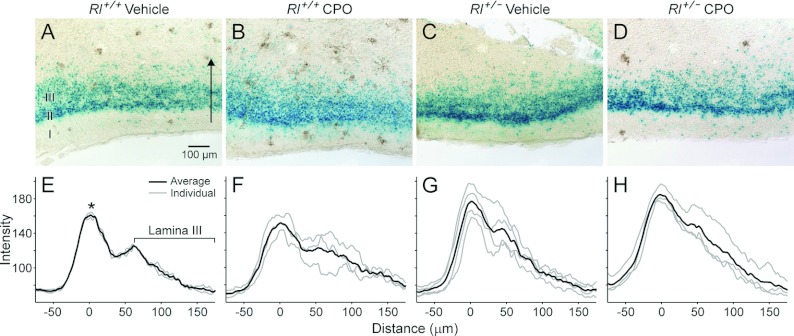
Figure 8. β-Galactosidase expression (A–D) and intensity plots (E–H) for the piriform cortex.
The arrow in (A) indicates the span and direction of the intensity plots in (E–H). The asterisk in (E) indicates the position of lamina II and the bracket indicates the position of lamina III. I, II, III, laminae I–III.
To quantify differences in lacZ distribution, we performed a density analysis of lacZ expression. To do this, the average labelling intensity was calculated from the internal edge of the granule cell layer to the external margin of the external plexiform layer using NIH ImageJ. Density plots were standardized by superimposing the peak of the mitral cell layer from each individual plot and an average density value was calculated for all animals in each genotype and treatment group. Plots from vehicle-treated Rl+/+ mice showed tight correlations in the position of the granule cell layer and the mitral cell layer, but some spread of lacZ distribution in the external plexiform layer (Figure 7E). The intensity of mitral cell layer labelling showed increased variability in other treatment conditions, particularly in vehicle-treated Rl+/− animals and in CPO-treated Rl+/+ animals (Figures 7F and 7G). In addition, labelling intensity was highly variable in the granule cell layer, confirming our qualitative observations. Thickness of the inner plexiform layer, defined as the distance from the granule layer peak to the mitral layer peak also increased in CPO-treated Rl+/+ animals to 67.33±6.81 μm (n=7) from 57±1.73 μm (n=3) in vehicle-treated Rl+/+ animals. Surprisingly, CPO-treated Rl+/− animals showed a relatively normal intensity profile (Figure 7H). This suggests possible opposing effects of reelin heterozygosity and CPO treatment, resulting in a more normal lacZ intensity distribution in CPO-treated Rl+/− mice than in either vehicle-treated Rl+/− mice or CPO-treated Rl+/+ mice.
The piriform cortex serves as the first cortical relay station for olfactory input from the mitral cells of the olfactory bulb (Haberly, 2001). The piriform cortex is trilaminar, with a largely cell-free superficial lamina (lamina I) and two cell-dense deeper laminae (Figure 8). Both vehicle-treated and CPO-treated Rl+/− animals showed an increase in the density and width of lamina II, and this cell layer appeared much more pronounced in Rl+/− animals as a result. The distinction between laminae II and III decreased in CPO-treated Rl+/+ and the combined width of both laminae increased. A few scattered β-gal expressing cells were observed in lamina I of vehicle-treated Rl+/+ animals, and there was a qualitative increase in the numbers of these cells in both Rl+/− and CPO-treated animals. In particular, CPO-treated Rl+/+ animals had far greater expression of β-gal in lamina I (Figure 8C).
The neocortex
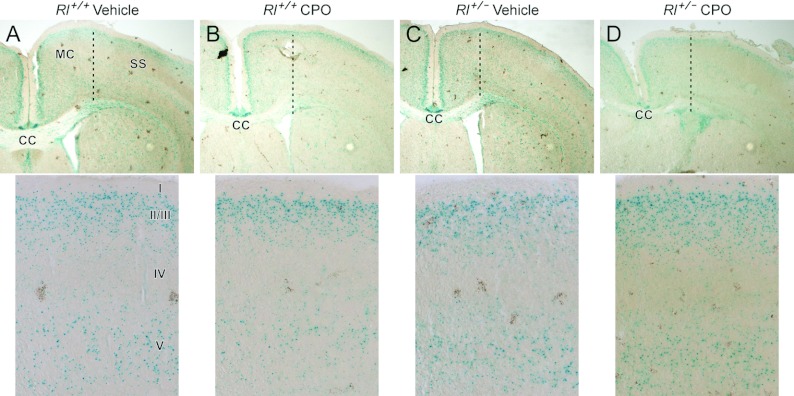
Reelin signalling has been extensively studied as a factor in neocortical patterning and loss of this signalling leads to altered cell positioning and inverted cortical lamination (reviewed in Rice and Curran, 2001). Moderate neocortical disruptions have also been observed in some autistic patients (Bailey et al., 1998; Casanova et al., 2002), although most studies fail to note any neocortical alterations (e.g. Bauman and Kemper, 2005). Neocortical organization was largely normal in our studies. Vehicle- and CPO-treated mice exhibited essentially normal neocortical layering regardless of genotype. In the somatosensory cortex, β-gal expression was noted in superficial cortical laminae (laminae II/III) and also in lamina V (Figure 9). Lamina IV was largely free of β-gal expression. In general, Dab1 expression, as judged by β-gal expression, was noticeably less pronounced in the neocortex than in the piriform cortex. This suggests several possibilities: that Dab1 expression may be required at earlier stages in the neocortex than in the piriform cortex and thus lacZ expression has dissipated from the neocortex by the postnatal stages examined in this study or that continued Dab1 expression is not required for cortical patterning and maintenance in the postnatal neocortex as it is in the piriform cortex.
Figure 9. β-Galactosidase expression in the neocortex.
(A–D) β-Galactosidase expression in the cortex; upper panels are low magnification overviews and lower panels are high magnification views of the somatosensory cortex. The dotted lines in the upper panels indicate the boundary between MC (motor cortex) and SS (somatosensory cortex). The position of the laminae is indicated by roman numerals I, II/III, IV and V.
The hippocampus
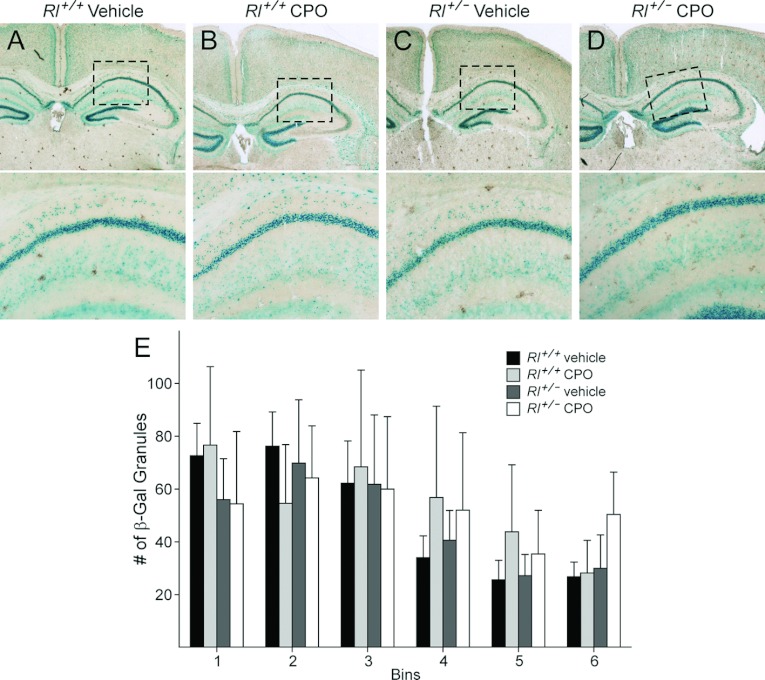
In the absence of reelin signalling, hippocampal layering is significantly altered. Pyramidal cells, which normally form a compact layer in the CA fields, become dispersed dorsally into the stratum oriens, while the granular dentate gyrus becomes extensively disorganized (Caviness and Sidman, 1973; Förster et al., 2006). In our studies, Dab1 β-gal expression was seen as discrete punctate labelling in a dense layer in the CA pyramidal cell layer (Figure 10). Punctate labelling was evident, but more dispersed, in the stratum oriens, whereas the radiatum exhibited sparse labelling. Qualitative assessment suggested that there was increased β-gal expression in the stratum oriens in both vehicle-treated Rl+/− animals and in CPO-treated Rl+/+ animals. To quantify these changes, the stratum oriens was divided into six bins from medial to lateral and the number of β-gal grains counted in each bin. Vehicle-treated Rl+/+ animals had β-gal expression at the highest levels in the three medial bins, whereas expression substantially decreased in the three more lateral bins (Figure 10E). Vehicle-treated Rl+/− animals showed a similar distribution of expression. In contrast, CPO-treated Rl+/+ animals showed approximately equal β-gal distribution across the first four bins, with moderate decreases in expression in the most lateral bins. CPO-treated Rl+/− animals also showed relatively equal β-gal expression across the first four bins. In contrast to all other groups, CPO-treated Rl+/− animals showed higher levels of β-gal expression in the most lateral bin. This suggests a difference in the distribution of Dab1-expressing cells in the stratum oriens in CPO-treated Rl+/− animals.
Figure 10. β-Galactosidase expression in the hippocampus.
(A–D) Low magnification (upper panels) and high magnification (lower panels) of the rostral hippocampus. Boxed areas in (A–D) are illustrated in the lower panels. (E) Distribution of ß-galactosidase reactivity in the stratum oriens in six bins. Bin 1 is the most medial and bin 6 is the most lateral.
The cerebellum
Reeler mutant mice are known to have cerebellar abnormalities, including lack of distinct lamination and foliation along with displaced Purkinje cells (Bauman and Kemper, 2005). We therefore examined cerebella for changes in either layering or cell placement. We examined both the central vermis and the more lateral ansiform and simple lobules. Dab1–β-gal expression was seen at high levels in Purkinje cells in both regions, and dispersed throughout the molecular layer (Figure 11) in vehicle-treated Rl+/+ animals. Small amounts of β-gal expression were also seen in the granule cell layer. There was a clear separation of Purkinje cell expression from β-gal expression in the molecular layer and expression in the Purkinje cell layer appeared continuous throughout the extent of the folia examined, without significant gaps (Figure 11A). Vehicle-treated, Rl+/− animals exhibited approximately the same distribution of β-gal, suggesting no apparent changes in cerebellar layering in heterozygous animals (Figure 11C). However, CPO treatment in Rl+/+ animals increased β-gal expression in the molecular layer of the lateral lobules, but not in the vermis (Figure 11B). To quantify these changes, sections were scored for the levels of β-gal expression (high, medium, low, using levels equivalent to those seen in the vermis as ‘high’). Assigning a value of 1 to low, 2 to medium and 3 to high suggested that vehicle-treated Rl+/+ animals had an average expression of 2±0 (n=3). Levels were decreased on average in vehicle-treated Rl+/− animals (1.375±0.48, n=4), increased in CPO-treated Rl+/+ animals (2.625±0.48, n=4), and seen at WT levels in CPO-treated Rl+/− animals (2.07±0.73, n=7). Although vehicle-treated Rl+/+ and Rl+/− animals and CPO-treated Rl+/+ animals had fairly consistent levels of β-gal expression in the lateral lobes, expression was much more variable in CPO-treated Rl+/− animals. This suggests some moderate changes in cerebellar layering specifically in the more lateral lobes.
Figure 11. β-Galactosidase expression in the cerebellum.
(A–D) β-Galactosidase expression in the simple (upper) and ansiform (lower) lobules of the vermis of the cerebellum. The arrow in the lower panel of (A) indicates the span and direction of the intensity plots in (E–H). (E–H) Intensity plots of ß-galactosidase expression in the ansiform lobule. The asterisk in (E) indicates the position of the Purkinje cell layer, and the position of the granule cell layer and molecular layer are indicated by the brackets. ML, molecular layer; P, Purkinje cell layer; GC, granule cell layer.
In summary, our anatomical studies made use of a Dab1lacZ reporter mouse to visualize all cells potentially responsive to reelin signalling. Anatomical studies have been conducted on Rl+/− animals and reveal subtle phenotypes in cell placement and dentritic arborization (Niu et al., 2008). However, all prior studies have relied on examining only specific cell types. Our current study provided a method to perform a survey of potentially all cell populations responsive to reelin signalling. We selected several brain regions known to be affected by disruptions in reelin signalling and assessed the organization of Dab1-expressing cells in these regions. We demonstrate subtle alterations in cell layering and positioning stemming from either reduced reelin expression or CPO exposure, but we found no evidence for combinatorial interactions between these variables leading to increased brain alterations.
DISCUSSION
Our study demonstrates clear but modest changes in behaviour and brain anatomy as a result of either decreased reelin expression or CPO exposure. Based on the identification of decreased reelin expression and organophosphate pesticide exposure as possible contributing factors to ASD (Abrahams and Geschwind, 2008; Keller and Persico, 2003), we examined mouse behaviours representative of the three key arenas in ASD – communication, social interaction, and restricted and repetitive behaviours. We found subtle alterations in each of these three behaviours. Some of the alterations, such as increased marble burying, appeared to be directed by genotype alone, whereas others, such as ultrasound vocalization and social interaction, could be altered by either decreased reelin or by exposure to CPO. Additionally, male and female mice often responded differently to these factors. Curiously, we failed to find an additive detrimental effect of decreased reelin combined with CPO exposure on either mouse behaviour or brain anatomy. In fact, we often observed the opposite result – combining decreased reelin and CPO exposure mitigated the effects of either factor alone.
Prior studies have demonstrated a functional interaction between reelin and organophosphate exposure, but the mechanisms for this interaction remain unclear. CPO exposure may have several effects on brain development. Exposure to lethal doses of CPO produces acute neurotoxicity, which likely stems from inhibition of ACHE, producing a cholinergic crisis due to synaptic accumulation of acetylcholine and the hyperstimulation of postsynaptic muscarinic and nicotinic receptors (Flaskos, 2012). However, exposure to sublethal doses of CPO also produces neurodevelopmental toxicity, although the mechanisms for this effect are less clear. Sublethal CPO exposure generates stable adducts on tyrosine and serine, altering the function of many proteins, although not in a predictable fashion. In one study, chronic exposure to low levels of CPO altered the structure of microtubules and affected the complement of microtubule-associated proteins (Jiang et al., 2010). These effects have the potential to alter cytoskeletal structure and to affect the migratory ability of developing neurons. Reelin also has known cytoskeletal effects, with disruption of the reelin signalling pathway resulting in hyperphosphorylation of tau, disturbing interactions with microtubules and destabilizing the cytoskeleton (Heisberger et al., 1999). If both reelin signalling and CPO exposure converge on the cytoskeleton, then we might expect that introduction of these two variables would produce more pronounced alterations in cell migration and brain anatomy. However, this does not appear to be the case in our studies. Alternatively, CPO may affect the structure and stability of reelin itself. Secreted reelin undergoes two cleavage events to produce three fragments (Lambert de Rouvroit et al., 1999; Jossin et al., 2007); as reelin is cleaved, its signalling ability diminishes (Kohno et al., 2009). The enzyme(s) responsible for these cleavages have not yet been identified, but preliminary evidence suggests the involvement of proprotein convertase 2 or ADAM (a disintegrase and metalloprotease) family members (Kohno et al., 2009; Hisanaga et al., 2012). It is possible that CPO may affect the function of these enzymes to inhibit the cleavage of reelin, thus potentially increasing the pool of full-length reelin; this possibility is consistent with our Western blotting results. In this case, animals with decreased reelin levels may have partial restoration of reelin signalling activity in the presence of CPO, at least to levels that allow generation of normal anatomical organization.
A prior study has also demonstrated specific behavioural changes in heterozygous reeler mice embryonically exposed to CPO (Laviola et al., 2006), but similar to our study, the results were also paradoxical. In Laviola et al. (2006) the number of ultrasound vocalizations was decreased in CPO-treated Rl+/+ mice and increased in CPO-treated Rl+/− mice. Additionally, scopolamine- and amphetamine-induced locomotion was decreased in CPO-treated Rl+/+ animals and increased in CPO-treated Rl+/− animals. While we did not test scopolamine- or amphetamine-induced locomotion, our ultrasound vocalization studies showed similar effects to those seen by Laviola et al. (2006) with CPO treatment decreasing ultrasound vocalization in WT mice and increasing it in Rl+/− male mice. However, in female mice, the number of ultrasound vocalizations was not affected by changes in reelin expression, as both vehicle-treated Rl+/+ and Rl+/− animals showed similar numbers of vocalizations. In contrast, CPO treatment significantly decreased the number of vocalizations in both Rl+/+ and Rl+/− females.
Additional behavioural tests also showed paradoxical results, with distinct differences between males and females. Open field testing showed no difference in centre against periphery time distribution in males, but did show decreased sniffing in the centre of the arena in both CPO-treated Rl+/+ mice and vehicle-treated Rl+/− mice, whereas CPO-treated Rl+/− mice showed no significant differences from vehicletreated Rl+/+ animals. Both CPO-treated Rl+/+ and vehicle-treated Rl+/− female mice showed modest, but not significantly different time distribution, whereas CPO-treated Rl+/− female mice showed a possible additive effect of both phenotypes and demonstrated a preference for the centre of the arena. In contrast, no sniffing differences were noted in any treatment condition in female mice. Social interactions were largely indistinguishable from control animals in both males and females, although a slight but significant increase in sniffing at stranger mice was demonstrated by CPO-treated Rl+/+ and Rl+/− females, suggesting that this behaviour is modulated by CPO treatment but not by reelin genotype. In contrast, marble burying appeared to be affected solely by genotype in male mice, with no effect of CPO treatment. These results suggest a complex effect of decreases in reelin expression and CPO treatment on behaviours related to ASDs.
Anatomical substrates for ASDs have been poorly defined in humans, probably due to the high variability of the disorder. By using a Dab1lacZ reporter mouse, we were able to identify brain regions that are potentially responsive to reelin signalling, by virtue of their expression of Dab1. Identifying brain structures that are altered in response to changes in genetic or environmental factors may represent a first step towards identifying possible anatomical substrates of ASDs. Reelin signalling has been widely recognized as a critical signal in establishing cortical layers, with reeler mutant mice exhibiting inverted cortical layering (reviewed in Rice and Curran, 2001). Surprisingly, decreased reelin expression and/or CPO exposure failed to induce measurable cortical alterations, at least in terms of cell populations expressing Dab1. It is possible that individual cell populations might be mispositioned even in the absence of gross abnormalities, but confirmation of this possibility in the future will require immunohistochemical studies using cell-specific markers.
Unlike the cortex, layering and cell positioning alterations were observed in the olfactory bulb, hippocampus and cerebellum. These alterations tended to take the form of more diffuse β-gal expression in specific cell layers (e.g. in the olfactory bulb), and decreased segregation between identified laminae (e.g in the piriform cortex). Additionally, we observed mispositioned Dab1-expressing cells, e.g. the extra cells present in lamina I of the piriform cortex, the extra cells in molecular layer of the cerebellum, and altered distribution of cells in the stratum oriens in the hippocampus. These changes are subtle and could probably only be visualized using a reporter gene system as we have done. Similar changes might be difficult to detect using imaging studies on human patients; this may be one reason why anatomical substrates of ASDs have to date gone largely undetected. Although we have identified alterations in regional layering and cell positioning, it remains to be seen what specific cell types are affected by reduced reelin expression and/or CPO exposure.
In summary, our findings show measurable effects of reducing reelin expression or early exposure to an organophosphate pesticide, but little exacerbation of phenotypes when both variables are combined. These results do not support the interaction of the reelin signalling pathway and organophosphate pesticides in the generation of ASD, but suggest that each factor has the potential to disrupt specific aspects of nervous system function independently and in an opposite fashion. Thus, when combined, phenotypes generated by each variable independently serve to restore behavioural and anatomical alterations in mice. While genetic and environmental factors are widely thought to act in concert to generate neurodevelopmental disorders, the specific combination of decreased reelin gene expression and CPO exposure appear not to function together in mice to generate the behavioural or anatomical changes similar to those appearing in ASD in humans.
ACKNOWLEDGEMENTS
We thank Ravikumar Ponnusamy, Casssandra Gonzalez and Kimberly Bui for help with behavioural testing. We also thank Donna Crandall for assistance with Figure preparation.
FUNDING
This work was supported by Autism Speaks! and pilot project funding from National Institute of Child Health and Human Development [grant number P50-HD055784 (to E.M.C.)].
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchelli E, Maestrini E. Autism spectrum disorders: molecular genetic advances. Am J Med Genet C Semin Med Genet. 2006;142C:13–23. doi: 10.1002/ajmg.c.30078. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Kuschner ES, Hyman SL. Olfaction and taste processing in autism. Biol Psychiatry. 2007;62:1015–1021. doi: 10.1016/j.biopsych.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–e1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based on [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Sidman RL. Retrohippocampal, hippocampal, and related structures of the forebrain in the reeler mutant mouse. J Comp Neurol. 1973;147:235–254. doi: 10.1002/cne.901470206. [DOI] [PubMed] [Google Scholar]
- Chloe A, Phun HQ, Tieu DD, Hu YH, Carpenter EM. Expression patterns of Hox10 paralogous genes during lumbar spinal cord development. Gene Expr Patterns. 2006;7:730–737. doi: 10.1016/j.modgep.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Ricci I, Sacco R, Liu X, D’Agruma L, Muscarella LA, Guaarnieri V, Militerni R, Bravaccio C, Elia M, Schneider C, Melmed R, Trillo S, Pascucci T, Puglisi-Allegra S, Reichelt K-L, Macciardi F, Holden JJ A, Persico AM. Paroxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiatry. 2005;10:1006–1016. doi: 10.1038/sj.mp.4001714. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Snow A V, Stary JM, Araghi-Niknam M, Reutiman TJ, Lee S, Brooks AI, Pearce DA. Reelin signaling is impaired in autism. Biol Psychiatry. 2005;57:777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Egan EA. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol Neurobiol. 2002;22:139–152. doi: 10.1023/a:1019857620251. [DOI] [PubMed] [Google Scholar]
- Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–21. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaskos J. The developmental neurotoxicity of organophosphorus insecticides: a direct role for the oxon metabolites. Toxicol Lett. 2012;209:86–93. doi: 10.1016/j.toxlet.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Förster E, Jossin Y, Zhao S, Chai X, Frotscher M, Goffinet AM. Recent progress in understanding the role of reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur J Neurosci. 2006;23:901–909. doi: 10.1111/j.1460-9568.2006.04612.x. [DOI] [PubMed] [Google Scholar]
- Gould GG, Seillier A, Weiss G, Giuffrida A, Burke TF, Hensler JG, Rock C, Tristan A, McMahon LR, Salazar A, O’Connor JC, Satsangi N, Satsangi RK, Gu TT, Treat K, Smolik C, Schultz ST. Acetominophen differentially enhances social behavior and cortical cannabinoid levels in inbred mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:260–269. doi: 10.1016/j.pnpbp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Heisberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disable-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- Hisanaga A, Morishita S, Suzuki K, Sasaki K, Koie M, Kohno T, Hattori M. A dinintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) cleaves reelin in an isoform-dependent manner. FEBS Lett. 2012;586:3349–3353. doi: 10.1016/j.febslet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the grain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Gui L, Goffinet AM. Processing of reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–4252. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Persico AM. The neurobiological context of autism. Mol Neurobiol. 2003;28:1–22. doi: 10.1385/MN:28:1:1. [DOI] [PubMed] [Google Scholar]
- Keshvara L, Magdaleno S, Benhayon D, Curran T. Cyclin-dependent kinase 5 phosphorylated disabled-1 independently of reelin signaling. J Neurosci. 2002;22:4869–4877. doi: 10.1523/JNEUROSCI.22-12-04869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khialeeva E, Lane TF, Carpenter EM. Disruption of reelin signaling alters mammary gland morphogenesis. Development. 2011;138:767–776. doi: 10.1242/dev.057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Kohno T, Nakano Y, Suzuki K, Ishii M, Tagami H, Baba A, Hattori M. Mechanism and significance of specific proteolytic cleavage of reelin. Biochem Biophys Res Commun. 2009;380:93–97. doi: 10.1016/j.bbrc.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet AM. reelin, the extracellular matrix protein deficient in Reeler mutant mice, is processed by a metalloproteinase. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- Lane AE, Dennis SJ, Geraghty ME. Brief report: further evidence of sensory subtypes in autism. J Autism Dev Disord. 2011;41:826–831. doi: 10.1007/s10803-010-1103-y. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Gaudino C, Marino R, Keller F. Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced sterotypies in reeler mutant mice. Psychopharmacology. 2006;187:331–344. doi: 10.1007/s00213-006-0426-z. [DOI] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci USA. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Jones P, O’Callaghan E, Takei N, Sham P. Genes, viruses and neurodevelopmental schizophrenia. J Psychiatry Res. 1992;26:225–235. doi: 10.1016/0022-3956(92)90029-n. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G. The reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild-type and heterozygous reeler mice by immunoelectron microscopy. J Neurocytol. 2001;30:413–425. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic, and environmental clues. Trends Neurosci. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Persico AM, D’Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Conciatori M, Marino R, Quattrocchi CC, Baldi A, Zelante L, Gasparini P, Keller F, Collaborative Linkage Study of Autism Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6:150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Chen K, Howell BW. A genetic interaction between the APP and Dab1 genes influences brain development. Mol Cell Neurosci. 2008;37:178–186. doi: 10.1016/j.mcn.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccieri L, Venerosi A, Capone F, Cometa MF, Lorenzini Pl, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorus pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windhan GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioral phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of Reln gene dosage and social isolation. Behav Neurosci. 2003;117:1257–1275. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Sigala S, Zoli M, Palazzolo F, Faccoli S, Zanardi A, Mercuri NB, Spano P. Selective disarrangement of the rostral telencephalic cholinergic system in heterozygous reeler mice. Neuroscience. 2007;144:834–844. doi: 10.1016/j.neuroscience.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Rowe A, Howlin P, Murphy DG. Impaired olfactory identification in Asperger's syndrome. J. Neuropsychiatry Clin Neurosci. 2003;15:105–107. doi: 10.1176/jnp.15.1.105. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Marin ED, Sahun I, Masachs N, Pujadas L, Corvelo A, Bosch C, Rossi D, Martinez A, Maldonado R, Dierssen M, Soriano E. Overexpression of reelin prevents the maifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology. 2011;36:2395–2405. doi: 10.1038/npp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and preservative behaviour more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Halley P, Hill R, Labots M, Martin S. Altered N-methyl-d-aspartate receptor function in reelin heterozygous mice: male-female differences with dopaminergic activity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:237–246. doi: 10.1016/j.pnpbp.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Ventruti A, Kazdoba TM, Niu S, D’Arcangelo G. Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience. 2011;189:32–42. doi: 10.1016/j.neuroscience.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci, USA. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]