Abstract
Background: Newborn screening (NBS) for long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) deficiency does not discriminate between isolated LCHAD deficiency, isolated long-chain keto acyl-CoA (LCKAT) deficiency and general mitochondrial trifunctional protein (MTP) deficiency. Therefore, screening for LCHAD deficiency inevitably comprises screening for MTP deficiency, which is much less amenable to treatment. Furthermore, absence of a clear classification system for these disorders is still lacking.
Materials and Methods: Two newborns screened positive for LCHAD deficiency died at the age of 10 and 31 days, respectively. One due to severe necrotizing enterocolitis (NEC), cardiomyopathy and multiorgan failure and the other due to severe infant respiratory distress syndrome (IRDS) and hypertrophic cardiomyopathy. (Keto)-acylcarnitine concentration and enzymatic analysis of LCHAD and LCKAT suggested MTP deficiency in both patients. Mutation analysis revealed a homozygous HADHB c.357+5delG mutation in one patient and a homozygous splice-site HADHB mutation c.212+1G>C in the other patient.
Data on enzymatic and mutation analysis of 40 patients with presumed LCHAD, LCKAT or MTP deficiency were used to design a classification to distinguish between these disorders.
Discussion: NEC as presenting symptom in MTP deficiency has not been reported previously. High expression of long-chain fatty acid oxidation enzymes reported in lungs and gut of human foetuses suggests that the severe NEC and IRDS observed in our patients are related to the enzymatic deficiency in these organs during crucial stages of development.
Furthermore, as illustrated by the cases we propose a classification system to discriminate LCHAD, LCKAT and MTP deficiency based on enzymatic analysis.
Electronic supplementary material:
The online version of this chapter (doi:10.1007/8904_2012_128) contains supplementary material, which is available to authorized users.
Introduction
In 2007, the newborn screening (NBS) program in the Netherlands was expanded with 13 inborn errors of metabolism, including the autosomal recessive long-chain fatty acid oxidation (FAO) disorder and long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) deficiency. LCHAD is part of mitochondrial trifunctional protein (MTP), which harbours two additional enzymes in long-chain FAO: long-chain enoyl-CoA hydratase (LCEH) and long-chain keto acyl-CoA thiolase (LCKAT). The enzyme active sites are located on different subunits, named alpha- and beta-, which together form an octameric complex of 4α- and 4β-subunits (Kamijo et al. 1994; Ushikubo et al. 1996). LCHAD and LCEH are located on the α-subunit, and are both encoded by the HADHA gene. LCKAT is located on the β-subunit, and is encoded by the HADHB gene (Kamijo et al. 1994; Ushikubo et al. 1996).
NBS for LCHAD deficiency is performed by measuring C16-OH-carnitine levels in dried blood spots. However, discrimination between isolated general MTP deficiency, LCHAD deficiency, isolated LCKAT deficiency (LCKAT deficiency) or isolated LCEH deficiency (LCEH deficiency; not identified yet) cannot be made on the basis of the acylcarnitine profile, but requires specific enzyme testing in lymphocytes or fibroblasts. Furthermore measurement of 3-keto-C18:1-carnitine and 3-keto-C18:2-carnitine, which accumulate in case of LCKAT deficiency but not LCHAD deficiency, might also be helpful (Wanders et al. 2010).
By far the most common mutation associated with LCHAD deficiency is the HADHA c.1528G>C mutation (p.Glu510Gln, allelic frequency 60%) (Das et al. 2006). Mutations associated with a deficient activity of all enzymes (MTP deficiency) are more heterogeneous.
While no patients with isolated LCEH deficiency have been reported, MTP deficiency has been reported in relatively large series of patients (Choi et al. 2007; den Boer et al. 2003; Olpin et al. 2005; Purevsuren et al. 2009; Saudubray et al. 1999; Spiekerkoetter et al. 2003; Ushikubo et al. 1996). Patients with MTP deficiency, including isolated LCHAD deficiency, most often present with acute metabolic decompensation consisting of hypoketotic hypoglycemia and rhabdomyolysis, generally followed by cardiomyopathy and later peripheral neuropathy. Hypotonia, areflexia and hepatic encephalopathy have also been described (Choi et al. 2007; den Boer et al. 2003; Olpin et al. 2005; Purevsuren et al. 2009; Saudubray et al. 1999; Spiekerkoetter et al. 2003; Ushikubo et al. 1996). In contrast, isolated LCKAT deficiency appears extremely rare and only one patient, who presented with lethal cardio-respiratory failure, has been reported (Das et al. 2006).
We present two patients identified by NBS with an abnormal screening result suggestive for LCHAD deficiency, who were subsequently diagnosed with MTP deficiency. Both patients were already severely ill at the time the results of the NBS became available, and showed remarkable clinical symptoms which are generally not observed in patients with a defect in FAO.
It is not possible to distinguish isolated LCHAD deficiency and LCKAT deficiency of MTP deficiency based on NBS results, clinical signs and symptoms or DNA mutation analysis. Therefore, we propose a classification based on enzymatic analysis of LCHAD and LCKAT.
Materials and Methods
Case 1
The patient, a girl, was the first child of consanguineous Caucasian parents. The pregnancy was complicated by eclampsia. The mother had 7 seizures, ALAT of 29 (normal 0–35), ASAT of 36 (0–30), and low platelets 127 × 10−9/L (normal 150–450). The pregnancy was therefore terminated at 35 weeks of gestation by caesarean section. Birth weight was 2,110 g (−0.4SD), length 40 cm (<−2.5SD) and head circumference 30 cm (<−2.5SD). APGAR scores were 4, 8 and 8 after 1, 5 and 10 min, respectively. Postpartum glucose was 9.6 mmol/L (normal 3.6–5.6), lactate 7.1 mmol/L (normal 0.0–2.2), ammonia 127 μmol/L (normal 0–75), LDH 899 U/L (normal 0–250), ASAT/ALAT were normal. CK at day 7 was 478 U/L (normal 0–145). With normal intake, plasma glucose levels remained above 4.2 mmol/L and lactate levels decreased to 2.6 mmol/L. On day 3, she had rectal blood loss. Upon suspicion of a necrotizing enterocolitis (NEC), she was admitted to the neonatal intensive care unit and parenteral feeding was initiated. On day 7, a sudden clinical deterioration suggested a gut perforation as a complication of the NEC and a laparotomy was performed. No perforation was found, but intestinal biopsies later showed the classical pathology of a NEC. There was no clinical improvement observed and echocardiography revealed a severely dilated cardiomyopathy with low cardiac output. On this same day (day 7), the NBS results from a dried blood spot, taken at day 4, became available and showed an elevated C16-OH-carnitine suggestive of LCHAD deficiency. Additional analysis in plasma revealed increased concentrations of hydroxy-acylcarnitines (Table 1). Subsequently, keto-acylcarnitine concentrations were analyzed and showed increased 3-keto-C18:1-carnitine and 3-keto-C18:2-carnitine, which is suggestive of LCKAT deficiency (Table 1). Enzymatic analysis showed reduced activities of both LCHAD and LCKAT (lymphocytes). DNA mutation analysis of the HADHB gene (GenBank accession number BC066963) showed a homozygous splice-site mutation in intron 4, c.212+1G>C, predicted to lead to aberrant splicing of the HADHB mRNA transcript. No mutation was found in the HADHA gene.
Table 1.
Acylcarnitine profile (NBS and plasma), enzymatic activity and mutations of both patients (controls ± SD)
| Acylcarnitine profile (bloodspot) | Patient 1: 35 weeks, 2,110 g (μmol/L) (day 4) | Patient 2: 30 weeks, 1,275 g (μmol/L) (day 3) | Control values (μmol/L) |
|---|---|---|---|
| C16-OH-carnitine | 1.44 | 0.63 | ≤0.08 |
| Acylcarnitine profile (plasma) | Patient 1 (μmol/L) (day 7) | Patient 2 (μmol/L) (day 7) | Control values (μmol/L) (N = 700) |
|---|---|---|---|
| Free carnitine | 6.9 | 9.82 | 22.35–54.80 |
| C14-carnitine | 0.2 | 0.36 | 0–0.08 |
| C14:1-carnitine | 0.1 | 0.42 | 0.02–0.18 |
| C14:1-OH-carnitine | 0.05 | 0.11 | 0–0.02 |
| C16-carnitine | 1.13 | 1.33 | 0.06–0.24 |
| C16-OH-carnitine | 0.59 | 0.44 | 0–0 |
| C16:1-carnitine | 0.32 | 0.45 | 0.02–0.08 |
| C16:1-OH-carnitine | 0.19 | 0.25 | 0–0.02 |
| C18:1-OH-carnitine | 0.6 | 1.12 | 0–0.02 |
| 3-Keto-C18:1-carnitine | Detected | Detected | Undetectable |
| 3-Keto-C18:2-carnitine | Detected | Detected | Undetectable |
| Activity | Patient 1 (nmol/(min.mg protein)) | Patient 2 (nmol/(min.mg protein)) | Control values (nmol/(min.mg protein)) | |
|---|---|---|---|---|
| HAD activity | C16 | 12L (23%) | 10L (19%) | 53 ± 18L (N = 88) |
| 11F (14%) | 79 ± 16F (N = 215) | |||
| C4 | 103L (69%) | 116L (78%) | 149 ± 46L (N = 135) | |
| 86F (76%) | 113 ± 29F (N = 215) | |||
| C16/C4 | 0.12L | 0.08L | 0.37 ± 0.20L (N = 88) | |
| 0.13F | 0.72 ± 0.14F (N = 215) | |||
| LCKAT activity | 0.9L (8%) | 1.7F (9%) | 10.2 ± 3.6L (N = 41) | |
| 18.3 ± 5.4F (N = 215) | ||||
| Mutation analysis | Patient 1 | Patient 2 |
|---|---|---|
| HADHA gene (allele 1) | Normal | Normal |
| HADHA gene (allele 2) | Normal | Normal |
| HADHB gene (allele 1)a | c.212+1G>C | c.357+5delG |
| HADHB gene (allele 2)a | c.212+1G>C | c.357+5delG |
L lymphocytes, F fibroblasts
aNumbering according to GenBank sequence BC066963
The patient developed seizures during prolonged hypotensive episodes. Cerebral ultrasound studies showed minimal flaring and a minor bleeding (grade II). Despite vigorous treatment, including high dose (8–10 mg/kg/min) intravenous glucose infusion, she died at 10 days of age because of severe multiorgan failure.
Case 2
The patient, a boy, was the first child of consanguineous parents. Pregnancy was complicated by pre-eclampsia and intrauterine growth retardation (IUGR). The pregnancy was terminated at 30 weeks by caesarean section because of foetal distress. Birth weight was 1,275 g (<−1.0SD), length 37 cm (−2.0SD) and head circumference 28 cm (1-0SD). APGAR scores were 7, 8 and 9 after 1, 5 and 10 min, respectively. On day 1, he became hypotensive and developed severe infant respiratory distress syndrome (IRDS; grades III and IV) despite multiple surfactant administrations. He was artificially ventilated. Despite continuous glucose infusion (8–10 mg/kg/min), he had multiple hypoglycaemic episodes (postpartum glucose 1.8 mmol/L) and a persistent lactic acidosis (>10 mmol/L, normal 0.0–2.2). Echocardiography performed on day 6 revealed a hypertrophic cardiomyopathy. Cerebral ultrasound showed no abnormalities. On day 10, the NBS results from a dried blood spot, taken at day 3, became available which revealed an elevated C16-OH-carnitine, suspicious for LCHAD deficiency. Additional analysis in plasma revealed increased concentrations of hydroxy-acylcarnitines (Table 1). Subsequently, keto-acylcarnitine concentrations were analyzed and showed increased 3-keto-C18:1-carnitine and 3-keto-C18:2-carnitine, which is suggestive of LCKAT deficiency (Table 1). Enzymatic analysis showed reduced activities of both LCHAD and LCKAT (fibroblasts). DNA mutation analysis of the HADHB gene (GenBank accession number BC066963) showed a homozygous mutation c.357+5delG, which was subsequently shown by cDNA analysis to result in a complete skipping of exon 6. No mutation was found in the HADHA gene.
Despite extensive treatment including ventilatory support, glucose infusion, parenteral feeding, carnitine supplementation (100 mg/kg/day), long-chain triglyceride restriction and medium-chain enriched feeding, he died 31 days after birth because of respiratory failure.
Classification
To be able to discriminate between LCHAD, LCKAT and MTP deficiency, we retrospectively analyzed data of 40 non-related patients in whom LCHAD and LCKAT activity was measured and DNA mutation analysis was performed. In addition, we analyzed data of 215 subjects in whom LCHAD and LCKAT activity was measured because of suspected FAO disorder, but in whom no FAO defect was found.
Hydroxyacyl-CoA dehydrogenase (HAD) activity was measured in homogenates of cultured fibroblasts by observing the decrease in absorbance at 340 nm (Wanders et al. 1990). 3-Keto-hexadecanoyl-CoA (C16) and acetoacetyl-CoA (C4) have been used as substrates for LCHAD activity measurements. LCHAD shows highest activity with C16 as substrate with virtually no reactivity with C4 as substrate (Wanders 1990; Wanders et al. 2010). However, another dehydrogenase – short chain hydroxyaxcyl-CoA dehydrogenase (SCHAD) – has activity with both C16 and C4. To be able to acquire an accurate approximation of LCHAD activity, the activities with C16 and C4 as substrate were used as a ratio (C16/C4).
A full deficiency of LCHAD will result in a C16/C4 HAD activity ratio of approximately 0.2, which is characteristic of the SCHAD enzyme, as it is five times more active with C4 than C16 as a substrate. A C16/C4 HAD activity ratio higher than approximately 0.2 is a result of (residual) LCHAD activity.
LCKAT activity was measured in homogenates of cultured fibroblasts by following the decrease in absorbance at 303 nm (Wanders et al. 1990).
All enzyme analyses were performed on a Cobas Centrifugal Analyzer (Hofmann-La Roche, Basel, Switzerland).
DNA was extracted from blood leukocytes and amplified by polymerase chain reaction (PCR). After amplification, 20 exons of HADHA and 16 exons of HADHB gene were sequenced.
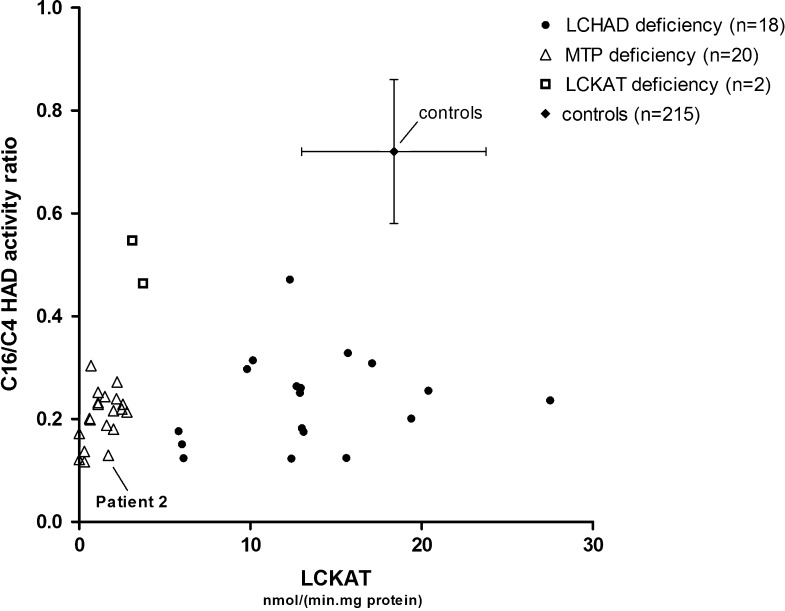
The results are shown in Fig. 1.
Fig. 1.
C16/C4 HAD activity ratio and LCKAT activity of patients and controls. C16/C4 HAD activity ratio and LCKAT activity have been analyzed in fibroblasts of 40 patients with HADHA and/or HADHB mutations and 215 controls as described in “Materials and Methods” section. Patients can be divided into three groups: LCHAD (filled circle), LCKAT (open square) and MTP (open triangle) deficiency. Mean C16/C4 HAD activity ratio of control group 0.72 ± 0.14 (mean ± SD; vertical error bars). Mean LCKAT activity of control group 18.3 ± 5.4 nmol/(min.mg protein) (mean ± SD; horizontal error bars)
Analytical Methods
Enzymatic activity measurements in lymphocytes and/or fibroblast were carried out as previously described (Wanders et al. 1990/1992). NBS acylcarnitine profiling was performed as described by Chace et al. (2003). Plasma acylcarnitine profiling was performed as described by Minkler et al. (2005). In addition, keto-acylcarnitine profiling was performed by incubating 50 μL of plasma with 5 μL of MOX Reagent (Pierce, Rockford, IL, USA) containing 2% methoxyamine⋅HCl in pyridine. The mixture was left to stand at room temperature for 2 h to allow the formation of methoxime-derivatives of all keto-containing substances. Following this incubation, the samples were treated in the usual manner for the isolation and derivatization of acylcarnitines, which were analyzed as their butyl-ester derivatives (Minkler et al. 2005).
Discussion
NEC as a presenting symptom in MTP deficiency has, to our knowledge, not been reported previously. Severe IRDS has been recognized as a rare early symptom in MTP deficiency (Olpin et al. 2005). Cardiomyopathy, which was present in both patients, is a frequently reported complication in MTP deficiency (den Boer et al. 2003; Olpin et al. 2005; Purevsuren et al. 2009; Spiekerkoetter et al. 2003).
Although it is generally assumed that FAO plays no or only a minor role during intrauterine life due to the abundance of glucose provided by the mother via the placenta (Oey et al. 2006; Shekhawat et al. 2003), the clinical course in our patients, as well as the patient with isolated LCKAT deficiency described by Das et al. (2006), suggests that normal function of the MTP complex is needed for normal intestinal and pulmonary development and function. Berger and Wood showed that complete disruption of long-chain FAO at the level of LCAD in animal models results in increased embryonic mortality (Berger and Wood 2004). It has also been shown that FAO enzymes are expressed abundantly in human placentas (Shekhawat et al. 2003). Furthermore, patients with long-chain defects in FAO may already display cardiomyopathy before and immediately after birth (den Boer et al. 2003; Olpin et al. 2005; Purevsuren et al. 2009; Spiekerkoetter et al. 2004), demonstrating a role for long-chain FAO during intrauterine life. Finally, the (pre)eclampsia, the premature delivery and the foetal distress seen in both hereby described patients, is also in line with this hypothesis (Oey et al. 2005).
While NEC is a relatively common complication in ill premature babies, it is rarely seen in newborns of 35 weeks gestational age, with a birth weight >1,500 g and in absence of a history of hypovolemic shock and/or asphyxia (Neu and Walker 2011). In addition, severe IRDS, not responding to multiple administration of surfactant, is rare in neonates born after 30 weeks gestation. We therefore hypothesize that both the NEC observed in patient 1 and the severe IRDS in patient 2 are linked to the defective long-chain FAO. Early foetal expression patterns of long-chain FAO enzymes, including VLCAD and LCHAD, demonstrate that these enzymes are expressed not only in myocardial tissue, but also abundantly in the foetal lung and gut (Oey et al. 2005). MTP deficiency during intrauterine life may therefore interfere with normal development or maturation of the foetal intestine and lungs. In the gut, this might result in decreased mucus synthesis, decreased intracellular junction integrity and increased permeability, both potentially related to the development of NEC (Neu and Walker 2011). In addition, intestinal villous atrophy and inflammation is observed in carnitine transport-deficient (OCTN2) mice (Shekhawat et al. 2007).
In the lungs, surfactant is secreted by alveolar type II cells, and decreased maturation or functioning of this process may lead to IRDS (Olpin et al. 2005). We therefore believe that MTP deficiency during intrauterine life may hamper normal surfactant synthesis.
We identified two novel mutations in the HADHB gene. The mutation found in patient 1, c.212+1G>C, affects the splice-donor site of intron 4 and is predicted to result in skipping of exon 4. Because fibroblasts were not available from patient 1, this could not be studied at the cDNA level. The G deletion at position c.357+5 found in patient 2 results in skipping of exon 6, as demonstrated by cDNA analysis prepared from mRNA isolated from fibroblasts of the patient.
Both mutations did not only result in a markedly reduced LCKAT activity, but also affected enzyme activity of LCHAD, which is possibly due to the loss of integrity of the MTP complex (Ushikubo et al. 1996). It is known that a single mutation in the HADHA or HADHB gene can result in either an isolated deficiency of LCHAD or LCKAT, or reduced activity of both enzymes. However, until now it has not been possible to clearly distinguish isolated LCHAD and LCKAT deficiency from MTP deficiency. We propose a classification system based on the C16/C4 HAD activity ratio and LCKAT residual enzyme activities measured in 40 patients. As shown in Fig. 1, the patients can be divided into three groups, which we have labelled as LCHAD, LCKAT and MTP deficiency. The LCHAD group contains patients with a mean C16/C4 HAD activity ratio of 0.24 ± 0.09 (mean ± SD) combined with a keto-thiolase activity of 13.5 ± 5.4 nmol/(min.mg protein) (mean ± SD). The MTP deficiency group consists of patients with a mean C16/C4 HAD activity ratio of 0.2 ± 0.05 (mean ± SD) and an LCKAT activity of 1.3 ± 0.88 nmol/(min.mg protein) (mean ± SD). The third group, isolated LCKAT deficiency, includes two patients with a C16/C4 HAD activity ratio of approximately 0.5 and an LCKAT activity of <5 nmol/(min.mg protein) (Fig. 1). The activity of the different enzymes could not be fully predicted based on the mutations. Although the LCHAD group includes 17 of the 18 patients homozygous for the HADHA c.1528G>C mutation, the MTP-deficient group includes patients with either alpha- or beta-subunit mutations. The two isolated LCKAT-deficient patients, one of which was reported previously (Das et al. 2006), have distinct beta-subunit mutations. Based on this classification, patient 2 will be categorized as MTP deficient. We could not obtain fibroblasts of the first patient and are therefore unable to classify this patient unambiguously. However, predicted is that the MTP protein of patient 1 is absent, because the mutation resulted in exon skipping. We therefore conclude patient 1 is also MTP deficient.
In summary, we present two patients in whom NBS results were consistent with LCHAD deficiency. They were eventually diagnosed with MTP deficiency, based on a novel proposed classification system. One of the patients presented with a severe NEC, which has not been associated previously with long-chain FAO defects. Furthermore, a severe IRDS was observed in the other patient. Both clinical presentations may be explained by high expression patterns of long-chain FAO enzymes not only in myocardial tissue, but in lung and gut tissue as well. Deficiency of MTP in the gut and lung might therefore explain the development of severe NEC and IRDS in addition to the cardiomyopathy found in our patients.
Acknowledgments
Eugene Diekman is paid by a grant of ZonMW, The Netherlands Organisation for Health Research and Development, dossier 200320006.
Synopsis
MTP-deficient patients may present with NEC or IRDS. Illustrated by two cases with these symptoms, a classification system for LCHAD, LCKAT and MTP deficiency based on enzymatic analysis is proposed.
Conflict of Interest
None.
Footnotes
Competing interests: None declared
References
- Berger PS, Wood PA. Disrupted blastocoele formation reveals a critical developmental role for long-chain acyl-CoA dehydrogenase. Mol Genet Metab. 2004;82:266–272. doi: 10.1016/j.ymgme.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–1817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- Choi J-H, Yoon H-R, Kim G-H, et al. Identification of novel mutations of the HADHA and HADHB genes in patients with mitochondrial trifunctional protein deficiency. Int J Mol Med. 2007;19:81–87. [PubMed] [Google Scholar]
- Das AM, Illsinger S, Lücke T, et al. Isolated mitochondrial long-chain ketoacyl-CoA thiolase deficiency resulting from mutations in the HADHB gene. Clin Chem. 2006;52:530–534. doi: 10.1373/clinchem.2005.062000. [DOI] [PubMed] [Google Scholar]
- Den Boer MEJ, Dionisi-Vici C, Chakrapani A, et al. Mitochondrial trifunctional protein deficiency: a severe fatty acid oxidation disorder with cardiac and neurologic involvement. J Pediatr. 2003;142:684–689. doi: 10.1067/mpd.2003.231. [DOI] [PubMed] [Google Scholar]
- Kamijo T, Wanders RJ, Saudubray JM, et al. Mitochondrial trifunctional protein deficiency. Catalytic heterogeneity of the mutant enzyme in two patients. J Clin Invest. 1994;93:1740–7. doi: 10.1172/JCI117158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler PE, Ingalls ST, Hoppel CL. Strategy for the isolation, derivatization, chromatographic separation, and detection of carnitine and acylcarnitines. Anal Chem. 2005;77:1448–57. doi: 10.1021/ac0487810. [DOI] [PubMed] [Google Scholar]
- Neu J, Walker WA. Necrotizing enterocolitis. N Eng J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey NA, Den Boer MEJ, Wijburg FA, et al. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005;57:755–759. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- Oey NA, Ruiter JPN, Attié-Bitach T, et al. Fatty acid oxidation in the human fetus: implications for fetal and adult disease. J Inherit Metab Dis. 2006;29:71–75. doi: 10.1007/s10545-006-0199-x. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Clark S, Andresen BS, et al. Biochemical, clinical and molecular findings in LCHAD and general mitochondrial trifunctional protein deficiency. J Inherit Metab Dis. 2005;28:533–544. doi: 10.1007/s10545-005-0533-8. [DOI] [PubMed] [Google Scholar]
- Purevsuren J, Fukao T, Hasegawa Y, et al. Clinical and molecular aspects of Japanese patients with mitochondrial trifunctional protein deficiency. Mol Genet Metab. 2009;98:372–377. doi: 10.1016/j.ymgme.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Saudubray JM, Martin D, De Lonlay P, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. doi: 10.1023/A:1005556207210. [DOI] [PubMed] [Google Scholar]
- Shekhawat P, Bennett MJ, Sadovsky Y, et al. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am J Physiol Endocrinol Metab. 2003;284:1098–105. doi: 10.1152/ajpendo.00481.2002. [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Srinivas SR, Matern D, et al. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(−/−)) mice. Mol Genet Metab. 2007;92:315–24. doi: 10.1016/j.ymgme.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter U, Sun B, Khuchua Z, et al. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum Mut. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Khuchua Z, Yue Z, et al. General mitochondrial trifunctional protein (TFP) deficiency as a result of either alpha- or beta-subunit mutations exhibits similar phenotypes because mutations in either subunit alter TFP complex expression and subunit turnover. Pediatr Res. 2004;55:190–196. doi: 10.1203/01.PDR.0000103931.80055.06. [DOI] [PubMed] [Google Scholar]
- Ushikubo S, Aoyama T, Kamijo T, et al. Molecular characterization of mitochondrial trifunctional protein deficiency: formation of the enzyme complex is important for stabilization of both alpha- and beta-subunits. Am J Hum Genet. 1996;58:979–988. [PMC free article] [PubMed] [Google Scholar]
- Wanders RJ, Ijlst L, van Gennip AH, et al. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 1990;13:311–4. doi: 10.1007/BF01799383. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Ijlst L, Poggi F, et al. Human trifunctional protein deficiency: a new disorder of mitochondrial fatty acid beta-oxidation. Biochem Biophys Res Commun. 1992;188:1139–1145. doi: 10.1016/0006-291X(92)91350-Y. [DOI] [PubMed] [Google Scholar]
- Wanders RJA, Ruiter JPN, Ijlst L, et al. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]