Abstract
Liver dysfunction usually accompanies metabolic decompensation in fatty acid oxidation disorders, including carnitine palmitoyltransferase (CPT) Ia deficiency. Typically, the liver is enlarged with raised plasma transaminase activities and steatosis on histological examination. In contrast, cholestatic jaundice is rare, having only been reported in long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. We report a 3-year-old boy with CPT Ia deficiency who developed hepatomegaly and cholestatic jaundice following a viral illness. No cause for the jaundice could be found, apart from the fatty acid oxidation disorder. Liver histology showed diffuse, predominately macrovesicular steatosis, hepatocellular and canalicular cholestasis but no bile duct paucity or evidence of large duct obstruction. The liver dysfunction resolved in 4–7 weeks.
Introduction
Long-chain fatty acids are activated to coenzyme A (CoA) esters at the outer mitochondrial membrane but they need to be converted to carnitine esters in order to cross the inner mitochondrial membrane. This reaction is catalysed by carnitine palmitoyltransferase I (CPT I, EC 2.3.1.21). Within the mitochondria, the acyl-groups are converted back to CoA esters by CPT II. Three different genetic isoforms of CPT I have been found in different tissues – CPT Ia in liver and kidney, CPT Ib in muscle and heart and CPT Ic in brain.
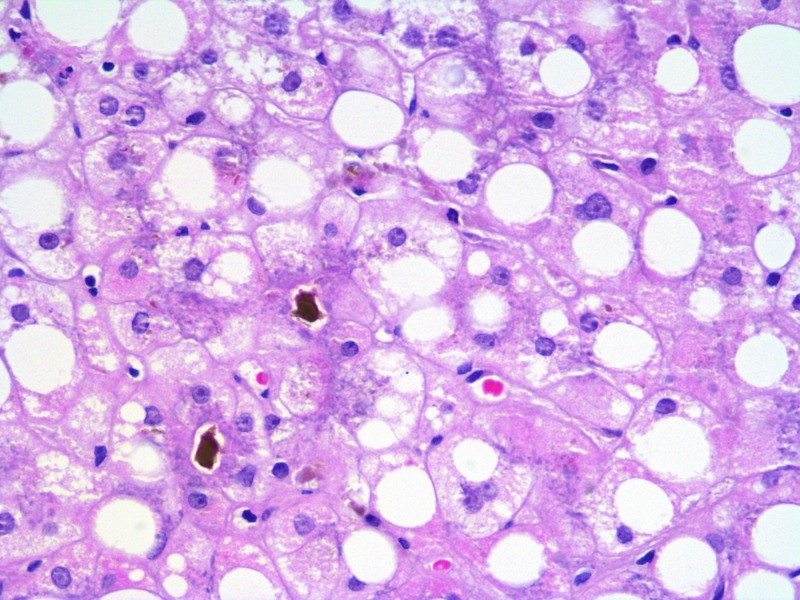
Fig. 1.
Liver histology showing steatosis (predominantly macrovesicular) and cholestasis. H&E ×400
Of the three isoforms, only CPT Ia deficiency has been identified in humans (OMIM 255120). Most patients present by the age of 2 years with hypoketotic hypoglycaemia, induced by fasting or illness. This is usually accompanied by liver dysfunction; there may also be transient lipaemia and renal tubular acidosis (Olpin et al. 2001). Paradoxically, although CPT Ia is not highly expressed in heart or muscle, several patients have had cardiac problems or raised plasma creatine kinase values (Olpin et al. 2001).
The liver dysfunction that accompanies hypoglycaemia in CPT Ia deficiency resembles that seen in other fatty acid oxidation disorders, such as MCAD deficiency, but it may persist for longer, often for several weeks. The liver is enlarged and histology has shown steatosis (Bougneres et al. 1981). Plasma transaminase activities are increased and there may be mild hyperammonaemia and hyperuricaemia. Here we report a patient who showed a different pattern of liver dysfunction, with cholestasis.
Case Report
The patient is the only child of healthy, non-consanguineous Caucasian parents. He presented at 18 months of age with a respiratory arrest and hypoglycaemia (0.4 mmol/l), associated with gastroenteritis. He made a full recovery but, at 2 years of age, a further episode of gastroenteritis led to a 5-min seizure associated with hypoglycaemia (2.1 mmol/l). Following correction of the hypoglycaemia, the patient had some brief focal seizures and was found to have left-sided weakness; over 24 h, he developed marked hepatomegaly. There was lipaemia (triglycerides 36 mmol/l, normal <1.7) with raised plasma levels of creatine kinase (1806 IU/l, normal 24–195) and urate (1088 umol/l, normal 120–390). Echocardiography, electrocardiography and cranial MRI were all normal. Urine organic acid analysis was unremarkable, as was blood acylcarnitine analysis, except for a raised free carnitine concentration (82 umol/l, normal 14–74). Tritium release assays subsequently showed reduced fatty acid oxidation flux (17–21% simultaneous controls for myristate, 12% for palmitate and 9–13% for oleate). Acylcarnitine measurements in cultured fibroblasts after 96 h incubation with 400 umol/l carnitine and 200 umol/l palmitate showed a low C16-acylcarnitine (0.26 umol/l, controls 1.47 ± 0.76, mean ± 2SD, n = 65) and a raised C5/C16 acylcarnitine ratio (7.3, controls 0.13–1.01, n = 70). CPT1a mutation analysis showed that the patient was a compound heterozygote for c.1766_1767insACATA (p.Tyr589X) and c.526G>T (p.Val176Phe). The patient made a full recovery, the hepatomegaly and weakness resolving over 6–8 weeks. The family was advised to give regular drinks containing glucose polymer during illnesses.
At the age of 3 years 9 months, the patient had a further admission to hospital. He had cough, diarrhoea, vomiting and fever, persisting for 6 days. There was an outbreak of influenza A H1N1 in the area and his general practitioner treated him with a course of oseltamivir, although the diagnosis was not confirmed by microbiological techniques. Glucose polymer was not given as he continued to eat. On admission, he was not hypoglycaemic but he had hepatomegaly and deranged liver function tests (bilirubin 79 μmol/l, alanine transaminase 336 IU/l, gamma glutamyltransferase 153 IU/l and normal clotting parameters). His symptoms settled after 2–3 days and he was allowed home with outpatient follow-up. After 10 days, he was readmitted with increasing jaundice, marked hepatomegaly and mild dehydration. He had no stigmata of chronic liver disease and was not encephalopathic. Initially, blood was lipaemic (triglycerides 12.2.mmol/l). Blood glucose concentrations were consistently normal. The plasma bilirubin concentration had increased to 226 μmol/l and stayed at this level for the next 7 days. Plasma activities were raised for alanine transaminase (maximum value 446 IU/l normal 9–36), alkaline phosphatase (maximum 826 IU/l normal 60–370) and gamma glutamyltransferase (maximum 368 IU/l normal <50). The albumin concentration was normal as were clotting studies apart from a marginally prolonged prothrombin time (14 s, controls 9–12 s).
No cause for the cholestatic liver disease was identified, apart from the fatty acid oxidation disorder. Serum alpha-1-antitrypsin, caeruloplasmin and immunoglobulins were normal and an autoantibody screen and serology for hepatitis A, B, C, cytomegalovirus (CMV), Epstein-Barr virus (EBV) and Varicella were negative. Polymerise chain reactions for CMV, EBV, entero- and adeno-viruses were also negative. Abdominal ultrasound examination showed an enlarged, echobright liver with no biliary dilatation; the spleen was normal, as was flow in the hepatic artery, hepatic veins and hepatic portal vein. A liver biopsy, undertaken 4 weeks after the initial admission, showed diffuse macrovesicular steatosis with minor microvesicular change in 60–80% hepatocytes. There was hepatocellular and canalicular cholestasis with focal acinar formation but no bile duct paucity, no evidence of large duct obstruction and no significant fibrosis (Fig. 1). Copper-associated protein was not identified.
The patient was given a diet high in carbohydrate from the time of admission. Supplements of medium-chain triglycerides, fat-soluble vitamins and ursodeoxycholic acid were added subsequently. The liver size and liver function tests gradually returned to normal 4–7 weeks after admission.
Discussion
It seems very likely that this boy’s cholestasis was caused by the metabolic derangement. Steatosis was the main finding on liver histology, as in previous patients with CPT Ia deficiency (Bougneres et al. 1981). The only additional features were hepatocellular and canalicular cholestasis, with focal acinar formation. The patient was thought to have had a viral illness, possibly influenza A H1N1, but the liver histology was not typical of a viral-induced hepatitis. Moreover, H1N1 influenza is associated with mild to moderate transaminitis but not severe cholestasis (Yingying 2011). The Medicines and Healthcare Products Regulatory Agency has received two reports of liver dysfunction after treatment with oseltamivir but there were alternative causes of liver dysfunction in these cases and a causal association with the drug was not established (www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con059973.pdf).
Liver dysfunction is common during acute illnesses in many fatty acid oxidation disorders. In contrast, cholestatic jaundice is rare, having only been reported previously in patients with LCHAD deficiency (Ibdah et al. 1999; Saudubray et al. 1999; Tyni et al. 1997). In these patients, the cholestasis has often accompanied an acute Reye-like illness but sometimes it has been an incidental finding (e.g. in a patient presenting with hypocalcaemia). Pregnant mothers carrying a foetus with LCHAD deficiency also have an increased risk of cholestasis (though the increase in risk is less than for HELLP syndrome and acute fatty liver of pregnancy) (Tyni et al. 1998).
CPT Ia deficiency is generally rare but a variant in the gene is extremely common in the Inuit population of Alaska, Canada and Greenland. In some regions, 70% of babies are homozygous for the c.1436C>T mutation, which reduces CPT Ia activity, estimates of residual activity ranging from 6–22% of control values. A few homozygous subjects present with hypoglycaemia as neonates or young children but most have been considered asymptomatic (Greenberg et al. 2009). Interestingly, however, a number of them have self-limiting cholestatic jaundice during infancy (S. Mercimek-Mahmutoglu, 2010, personal communication).
Our patient’s cholestasis might be due to impaired energy production preventing ATP-dependent bile acid secretion; alternatively, toxic metabolites might interfere with the canalicular transport proteins for bile acids or phospholipids. Though cholestasis is unusual in fatty acid oxidation disorders, it accompanies hepatic steatosis in a number of other genetic metabolic disorders, including mitochondrial liver disease, galactosaemia, tyrosinaemia type 1, Wilson disease and cystic fibrosis. Mild cholestasis also occurs in a rat model of non-alcoholic fatty liver disease (Pizarro et al. 2004) but the pathogenesis is unlikely to resemble that in our patient.
Our patient’s liver dysfunction resolved over 4–7 weeks on treatment with a high carbohydrate diet, medium chain triglycerides and ursodeoxycholic acid. We do not know whether the treatment contributed to the recovery, but we suspect that the jaundice would have resolved without intervention.
Abbreviations
- CMV
Cytomegalovirus
- CPT
Carnitine palmitoyltransferase
- EBV
Epstein-Barr virus
- LCHAD
Long-chain 3-hydroxyacyl-CoA dehydrogenase
Footnotes
Competing interests: None declared
References
- Bougneres PF, Saudubray JM, Marsac C, Bernard O, Odievre M, Girard J. Fasting hypoglycemia resulting from hepatic carnitine palmitoyl transferase deficiency. J Pediatr. 1981;98:742–746. doi: 10.1016/S0022-3476(81)80834-7. [DOI] [PubMed] [Google Scholar]
- Greenberg CR, Dilling LA, Thompson GR, et al. The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian aboriginal populations. Mol Genet Metab. 2009;96:201–207. doi: 10.1016/j.ymgme.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Dasouki MJ, Strauss AW. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: variable expressivity of maternal illness during pregnancy and unusual presentation with infantile cholestasis and hypocalcaemia. J Inherit Metab Dis. 1999;22:811–814. doi: 10.1023/A:1005506024055. [DOI] [PubMed] [Google Scholar]
- Olpin SE, Allen J, Bonham JR, et al. Features of carnitine palmitoyltransferase type I deficiency. J Inherit Metab Dis. 2001;24:35–42. doi: 10.1023/A:1005694320063. [DOI] [PubMed] [Google Scholar]
- Pizarro M, Balasubramaniyan N, Solis N, et al. Bile secretory function in the obese Zucker rat: evidence of cholestasis and altered canalicular transport function. Gut. 2004;53:1837–1843. doi: 10.1136/gut.2003.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudubray JM, Martin D, de Lonlay P, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. doi: 10.1023/A:1005556207210. [DOI] [PubMed] [Google Scholar]
- Tyni T, Ekholm E, Pihko H. Pregnancy complications are frequent in long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Am J Obstet Gynecol. 1998;178:603–608. doi: 10.1016/S0002-9378(98)70446-6. [DOI] [PubMed] [Google Scholar]
- Tyni T, Palotie A, Viinikka L, et al. Long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency with the G1528C mutation: clinical presentation of thirteen patients. J Pediatr. 1997;130:67–76. doi: 10.1016/S0022-3476(97)70312-3. [DOI] [PubMed] [Google Scholar]
- Yingying C. Abnormal liver chemistry in patients with influenza A H1N1. Liver Int. 2011;31:902. doi: 10.1111/j.1478-3231.2011.02519.x. [DOI] [PubMed] [Google Scholar]