Abstract
Background. Enzyme Replacement Therapy (ERT) is the standard of care in Gaucher disease. The effects of withdrawal or reduced doses are debated, thus a retrospective cohort study was conducted to investigate clinical and laboratory differences in 34 Gaucher type 1 patients experiencing an ERT dosage reduction after the forced temporary imiglucerase shortage in 2009.
Methods. Haemoglobin concentration, leukocytes and platelets counts, and chitotriosidase activity were assessed at baseline and after 6 and 12 months (t0, t6, t12), while bone pain, energy, work or school performance, concentration, memory and social life every 3 months.
Results. The cohort was made up of 18 males and 16 females (medians: age 41.8 years, therapy duration 14.1 years, dosage reduction 35.5%). Haemoglobin, leukocytes and platelets remained substantially stable, while chitotriosidase activity showed an increase, especially after t6. Age, splenectomy or genotype were not associated with laboratory parameters changes, except for a significant median increase of chitotriosidase activity in non-splenectomised patients after 12 months (p = 0.01). At 3, 6, 9 and 12 months, more than 50% patients reported at least one problem in subjective well-being (56%, 65%, 70%, 58%, respectively), while bone pain occurred or worsened in 13/33, 13/32, 7/28 and 5/26 patients, respectively. No bone crises were reported.
Conclusions. Drug reduction did not induce substantial modification in the laboratory values but seems to have influenced the well-being perception of some Gaucher patients. Thus, bone pain, general health and quality of life should be carefully monitored during ERT reductions.
Keywords: Enzyme Replacement Therapy, Bone Pain, Gauche Disease, Splenectomised Patient, Gauche Disease Patient
Background
Gaucher disease (GD), the most common lysosomal storage disorder, is a recessive autosomal disease due to mutations in the gene encoding the lysosomal enzyme acid beta glucosidase (GBA1). The deficient activity of this enzyme results in the accumulation of glucosylceramide (GlcCer) within the lysosomes. Type I (GD1) is the most common form of GD and may imply a large variety of symptoms, ranging from completely asymptomatic to child-onset forms (Beutler and Grabowski 2001; Jmoudiak and Futerman 2005).
Several therapeutic approaches are being examined with the aim to reduce the GlcCer intracellular burden, but Enzyme Replacement Therapy (ERT) with human recombinant acid beta glucosidase still remains the standard of care in these patients (Hughes and Pastores 2010).
In June 2009, the European Medicines Agency (EMEA) published a press release to communicate that after the viral contamination (calicivirus of the type Vesivirus 2117) of Genzyme’s manufacturing plant in Allston Landing (USA), the company had to shut down the production of imiglucerase (Cerezyme ®, Genzyme Corporation, MA, USA) (EMEA 2009), causing the temporary worldwide shortage of the drug.
In 2009, imiglucerase was the only registered enzyme available for ERT in GD (Hollak et al. 2010); consequently, many patients under treatment were forced to withdraw or reduce doses.
So far, several studies have been published to describe the clinical consequences of this shortage (Giraldo et al. 2011; Goldblatt et al. 2011; Zimran et al. 2011). Moreover, although no trials were performed to ascertain the possible effects of a temporary dosage reduction or withdrawal, this issue had already been dealt with before the occurrence of imiglucerase shortage (Drelichman et al. 2007; Elstein et al. 2000; Grinzaid et al. 2002; Schwartz et al. 2001; Vom Dahl et al. 2001). However, each study analysed only a few patients and the results among the studies were not homogeneous.
The aim of this study was to assess possible differences in selected laboratory values and clinical aspects in patients experiencing an ERT dosage reduction after the temporary imiglucerase shortage in 2009.
Methods
A retrospective cohort study was conducted on a group of GD1 patients. The inclusion criteria were having experienced a drug reduction due to imiglucerase shortage in 2009 for at least 1 year and being followed up at the Regional Coordinator Centre for Rare Diseases of the University Hospital “Santa Maria della Misericordia” (Udine, Italy) until July 2011.
The haemoglobin concentration (Hb) and white blood cells (WBC) and platelets (Plt) counts were examined. Chitotriosidase (Ct) activity, acknowledged as a marker of disease (Hollak et al. 1994), was measured as previously described (Hollak et al. 1994). These parameters were evaluated no more than 6 months before the drug reduction and after 6 and 12 months (t0, t6, t12, respectively). Since a closer observation was performed after the shortage (a phone call was made by the charge nurse every 3 months for 1 year), it was also possible to evaluate their subjective condition after the drug dosage reduction. In particular, patients were asked about the occurrence or the worsening of bone pain. Several specific questions (five for the adults and three for the paediatric patients) were also asked about a decline in vitality/strength, work/school performance, concentration/memory and social life after the dosage decrease.
The Ethics Committee of the University Hospital “Santa Maria della Misericordia” (Udine, Italy) approved this study.
Statistical Analysis
Continuous variables are described as median and first and third quartile. Categorical variables are presented as frequency and percentage. The Shapiro Wilk test was used to check the normality assumption and since the variables were not normally distributed, the signed rank test was used to compare two groups. All the analyses were performed using the statistical package Stata 11.0 (Stata Statistical Software: Release 11.0, 2009. StataCorp LP, College Station, TX, USA).
Results
Thirty-four out of the 38 Gaucher patients followed up until July 2011 fulfilled the inclusion criteria and constituted the study retrospective cohort. Eighteen patients were males and 16 females, the median age was 41.8 years and 4 of them were children (age < 16 years). Their main characteristics are described in Table 1.
Table 1.
Characteristics of the patients
| Variables | n (%) | Median (IQR) |
|---|---|---|
| Gender | ||
| Male | 18 (53) | |
| Female | 16 (47) | |
| Genotype | ||
| N370S/N370S | 5 (15) | |
| N370S/other | 18 (53) | |
| Other/other | 11 (32) | |
| Previous spleen removal | ||
| Yes | 10 (29) | |
| No | 24 (71) | |
| Age, at therapy start (years) | 27.8 (13.2–37.3) | |
| Age, at reduction (years) | 41.8 (29.2–50.5) | |
| Duration of therapy, at reduction (years) | 14.1 (10.5–15.6) | |
| ERT dose (IU/kg/month) | ||
| Before reduction | 55.5 (48–63) | |
| After reduction | 15.8 (15–30) | |
| % variation | 35.5 (28–47) |
All patients were treated with imiglucerase when the drug shortage occurred and all experienced one or two drug reductions in July–August 2009. Their pre-reduction median dosage was 55.5 units/kg/month, while the median dosage after the decrease was 15.8 units/kg/month (signed rank test p < 0.0001). The median percentage variation between the pre- and post-reduction doses was 35.5% (Table 1).
Laboratory Values
Levels of Hb, WBC, Plt and Ct activity at baseline and at 6 and 12 months after drug reduction are summarised in Table 2. Results of the comparisons t0-t6, t6-12, t0-t12 (signed rank test) are also reported.
Table 2.
Laboratory values at t0, t6 and t12 and their differences between t0-t6, t6-12, t0-t12
| Parameter (unit) | t0 | t6 | t12 | t0-t6 | t6-t12 | t0-t12 |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | p-value | p-value | p-value | |
| n | n | n | n | n | n | |
| Haemoglobin (g/dL) | 14.0 (13.1–15.2) | 14.6 (12.9–15.5) | 14.1 (13.1–15.0) | 0.28 | 0.02 | 0.20 |
| n = 30 | n = 22 | n = 25 | n = 22 | n = 16 | n = 23 | |
| White blood cells (n/μL) | 5550 (4690–7255) | 5460 (4780–7940) | 6130 (5080–7790) | 0.29 | 0.96 | 0.45 |
| n = 30 | n = 22 | n = 26 | n = 22 | n = 17 | n = 24 | |
| Platelets (103/μL) | 193 (150–292) | 198 (149–301) | 231 (152–292) | 0.75 | 0.78 | 0.97 |
| n = 30 | n = 22 | n = 24 | n = 22 | n = 16 | n = 22 | |
| Chitotriosidase (nmol/mL/h) | 568 (349–919) | 642 (467–787) | 1,057 (543–1405) | 0.88 | 0.0004 | 0.19 |
| n = 21 | n = 28 | n = 25 | n = 20 | n = 24 | n = 21 | |
| Chitotriosidase (nmol/mL/h) (patients without missing values) | 555 (334–940) | 703 (511–802) | 1,114 (516–1391) | 0.88 | 0.04 | 0.01 |
| n = 20 | n = 20 | n = 20 | n = 20 | n = 20 | n = 20 |
Hb concentration did not show important variations between t0 and t6 (median variation 0.1 g/dL, IQR −0.3 ÷ 0.5, n = 22), t6 and t12 (median −0.4 g/dL, IQR −0.7 ÷ 0.1, n = 16) and t0-t12 (median −0.1, IQR −0.7 ÷ 0.4, n = 23). A decrease was evidenced in 7/22 patients at t6 and in 14/23 at t12, but none ever suffered from anaemia, as defined by Pastores et al. (2004).
Overall, the WBC and platelets counts after 6 and 12 months were not statistically different from the baseline values (Table 2). When compared to t0, WBC showed a decrease in 8/22 patients at t6 and in 12/24 patients at t12, while platelets were reduced at t6 in 10/22 patients and at t12 in 13/22. However, only one reached a value lower than 100,000/μL (89,000 at t6).
When only the patients without missing values in the measurements of Hb (n = 16), WBC (n = 17) and Plt (n = 16) were analysed separately, the results did not change.
Chitotriosidase activity significantly increased between t6 and t12 (p = 0.0004), with a median variation of 398 nmol/mL/h (IQR 55 ÷ 590) in the 24 patients examined, but did not vary between t0 and t6 (median 138, IQR −383 ÷ 280, n = 20) nor between t0 and t12 (median 311, IQR −49 ÷ 634, n = 21). Nevertheless, when the patients without missing values were analysed (n = 20), Ct after 6 months (median 703; IQR 511–802) was not different from Ct at t0 (median 555; IQR 334–940), but statistically significant increases were noticed between t0 and t12 (median 1,114; IQR 516–1,391; p = 0.04) and between t6 and t12 (p = 0.01).
Comparisons were also made to assess whether being a child, being splenectomised or having a particular genotype (N370S/N370S vs N370S/other and other/other; N370S/N370S and N370S/other vs other/other) might affect the response to ERT shortage. No differences were found, except when Ct activity at t0-t12 was compared between splenectomised and non-splenectomised patients. While splenectomised patients showed a non-significant median decrease from 1,322 to 834 nmol/mL/h (p = 0.17), the non-splenectomised patients showed a significant median increase from 543 to 1,191 nmol/mL/h (p = 0.01).
Subjective Well-Being
Table 3 shows the number of patients who reported a worsening in selected aspects of their life (energy, work or school performance, concentration, memory, social life) at different times after the drug reduction (3, 6, 9, 12 months). More than 50% of patients declared at least one subjective problem that arose 3, 6, 9 and 12 months after the drug reduction (56%, 65%, 70%, 58%, respectively).
Table 3.
Problems that impaired the perception of well-being after drug reduction, as reported by patients (compared to t0)
| Problems | t3 | t6 | t9 | t12 |
|---|---|---|---|---|
| ↓Energy | 15/33 | 12/32 | 11/28 | 3/26 |
| ↓Performance (work/school) | 7/33 | 7/32 | 3/28 | 4/26 |
| ↓Concentrationa | 4/29 | 8/29 | 9/24 | 8/24 |
| ↓Memorya | 6/29 | 14/29 | 11/24 | 9/24 |
| ↓Social lifea | 4/28 | 5/28 | 3/25 | 2/24 |
| ↑Tirednessb | 1/4 | 1/3 | 1/3 | 0/2 |
| ≥ 1 problem | 19/33 | 21/32 | 20/28 | 15/26 |
aOnly adults
bOnly children
Figure 1 shows the distribution of the number of problems that occurred after the drug reduction and reported at the four different time points by the adult patients who answered the five questions on subjective well-being. As for children, at t3, one child out of four reported one problem (tiredness), at t6 and t9, one child out of three reported two problems (tiredness and loss of energy in the same child), while at t12 none of the two children reported problems.
Fig. 1.
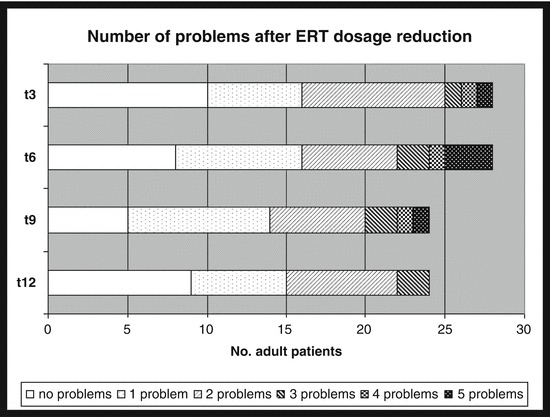
Number of subjective problems reported by adult patients after the drug reduction. Note: only patients who answered all five questions
Bone Pain
Thirty-three patients answered the questions about bone pain; among these, 13 (2 children) already suffered from bone pain before the drug reduction. The frequency of bone pain onset in asymptomatic patients and its worsening in patients already suffering from bone symptoms is reported in Table 4. No patients suffered from bone crises.
Table 4.
Onset and worsening of bone pain after drug reduction (compared to t0)
| Bone pain | t3 | t6 | t9 | t12 |
|---|---|---|---|---|
| Onset | ||||
| Overall | 5/20 | 6/19 | 4/18 | 4/17 |
| Adults | 5/18 | 6/17 | 4/16 | 4/15 |
| Children | 0/2 | 0/2 | 0/2 | 0/2 |
| Worsening | ||||
| Overall | 8/13 | 7/13 | 3/10 | 1/9 |
| Adults | 8/11 | 6/12 | 2/9 | 1/9 |
| Children | 0/2 | 1/1 | 1/1 | 0/0 |
| Onset or worsening | ||||
| Overall | 13/33 | 13/32 | 7/28 | 5/26 |
| Adults | 13/29 | 12/29 | 6/25 | 5/24 |
| Children | 0/4 | 1/3 | 1/3 | 0/2 |
Discussion
The aim of this study was to investigate whether the imiglucerase dosage reduction due to the temporary shortage in supplies had an effect on the clinical conditions and laboratory parameters of 34 GD1 patients, four of whom were children.
This is the only Italian study investigating the possible consequences of this forced ERT dose reduction. Furthermore, even when considering the international literature, only one previous study (Giraldo et al. 2011), focusing on the effects of a dosage reduction rather than those of a complete withdrawal, was performed. These authors described a group of 17 Spanish GD patients and reported stable values of Hb and Plt 6 months after ERT reduction, consistently with the results of the present study. However, a statistically significant increase of the chitotriosidase activity was reported (p = 0.03) after 6 months (Giraldo et al. 2011), while the increase observed in the Italian patients at the same time point did not reach statistical significance but became significant between 6 and 12 months. The increase was even more evident when only patients without missing values were taken into account, since Ct increased from 555 at baseline to 703 at 6 months and 1,114 at 12 months.
About 40 % (7/17) of the Spanish patients complained of diffuse bone pain (Giraldo et al. 2011), consistent with the fraction of patients of the present study that reported the onset or worsening of bone pain at 3 and 6 months. While no bone crises were observed in the Italian patients, Giraldo et al. reported a bone crisis in three cases (Giraldo et al. 2011). This difference could be due to a different disease severity in the two groups of patients, the Italian cohort being milder.
As for ERT withdrawal, Elstein et al. reported stable clinical conditions and laboratory values in 15 patients after 4 years of follow-up (Elstein et al. 2000), consistent with the results of a Brazilian case report describing clinical stability after a 3-month withdrawal (Schwartz et al. 2001). However, several authors reported the deterioration of haematological parameters (Hb, Plt, Ct) and an increase in organomegaly (Zimran et al. 2011; Grinzaid et al. 2002; Vom Dahl et al. 2001) after an ERT withdrawal lasting from 3 to more than 24 months. Moreover, Giraldo et al. reported conflicting results on 23 patients who withdrew ERT for 6 months, describing stable Hb and Plt in the great number of patients but at the same time the occurrence of a bone crisis in one patient and of mild anaemia in another one (Giraldo et al. 2011). Two recent studies (Giraldo et al. 2011; Goldblatt 2011) showed that even if laboratory parameters remained stable in most GD patients after 5–6 month of ERT withdrawal, some of them worsened their clinical condition.
Therefore, it may be argued that some characteristics of GD patients could play a role in the response to dosage variation. Thus, in this study, the possible role of genotype, age and splenectomy on the laboratory parameters after ERT reduction was investigated. Among the four patients described by Grinzaid et al., a better clinical and laboratory behaviour was seen in the one with a N370S/N370S genotype after a therapy discontinuation of ≥1 year (Grinzaid 2002). On the contrary, among the Italian patients, no differences were found in laboratory values variations according to genotype.
No differences were even found when comparing paediatric and adult patients, in contrast with a study conducted in Argentina that reported a clear deterioration of the clinical condition in five children forced to withdraw for 15–36 months (Drelichman et al. 2007).
Finally, the non-splenectomised patients showed a significant increase in chitotriosidase activity between t0 and t12, while in splenectomised patients a non-significant decrease was observed. This is in contrast with the study by Czartoryska et al., reporting a more evident increase in chitotriosidase activity after ERT cessation in two splenectomised patients when compared to six non-splenectomised patients (Czartoriska et al. 2000). However, the reduced Ct activity in splenectomised patients could be explained with the spleen removal itself, which leads to a reduction of the number of macrophages and, in turn, to their Ct production.
In spite of the substantial maintenance of laboratory values, more than one half of the studied patients reported at least one problem that worsened their perception of well-being during the follow-up period. Moreover, a variable number of patients reported the onset or worsening of bone pain (from about 40 % at t3 and t6 to about 20% at t12). However, consideration of the psychological impact of experiencing a forced reduction of a drug, that is thought to be indispensable by the patient, cannot be underestimated. In fact, the majority of these patients had good control of GD under ERT treatment for years, being allowed to live normal lives. When the shortage occurred, many patients reported to be worried about a possible influence on the outcome of their disease. Therefore, the subjective problems they reported could be influenced by this psychological attitude. Finally, it is important to point out that the information on both well-being and bone pain was not gathered using a validated questionnaire and it is difficult to evaluate the relevance of the problems reported.
Conclusions
In summary, the ERT dosage reduction did not cause important changes in the laboratory parameters, except for an increase of the Ct activity. Nevertheless, a part of GD patients experienced some modification in their general conditions. Therefore, when a decrease in ERT dosage needs to be introduced, a careful evaluation of the general health (with a special attention to bone pain) and quality of life of each patient should be carried out.
Synopsis
Bone pain, general health and quality of life should be carefully monitored during ERT reductions.
Authors’ Contributions
LD participated in the study design, performed the statistical analysis and drafted the manuscript. AS participated in the study design and in the draft of the manuscript. AD carried out the chitotriosidase activity and genotype analyses and participated in the draft of the manuscript. DM acquired the data and participated in the draft of the manuscript. GL acquired the data and participated in the draft of the manuscript. GC participated in the design of the study. BB conceived the study, participated in its design and coordination and in the draft of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Competing interests: None declared
Contributor Information
Laura Deroma, Email: deroma.laura@aoud.sanita.fvg.it.
Annalisa Sechi, Email: sechi.annalisa@aoud.sanita.fvg.it.
Andrea Dardis, Email: dardis.andrea@aoud.sanita.fvg.it.
Daniela Macor, Email: macor.daniela@aoud.sanita.fvg.it.
Giulia Liva, Email: liva.giulia@aoud.sanita.fvg.it.
Giovanni Ciana, Email: ciana.giovanni@aoud.sanita.fvg.it.
Bruno Bembi, Email: bembi.bruno@aoud.sanita.fvg.it.
References
- Beutler E, Grabowski GA (2001) Gaucher disease. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic & molecular bases of inherited disease. McGraw-Hill Medical Publishing Division, New York, pp 3635–3668
- Czartoriska B, Tylki-Szymanska A, Lugowska A. Changes in serum chitotriosidase activity with cessation of replacement enzyme (cerebrosidase) administration in Gaucher disease. Clin Biochem. 2000;33(2):147–149. doi: 10.1016/S0009-9120(99)00098-3. [DOI] [PubMed] [Google Scholar]
- Drelichman G, Ponce E, Basack N, et al. Clinical consequences of interrupting enzyme replacement therapy in children with type 1 Gaucher disease. J Pediatr. 2007;151:197–201. doi: 10.1016/j.jpeds.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Elstein D, Abrahamov A, Hadas-Halpern I, Zimran A. Withdrawal of enzyme replacement therapy in Gaucher’s disease. Br J Haematol. 2000;110:488–492. doi: 10.1046/j.1365-2141.2000.02177.x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (EMEA) (2009) Supply shortages of Cerezyme and Fabrazyme – priority access for patients most in need of treatment recommended. Doc. Ref. EMEA/389995/2009. London, 25-6-2009
- Giraldo P, Irun P, Alfonso P, et al. Evaluation of Spanish Gaucher disease patients after a 6-month imiglucerase shortage. Blood Cells Mol Dis. 2011;46:115–118. doi: 10.1016/j.bcmd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Goldblatt J, Fletcher JM, McGill J, Szer J, Wilson M. Enzyme replacement therapy “drug holiday”: results from an unexpected shortage of an orphan drug supply in Australia. Blood Cells Mol Dis. 2011;46:107–110. doi: 10.1016/j.bcmd.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Grinzaid KA, Geller E, Hanna SL, Elsas LJ., II Cessation of enzyme replacement therapy in Gaucher disease. Genet Med. 2002;4(6):427–433. doi: 10.1097/00125817-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Hollak CEM, van Weely S, van Oers MHJ, Aerts JMFG. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollak CEM, vom Dahl S, Aerts JMFG, et al. Forze majeure: therapeutic measures in response to restricted supply of imiglucerase (Cerezyme) for patients with Gaucher disease. Blood Cells Mol Dis. 2010;44:41–47. doi: 10.1016/j.bcmd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Hughes DA, Pastores GM. The pathophysiology of GD – current understanding and rationale for existing end emerging therapeutic approaches. Wien Med Wochenschr. 2010;160:594–599. doi: 10.1007/s10354-010-0864-4. [DOI] [PubMed] [Google Scholar]
- Jmoudiak M, Futerman AH. Gaucher disease: pathological mechanisms and modern management. Br J Haematol. 2005;129:178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Weinreb NJ, Aerts H, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41(Suppl 5):4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Schwartz IVD, Karam S, Ashton-Prolla P, et al. Effects of imilglucerase withdrawal on an adult with Gaucher disease. Br J Haematol. 2001;113:1089. doi: 10.1046/j.1365-2141.2001.02821-13.x. [DOI] [PubMed] [Google Scholar]
- Vom Dahl S, Poll LW, Haussinger D. Clinical monitoring after cessation of enzyme replacement therapy in M Gaucher. Br J Haematol. 2001;113:1084–1085. doi: 10.1046/j.1365-2141.2001.02821-9.x. [DOI] [PubMed] [Google Scholar]
- Zimran A, Altarescu G, Elstein D. Nonprecipitous changes upon withdrawal from imiglucerase for Gaucher disease because of a shortage in supply. Blood Cells Mol Dis. 2011;46:111–114. doi: 10.1016/j.bcmd.2010.05.001. [DOI] [PubMed] [Google Scholar]


