Abstract
Background. The diagnosis of autosomal dominant GTP-cyclohydrolase deficiency relies on the examination of the GCH1 gene and/or pterins and neurotransmitters in CSF. The aim of the study was to assess the diagnostic value, if any, of pterins in urine and blood phenylalanine (Phe) and tyrosine (Tyr) under oral Phe loading test.
Methods. We report on two new pedigrees with four symptomatic and four asymptomatic carriers whose pattern of urinary pterins and blood Phe/Tyr ratio under oral Phe loading pointed to GTP-cyclohydrolase deficiency. The study was then extended to 3 further patients and 90 controls. The diagnostic specificity and sensitivity of these metabolic markers were analysed by backwards logistic analysis.
Results. Two genetic alterations segregated alternatively in Family 1 (c.631-632 del AT and c.671A > G), while exon 1 deletion was transmitted along three generations in Family 2. Neopterin and biopterin concentrations in urine clustered differently in controls under and over the age of 15. Therefore patients and controls were sub grouped according to this age. Neopterin was significantly reduced in GCH1 mutated subjects younger than 15, and both neopterin and biopterin in those older than 15. Moreover, the Phe/Tyr ratios at the second and third hour were both significantly higher in patients than in controls. Backwards logistic regression demonstrated the high diagnostic sensitivity and specificity of combined values of neopterin concentration and Phe/Tyr ratio at the second hour.
Conclusions. Pterins in urine and Phe loading test are non-invasive and reliable tools for the biochemical diagnosis of GTP-cyclohydrolase deficiency.
Introduction
Autosomal dominant (AD) GTP-cyclohydrolase (GTP-CH EC 3.5.4.16) deficiency (DYT5a; MIM # 600225) presents with a wide spectrum of Dopa-responsive movement disorders (DRD) and a variable and sex-conditioned penetrance (Segawa et al. 2003; Segawa 2009). GCH1 gene alterations are found in about 60% of patients (Segawa 2009), while in almost all cases neopterin and biopterin are low in CSF (Fink et al. 1988; Furukawa et al. 1993; LeWitt et al. 1986).
Pterins in urine, even though rarely assessed, are generally considered normal (Furukawa et al. 1998). We report on two new families with genetically confirmed DYT5a whose pattern of urinary pterins coupled with phenylalanine (Phe)/tyrosine (Tyr) ratio under oral Phe loading pointed to GTP-GH deficiency. On this perspective, the biochemical hallmarks of the disease were reviewed and the potential diagnostic value of Phe loading test and pterins in urine was explored.
Patients
Family 1. The proband, a 12-year-old girl, was born after a normal pregnancy and delivery from Italian unrelated parents. Mother and a 9-year-old brother were normal. Her 36-year-old father had been suffering since adolescence from gait fatigability and foot rigidity that increased in the evening period. The patient’s early psychomotor development was considered normal. At the age of 3 years, she suffered from atonic fits and was treated with antiepileptic medicaments. When 8 years old, she was again examined because of learning difficulties that were ascribed to a mild mental retardation. Starting from the age of 9 years, she complained of fatigability and instable gait emerging during the evening period. On examination, at the age of 11, she exhibited generalised choreoathetosis, which was exacerbated by exercise and in the late afternoon and evening when she was no longer capable of walking unsupported. She was mildly mentally retarded (WISC-R IQ 51), depressed and anxious. On neurological examination her father showed a mild foot dystonia with diurnal fluctuation.
Molecular analysis of the GCH1 gene (see below for the methods) disclosed a frameshift mutation in exon 6 (c.631-632 del AT, p. M211fs) in the girl and in her father (case 1a and b, respectively, Table 1). Unexpectedly, a different point mutation was found in the patient’s mother and brother (c.671A>G, p.K224R) (case 1c and 1d, Table 1).
Table 1.
Genotype and biochemical phenotype in CGH1 mutated subjects enclosed in the study (see text for clinical details)
| ID | Genotype | Sex | Age (years) | CSF HVAa (r.v.)b | CSFa 5-HIAA (r.v.)b | CSF NEOa (r.v.)b | CSF BIOa (r.v.)b | Urine NEOa (r.v.)c | Urine BIOa (r.v.)c | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | c.631-632 del AT [p.M211fs] | F | 11 | 229 | 143 | 7.7 | 11.8 | 0.27 | 1.21 | Pedigree 1: proband |
| (148–434) | (68–115) | (9.1–20.1) | (10.1–29.9) | (0.30–1.84) | (0.53–2.05) | |||||
| 1b | c.631-632 del AT [p.M211fs] | M | 36 | 0.23 | 0.69 | Pedigree 1: father of 1a | ||||
| (0.19–0.91) | (0.28–0.86) | |||||||||
| 1c | c.671A>G [p.K224R] | M | 8 | 0.22 | 0.65 | Pedigree 1: brother of 1a | ||||
| (0.30–1.84) | (0.53–2.05) | |||||||||
| 1d | c.671A>G [p.K224R] | F | 34 | 0.41 | 0.36 | Pedigree 1: mother of 1a | ||||
| (0.19–0.91) | (0.28–0.86) | |||||||||
| 2a | Exon 1 deletion | F | 7 | 188 | 74 | 5.4 | 11.1 | 0.23 | 0.42 | Pedigree 2: proband |
| (137–582) | (68–220) | (9.1–20.1) | (10.1–29.9) | (0.30–1.84) | (0.53–2.05) | |||||
| 2b | Idem | F | 36 |
0.13 (0.19–0.91) |
0.16 (0.28–0.86) |
Pedigree 2: mother of 2a | ||||
| 2c | Idem | M | 5 | 0.25 | 0.60 | Pedigree 2: brother of 2a | ||||
| (0.30–1.84) | (0.53–2.05) | |||||||||
| 2d | Idem | F | 58 | 0.13 | 0.16 | Pedigree 2: maternal grandmother 2a | ||||
| (0.19–0.91) | (0.28–0.86) | |||||||||
| 3 | c.68 C>T [p.P23L] | M | 25 | 81 | 45 | 5.1 | 10.5 | 0.11 | 0.19 | Leuzzi, unpublished case |
| (98–450) | (45–135) | (9.1–20.1) | (10.1–29.9) | (0.19–0.91) | (0.28–0.86) | |||||
| 4 | c.671A>G [p.K224R] | M | 17 | 80 | 42 | 8.7 | 16.9 | 0.16 | 0.47 | Leuzzi et al. 2002 |
| (98–450) | (45–135) | (9.1–20.1) | (10.1–29.9) | (0.30–1.84) | (0.53–2.05) | |||||
| 5 | c.262 C>T [p.R88W] | F | 45 | 0.14 | 0.4 | Leuzzi, unpublished case | ||||
| (0.19–0.91) | (0.28–0.86) |
HVA homovanillic acid, 5-HIAA 5-hydroxyndoleacetic acid, NEO neopterin, BIO biopterin, BH4 tetrahydrobiopterin
a Pathological values are in bold
bAge-related reference values (r.v.) (nmol/l)
cAge-related reference values (r.v) (2.5–98 percentile of control values; mmol/mol creatinine)
L-Dopa/Carbidopa treatment (up to 8/2 mg/kg bw/day) resulted in a dramatic improvement in the father and in the proband. At the same time her WISC-R IQ improved to 63.
Family 2. A 7.5-year-old girl of Swedish and Italian descent presented with a progressive long-lasting severe gait disorder with diurnal fluctuation. She was the first offspring of two unrelated parents. The 32-year-old mother had been complaining during childhood of remarkable fatigability that disappeared in the following years. The proband was born after a normal pregnancy and delivery. She was able to walk unsupported at the age of 13 months when the parents first noticed fatigability and protracted uncertainty in the gait. At the age of 3, a mild paraparesis was diagnosed. During the following few years paraparesis worsened and a diurnal fluctuation of the symptoms became manifest. On examination, at the age of 7, she showed normal mental development (WISC-III IQ 115), generalised hypo- and bradykinesia, dystonic paraparetic gait with extra rotation of the hip and feet drop in the propulsive phase of gait and masked face with rigid fixed posture of head and neck. Exon 1 deletion in the GCH1 gene was detected in the proband, her brother, mother and unaffected 58-year-old maternal grandmother of Swedish origin (respectively cases 2a-2d in Table 1) (see below for the methods).
The only neurological sign detected in the mother was an exaggerated dorsal flexion of the big toe in the oscillatory phase of the gait.
Under l-Dopa/Carbidopa treatment (2/0.5 mg/kg bw/day), the girl experienced a marked improvement of both hypo-bradykinesia and lower limb dystonia.
Methods
Genomic analysis included the following: exon and intron–exon boundaries sequencing (Bandmann et al. 1996), screening for intra-gene deletions or duplications of the alleles negative to sequencing analysis (Zirn et al. 2008) (SALSA MLPA KIT, MRC-Holland, Amsterdam, The Netherlands) (Schouten et al. 2002) and retesting of the patients positive to MLPA analysis with Real-Time PCR (SYBR Green dye chemistries).
Informed consent was obtained from all subjects examined and (if minor) their parents.
5-Hydroxyindoleacetic acid [5-HIAA], homovanillic acid [HVA], and 3-methoxy-4-hydroxyphnylglycol were assessed in CSF by high-performance liquid chromatography with electrochemical detection. Neopterin and biopterin were determined in the first morning urine sample and CSF according to the method published elsewhere (Antonozzi et al. 1988). Phe and Tyr were measured by ESI-MS/MS (Chace et al. 1993) in dried blood spots at baseline and 1, 2, 3, 4, 5 and 6 h after oral Phe loading (100 mg/kg body weight) (Hyland et al. 1997).
To evaluate specificity and sensitivity of these tests for the diagnosis of Segawa disease, the study was extended to 3 previously diagnosed patients (Table 1, cases 3–5) and 90 subjects with primary and secondary movement disorders not due to defects of biogenic amine metabolism who acted as control samples. In the whole, pterins in urine were assessed in 101 subjects, while 9 subjects with GCH1 mutations and 39 controls also underwent Phe loading test. No subject among patient and control population showed clinical and/or haematological features of concurrent infection disease when the urine sample was collected.
Statistical analysis. Quantitative data are presented as means ± standard deviations, and were analyzed by Mann–Whitney U test to assess differences between groups within each age class. Stepwise logistic regression analysis (backwards selection) was performed to select the variables associated with the diagnosis, separately in the two age groups. Potential diagnostic factors were biopterin, neopterin, Phe peak, Phe/Tyr at the second and third hour after Phe loading. The estimated Receiver Operating Characteristic (ROC) curve was then plotted for the selected model and for the models including each of the selected variables, separately considered. The areas under the ROC curves were calculated as an overall measure of diagnostic efficiency of the selected criterion. Sensitivity (Se), specificity (Sp) and positive and negative predictive values (PPV and NPV, respectively) were computed. Finally, a curve representing the diagnostic test based on the selected model was determined.
All the statistical analyses were performed using STATA Statistical Software (Release 8.0).
Results
Table 1 shows GCH1 genotype and biochemical phenotype of patients and asymptomatic carriers from the two families. Neopterin was low in CSF of both propositi (1a, 2a). The pattern of pterin excretion in urine (Table 1 cases 1a-2d) and Phe/Tyr ratio under oral Phe loading in symptomatic (4) and pre-symptomatic (4) carriers from the two families pointed both to GTP-GH deficiency.
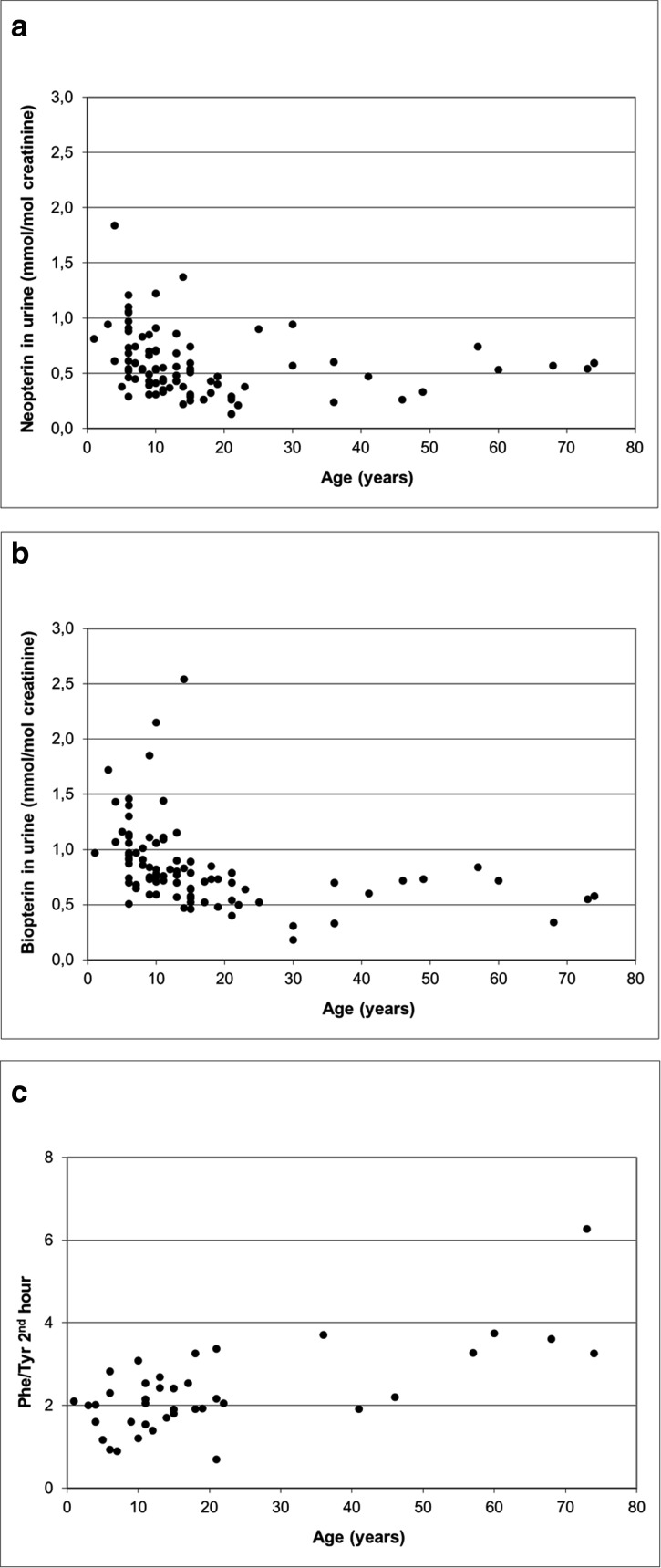
The preliminarily exploration of the levels of neopterin and biopterin in urine from the controls showed they clustered differently before and over the age of 15 (Fig. 2a and b). Mean values of biopterin and neopterin in the “15–17 years” group were significantly different from those observed in the “0–14 years” group (p = 0.0003 and p = 0.0137, respectively), while they did not differ from those observed in the “18–74 years” group (p = 0.8121 and p = 0.7979, respectively). At the ratio Phe/Tyr under Phe loading test at second hour, no significant differences were observed in both comparisons (p = 0.4857 and p = 0.1615, respectively (Fig. 1c shows the distribution of Phe/Tyr at second hour). Therefore, for the following statistical analysis, GCH1 mutated subjects and controls were all grouped according to their age (pterin analysis: 59 < 15 and 42 ≥ 15 years; Phe loading test: 23 < 15, 25 ≥ 15 years).
Fig. 2.
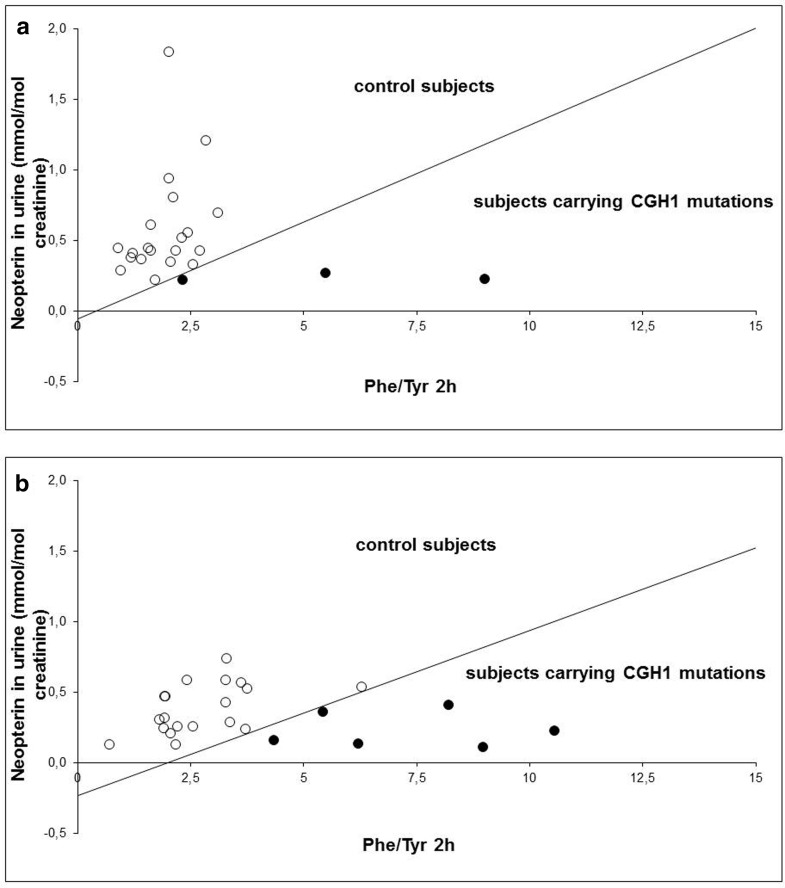
Scatter plot of Phe/Tyr at second hour and neopterin values. The white dots represent controls (39) and the black dots patients (9) included in the ROC analysis. The curve divides the space into two areas, namely the area of control subjects above the curve and the area of subjects carrying GCH1 mutations below the curve. Each patient can be represented as a point in this space according to their values of neopterin and Phe/Tyr ratio 2 h after Phe loading: if the point lies in the area below the curve, the patient has a high probability of carrying a GCH1 mutations, whereas if they lie in the area above the curve, the patient has a high probability of not carrying a GCH1 mutation. Decision rule can be expressed by the following analytical expressions. The test is positive if “-27.47 - 464.38 * [neopterin] + 63.93 * Phe/Tyr2h > 0” for subjects younger than 15 years (a) and “-106.91 - 456.85 * [neopterin] + 53.48 * Phe/Tyr2h > 0” for subjects older than 15 years (b), where [neopterin] is the concentration of neopterin in urine (expressed in mmol/mol creatinine) and Phe/Tyr2h is the ratio between Phe and Tyr concentrations in dried blood spot (each expressed in μmol/l of blood) 2 h after Phe loading
Fig. 1.
Scatter plot of neopterin concentrations in urine vs age in controls (n = 90; r = −0.19; p = 0.0748) (a), biopterin concentrations in urine vs age in controls (n = 90; r = −0.37; p = 0.0003) (b) and Phe/Tyr ratio 2 h after Phe loading vs age in controls (c) (n = 39; r = 0.68; p < 0.0001)
Neopterin and biopterin concentrations (mmol/mol creatinine) in urine were, respectively, 0.66 ± 0.31 (range 0.22–1.84) and 0.99 ± 0.39 (range 0.47–2.54) in controls younger than 15, and 0.44 ± 0.20 (range 0.13–0.94) and 0.60 ± 0.16 (range 0.18-0.89) in those aged >15. In GCH1 mutated subjects, they were respectively 0.24 ± 0.02 (range 0.22–0.27) and 0.72 ± 0.34 (range 0.42–1.21), and 0.22 ± 0.11 (0.11-0.41) and 0.41 ± 0.19 (range 0.16-0.69) (GCH1 mutated subjects vs controls: <15 years neopterin p = 0.0013, biopterin p = 0.1066; ≥ 15 years neopterin p = 0.0053, biopterin p = 0.0216). Phe/Tyr ratio at seconnd and third hour after the loading of Phe were both significantly higher in patients than in controls (< 15 years: 5.59 ± 3.34 vs 1.91 ± 0.62, p = 0.0285; and 4.48 ± 3.88 vs 1.43 ± 0.47, p = 0.0621, respectively; ≥ 15 years: 7.27 ± 2.44 vs 2.73 ± 1.18, p = 0.0006; and 5.69 ± 2.36 vs 2.07 ± 0.84, p = 0.0007, respectively).
Backwards logistic regression identified Phe/Tyr at second hour and urine neopterin as significantly associated to GCH1 gene alteration. The combined criterion appeared to perform better in terms of specificity and sensitivity than the criteria based on Phe/Tyr at second hour and neopterin separately considered (Table 2). Considering both Phe/Tyr at second hour and neopterin values, the decision rule can be based on the following analytical expressions, obtained by the multivariate logistic regression (ROC analysis). The test is positive if:
Table 2.
Results of the logistic regression analyses assessing the diagnostic value of Phe/Tyr ratio 2 h after Phe loading and neopterin concentration in urine
| Area under the ROC curve | Phe/Tyr 2nd hour | Neopterino | Phe/Tyr 2nd hour + Neopterino | |||
|---|---|---|---|---|---|---|
| Age | ||||||
| <15 years (n = 23) | ≥15 years (n = 25) | <15 years (n = 59) | ≥15 years (n = 42) | <15 years (n = 23) | ≥15 years (n = 25) | |
| Se % | 66.7 | 83.3 | 75.0 | 42.9 | 100.0 | 100.0 |
| Sp % | 100.0 | 94.7 | 98.2 | 94.3 | 100.0 | 100.0 |
| PPV % | 100.0 | 83.3 | 75.0 | 60.0 | 100.0 | 100.0 |
| NPV % | 95.2 | 94.7 | 98.2 | 89.2 | 100.0 | 100.0 |
| Correctly classified % | 95.7 | 92.0 | 96.6 | 85.7 | 100.0 | 100.0 |
| Cut-off a | 3.84 | 5.24 | 0.26 | 0.15 | See Fig. 2a | See Fig. 2b |
| Logistic regression coefficients | See ‘Results’ and Fig. 2a | See ‘Results’ and Fig. 2b | ||||
| Constant | −6.90 | −7.59 | 11.48 | 1.48 | ||
| Independent variable | 1.79 | 1.45 | −44.12 | −10.09 | ||
| p for the coefficient of the selected independent variable | 0.145 | 0.022 | 0.053 | 0.022 | ||
Se sensitivity, Sp specificity, PPV positive predictive values, NPV negative predictive values
° mmol/mol creatinine
aCut-off values were obtained by logistic regression coefficients
“-27.47 - 464.38 * [neopterin] + 63.93 * Phe/Tyr 2 h > 0” for subjects younger than 15 years (Fig. 2a), and “-106.91 - 456.85 * [neopterin] + 53.48 * Phe/Tyr2h > 0” for subjects older than 15 years (Fig. 2b), where [neopterin] is the concentration of neopterin in urine (expressed in mmol/mol creatinine) and Phe/Tyr2h is the ratio between Phe and Tyr concentrations in dried blood spot (each expressed in μmol/l of blood) 2 h after Phe loading.
Discussion
The GCH1 gene mutation is the most common cause of early onset DRD (Hagenah et al. 2005; Clot et al. 2009), involving about 85% of all patients with DRD (Furukawa 2004). More than 140 alterations have been so far reported on the GCH1 gene (www.hgmd.cf.ac.uk, accessed 17 November 2011). Two pathogenetic mutations occurred independently in family 1. p.M211fs was detected in the proband, who presented moderate mental retardation years before developing generalized choreoathetosis with diurnal fluctuation. Both symptoms, and remarkably movement disorders, improved with l-Dopa/Carbidopa therapy. A mild dystonic phenotype was detected in her father. p.M211fs has been so far associated with a typical DRD presentation (Hagenah et al. 2005; Clot et al. 2009; Trender-Gerhard et al. 2009). Different to what happens in the recessive disorders of biogenic amine metabolism (Leuzzi et al. 2010), mental retardation is not part of DYT5a phenotype. It has been reported in the context of a complex presentation due to a 2.3 Mb deletion on chromosome 14q21-22 (family ITD612 in Clot et al. 2009) and in a single patient with a biochemically confirmed diagnosis but no alteration on the GCH1 gene (Nagata et al. 2007). Two other members of Family 1 carried p.K224R mutation and were clinically normal in spite of some biochemical alterations suggesting GTP-CH deficiency (see below). p.K224R has been so far reported in three pedigrees associated with athetoid cerebral palsy (family Hu in Bandmann et al. 1998), myoclonic dystonia (Leuzzi et al. 2002) and adult-onset gait dystonia, rigidity and bradykinesia (Patient D97 in Garavaglia et al. 2004). Since the first report (Furukawa et al. 2000), few pedigrees harbouring gross deletion of the GCH1 gene have been reported (Klein et al. 2002; Hagenah et al. 2005; Ohta et al. 2006; Clot et al. 2009). Their phenotype overlaps with that of patients with less severe alterations.
Since the availability of an aetiological treatment, the diagnosis of DYT5a should be considered for each patient presenting with an early onset dystonia-parkinsonism syndromes. Tables 1 and 3 summarize the GCH1 genotype and biochemical phenotype in the few cases in which both were examined. CSF examination was performed on 23 patients (including ours) from 16 pedigrees. Neopterin was low in 21/23 cases, biopterin or tetrahydrobiopterin in 18/22, HVA in 11/15, and 5-HIAA in 8/15. As a group, the subjects carrying GCH1 mutations showed also a lower level of neopterin in urine in comparison with the controls.
Table 3.
CGH1 genotype and biochemical phenotype in patients with Segawa disease from the literature
| IDa | Genotype | Sex | CSF HVAb | CSF 5-HIAAb | CSF NEOb | CSF BIO/BH4b | Ref. |
|---|---|---|---|---|---|---|---|
| (r.v.)c | (r.v.)c | (r.v.)c | (r.v.)c | ||||
| 6 | c.218C>A [p.A73D] |
M |
145 (364–870) |
151 (155–359) |
7 (5–53) |
<2 (20–61) |
Opladen et al. 2010 (pt 1) |
| 7 | c.623 + 3insT [IVS5 + 3insT] |
F |
95 (217–507) |
62 (75–203) |
3 (7–27) |
3 (20–49) |
Ibidem (pt 2) |
| 8 | GCH1 mutation |
F | 414 (220–560) |
174 (90–237) |
4 (7–27) |
6 (20–49) |
Ibidem (pt 6) |
| 9 | Heterozygous deletion of all 6 exons | M |
112 (217–507) |
34 (75–203) |
2 (7–27) |
2 (20–49) |
Ibidem (pt 7) |
| 10 | c.547G>A [p.E183K] |
F |
18.8 (25 ± 5.0) |
9.7 (25 ± 5.0) |
Ikeda et al. 2009 | ||
| 11 | c.64_65delGGi nsAACC [p.G21fsX66] |
F |
48 (115–455) |
20 (51–204) |
6 (10–31) |
n.d. (18–53) |
von Mering et al. 2008 |
| 12 | c.159delG | F |
3.1 (22.6 ± 1.5) |
3.8 (20.6 ± 1.4) |
Furuya et al. 2006 | ||
| [p.W53fs] | |||||||
| 13 | c.265C>T [p.Q89X] |
M |
268 (334–906) |
236 (170–420) |
2 (8–43) |
8 (11–39) |
Lopez-Laso et al. 2006 |
| 14a | c.618del15bp [p.V206fs] |
F |
8.5 (22.1 ± 7.0) |
8.2 (26.4 ± 8.5) |
Ohta et al. 2006 | ||
| 14b | c.626 + 1G>A [IVS5 + 1G>A] |
F |
3.9 (22.1 ± 7.0) |
1.3 (26.4 ± 8.5) |
Ibid (pt 4) |
||
| 14c | Exons 3-4 deletion |
F |
8.6 (22.1 ± 7.0) |
15.7 (26.4 ± 8.5) |
Ibid (pt 5) |
||
| 15a | c.411del 37 bp [p.F138fs] |
M |
142 (395 ± 56) |
50 (126 ± 22) |
1.1 (> 20) |
5.4 (> 20) |
Hahn et al. 2001 |
| 15b | c.411del 37 bp [p.F138fs] |
M |
85 (268 ± 24) |
43 (108 ± 15) |
4.8 (> 20) |
6.8 (>20) |
Ibid (III:3) |
| 15c | c.411del 37 bp [p.F138fs] |
F |
447 (528 ± 75) |
161 (133 ± 14) |
18.5 (> 20) |
27.8 (> 20) |
Ibidem (IV:6) |
| 16a | c.671A>G [p.K224R] |
F |
5.9 (7–65) |
Bandmann et al. 1996; (Family Hu, II-3) |
|||
| 16b | c.671A>G [p.K224R] |
F | 91 (72–656) |
65 (58–222) |
5.9 (7–65) |
6.6 (BH4) (9–40) |
Robinson et al. 1999; (Family Hu, II-4) |
| 16c | c.671A>G [p.K224R] |
M | 152 (72–256) |
49 (58–222) |
4.3 (7–65) |
16 (BH4) (9–40) |
Ibid (Family Hu, II-5) |
| 17a | c.344-1G>A [IVS1-1G>A] |
4.4 (13.0–38.3) |
7.1 (9.6–21.2) |
Furukawa et al. 1996; (pt 2) |
|||
| 17b | c.341C>A [p.S114X] |
8.9 (13.0–38.3). |
5.9 (9.6–21.2) |
Ibid (pt 3) |
HVA homovanillic acid, 5-HIAA 5-hydroxyndoleacetic acid, NEO neopterin; BIO biopterin, BH4 tetrahydrobiopterin
nd: not detectable
aNumeration follows that of Table 1
bPathological values are in bold
cr.v.: reference values (nmol/l)
GTP-CH is the limiting enzyme for the synthesis of tetrahydrobiopterin, which is the cofactor of phenylalanine, tyrosine and tryptophan hydroxylases (Blau et al. 2001). Based on this, a Phe loading procedure has been proposed (Hyland et al. 1997) and different values of Phe/Tyr 4 hours after the loading have been suggested as a critical threshold for the diagnosis: 4.5 (Saunders-Pullman et al. 2000), 7.5 (Bandmann et al. 2003), 5.25 (Saunders-Pullman et al. 2004). In spite of high sensitivity and specificity of the test, false negatives (Saunders-Pullman et al. 2004) and positives (Bandmann et al. 2003) were reported. Recent data suggest that the ratio obtained at the second hour may be more sensitive in young patients with DRD (Opladen et al. 2010; Lopez-Laso et al. 2006). The test reliability improves if the peak value of blood Phe exceeds 600 μmol/L and the plasma and blood spot biopterin variations during the loading are monitored (Opladen et al. 2010, Saunders-Pullman et al. 2004).
We have showed that the analysis of neopterin in first morning urine leads to a correct classification in 96.6% (age < 15aa ) and 85.7% (age > = 15 years) of 101 analyzed samples (Table 3) and that the evaluation of both Phe/Tyr ratio at the second hour and neopterin in urine leads to the highest specificity and sensitivity in order to detect young and adult subjects with GCH1 alterations, even though it cannot discriminate between symptomatic and pre-asymptomatic carriers (Hyland et al. 1999).
A pattern of Phe/Tyr ratio mimicking that found in GTP-CH deficiency may be found in the carriers of Phenylalanine Hydroxylase gene mutations (Driscoll and Hsai 1956; Guldberg et al. 1998), who, however, have a normal excretion of pterins in urine.
Caution should be adopted in interpreting urine and serum neopterin values in the presence of concomitant conditions potentially leading to T cell–mediated immune response activation (Huber et al. 1984) (such as viral infections, autoimmune diseases, malignancies, pregnancy, etc) (Werner-Felmayer et al. 2002): they could stimulate neopterin synthesis, so masking a possible partial defect of GTP-CH.
In conclusion, even though conducted in a restricted number of subjects, our study suggests a new simple, reliable and non-invasive approach to the diagnosis of DYT5a. It could be part of the diagnostic workup for patients presenting with idiopathic movement disorders of unknown origin.
Synopsis
The authors propose a rapid, simple and non-invasive method for the diagnosis of AD-DRD and suggest it as part of the diagnostic workup for patients presenting with idiopathic movement disorders of unknown origin.
Author Roles
Vincenzo Leuzzi: Conception, Organization, Execution of Research project; Writing of the first draft, Review and Critique of Statistical Analysis.
Claudia Carducci: Conception, Organization, Execution of Research project, Biochemical Studies; Review and Critique of Statistical Analysis.
Flavia Chiarotti: Review and Critique of experimental design, Statistical Analysis,
Daniela D'Agnano: Organization, Execution of Research project; Clinical data collection and patient follow-up; Review and Critique of Statistical Analysis.
Italo Antonozzi: Conception, Organization, Execution of Research project; Writing of the first draft, Review and Critique of Statistical Analysis
Maria Teresa Giannini: Organization, Execution of Research project; Clinical data collection and patient follow-up. Review and Critique of Statistical Analysis.
Carla Carducci: Organization, Execution of Research project; Molecular analysis; Review and Critique of Statistical Analysis.
Guarantor
Vincenzo Leuzzi
Competing Interests
The authors have declared that no competing interests exist.
Disclosure Information on Financial Support
No financial support.
Written Consent
A written consent of the legal substitute of the patients was obtained for genetic analysis.
Footnotes
Competing interests: None declared
References
- Antonozzi I, Carducci C, Vestri L, Pontecorvi A, Moretti F. Rapid and sensitive method for high-performance liquid chromatographic analysis of pterins in biological fluids. J Chromatogr. 1988;459:319–424. doi: 10.1016/S0021-9673(01)82042-2. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Nygaard TG, Surtees R, Marsden CD, Wood NW, Harding AE. Dopa responsive dystonia in British patients: new mutations of the GTP-cyclohydrolase I gene and evidence for genetic heterogeneity. Hum Mol Genet. 1996;5:403–406. doi: 10.1093/hmg/5.3.403. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Valente EM, Holmans P, et al. Dopa-responsive dystonia: a clinical and molecular genetic study. Ann Neurol. 1998;44:649–656. doi: 10.1002/ana.410440411. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Goertz M, Zschocke J, et al. The phenylalanine loading test in the differential diagnosis of dystonia. Neurology. 2003;60:700–702. doi: 10.1212/01.WNL.0000048205.18445.98. [DOI] [PubMed] [Google Scholar]
- Blau N, Thony B, Cotton RGH, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw Hill; 2001. pp. 1725–1776. [Google Scholar]
- Chace DH, Millington DS, Terada N, Kahler SG, Roe CR, Hofman LF. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin Chem. 1993;39:66–71. [PubMed] [Google Scholar]
- Clot F, Grabli D, Cazeneuve C, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with Dopa-responsive dystonia. Brain. 2009;132:1753–1763. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- Driscoll KW, Hsia DY. Detection of the heterozygous carriers of phenylketonuria. Lancet. 1956;217(6957):1337–1338. doi: 10.1016/s0140-6736(56)91489-1. [DOI] [PubMed] [Google Scholar]
- Fink JK, Barton N, Cohen W, Lovenberg W, Burns RS, Hallett M. Dystonia with marked diurnal variation associated with biopterin deficiency. Neurology. 1988;38:707–711. doi: 10.1212/WNL.38.5.707. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Nishi K, Kondo T, Mizuno Y, Narabayashi H. CSF biopterin levels and clinical features of patients with juvenile parkinsonism. Adv Neurol. 1993;60:562–567. [PubMed] [Google Scholar]
- Furukawa Y, Shimadzu M, Rajput AH, et al. GTP-cyclohydrolase I gene mutations in hereditary progressive and Dopa-responsive dystonia. Ann Neurol. 1996;39:609–617. doi: 10.1002/ana.410390510. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Shimadzu M, Hornykiewicz O, Kish S. Molecular and biochemical aspects of hereditary progressive and Dopa-responsive dystonia. Adv Neurol. 1998;78:267–282. [PubMed] [Google Scholar]
- Furukawa Y, Guttman M, Sparagana SP, et al. Dopa-responsive dystonia due to a large deletion in the GTP cyclohydrolase I gene. Ann Neuro. 2000;l47:517–520. doi: 10.1002/1531-8249(200004)47:4<517::AID-ANA17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Furukawa Y. Update on dopa-responsive dystonia: locus heterogeneity and biochemical features. Adv Neurol. 2004;94:127–138. [PubMed] [Google Scholar]
- Furuya H, Murai H, Takasugi K, et al. A case of late-onset Segawa syndrome (autosomal dominant dopa-responsive dystonia) with a novel mutation of the GTP-cyclohydrase I (GCH1) gene. Clin Neurol Neurosurg. 2006;108:784–786. doi: 10.1016/j.clineuro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Garavaglia B, Invernizzi F, Carbone ML, et al (2004) GTP-cyclohydrolase I gene mutations in patients with autosomal dominant and recessive GTP-CH1 deficiency: identification and functional characterization of four novel mutations. J Inherit Metab Dis 27: 455–463 [DOI] [PubMed]
- Guldberg P, Henriksen KF, Lou HC, Guttler F. Aberrant phenylalanine metabolism in phenylketonuria heterozygotes. J Inherit Metab Dis. 1998;21:365–372. doi: 10.1023/A:1005398406988. [DOI] [PubMed] [Google Scholar]
- Hagenah J, Saunders-Pullman R, Hedrich K, et al. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology. 2005;64:908–911. doi: 10.1212/01.WNL.0000152839.50258.A2. [DOI] [PubMed] [Google Scholar]
- Hahn H, Trant MR, Brownstein MJ, Harper RA, Milstien S, Butler IJ. Neurologic and psychiatric manifestations in a family with a mutation in exon 2 of the guanosine triphosphate-cyclohydrolase gene. Arch Neurol. 2001;58:749–755. doi: 10.1001/archneur.58.5.749. [DOI] [PubMed] [Google Scholar]
- Hyland K, Fryburg JS, Wilson WG, et al. Oral phenylalanine loading in Dopa-responsive dystonia: a possible diagnostic test. Neurology. 1997;48:1290–1297. doi: 10.1212/WNL.48.5.1290. [DOI] [PubMed] [Google Scholar]
- Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland K, Nygaard TG, Trugman JM, Swoboda KJ, Arnold LA, Sparagana SP. Oral phenylalanine loading profiles in symptomatic and asymptomatic gene carriers with dopa-responsive dystonia due to dominantly inherited GTP cyclohydrolase deficiency. J Inherit Metab Dis. 1999;22:213–215. doi: 10.1023/A:1005532610051. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Kanmura K, Kodama Y, Sawada K, Hunoi H, Hasegawa K. Segawa disease with a novel heterozygous mutation in exon 5 of the GCH-1 gene (E183K) Brain Dev. 2009;31(2):173–175. doi: 10.1016/j.braindev.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Klein C, Hedrich K, Mohrmann K, et al. Exon deletions in the GCHI gene in two of four Turkish families with dopa-responsive dystonia. Neurology. 2002;59:1783–1786. doi: 10.1212/01.WNL.0000035629.04791.3F. [DOI] [PubMed] [Google Scholar]
- Leuzzi V, Carducci CA, Carducci CL, et al. Phenotypic variability, neurological outcome and genetics background of 6-pyruvoyl-tetrahydropterin synthase deficiency. Clin Genet. 2010;77:249–257. doi: 10.1111/j.1399-0004.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- Leuzzi V, Carducci C, Carducci C, Cardona F, Artiola C, Antonozzi I. Autosomal dominant GTP-CH deficiency presenting as a dopa-responsive myoclonus-dystonia syndrome. Neurology. 2002;59:1241–1243. doi: 10.1212/WNL.59.8.1241. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Miller LP, Levine RA, et al. Tetrahydrobiopterin in dystonia: identification of abnormal metabolism and therapeutic trials. Neurology. 1986;36:760–764. doi: 10.1212/WNL.36.6.760. [DOI] [PubMed] [Google Scholar]
- Lopez-Laso E, Ormazabal A, Camino R, et al. Oral phenylalanine loading test for the diagnosis of dominant guanosine triphosphate cyclohydrolase I deficiency. Clin Biochem. 2006;39:893–897. doi: 10.1016/j.clinbiochem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Nagata E, Kosakai A, Tanaka K, et al. Dopa-responsive dystonia (Segawa disease)-like disease accompanied by mental retardation: a case report. Mov Disord. 2007;22:1202–1203. doi: 10.1002/mds.21517. [DOI] [PubMed] [Google Scholar]
- Ohta E, Funayama M, Ichinose H, et al. Novel mutations in the guanosine triphosphate cyclohydrolase 1 gene associated with DYT5 dystonia. Arch Neurol. 2006;63:1605–1610. doi: 10.1001/archneur.63.11.1605. [DOI] [PubMed] [Google Scholar]
- Opladen T, Okun JG, Burgard P, Blau N, Hoffmann GF. Phenylalanine loading in pediatric patients with Dopa-responsive dystonia: revised test protocol and pediatric cut off values. J Inherit Metab Dis. 2010;101:48–54. doi: 10.1007/s10545-010-9164-9. [DOI] [PubMed] [Google Scholar]
- Robinson R, McCarthy T, Bandmann O, Dobbie M, Surtees R, Wood NW. GTP cyclohydrolase deficiency; intrafamilial variation in clinical phenotype, including levodopa responsiveness. J Neurol Neurosurg Psychiatry. 1999;66:86–89. doi: 10.1136/jnnp.66.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Hyland K, Blau N, et al. Phenylalanine loading in the diagnosis of Dopa-responsive dystonia: the necessity for measuring biopterin. Ann Neurol. 2000;48:466. [Google Scholar]
- Saunders-Pullman R, Blau N, Hyland K, et al. Phenylalanine loading as a diagnostic test for DRD: interpreting the utility of the test. Mol Genet Metab. 2004;83:207–212. doi: 10.1016/j.ymgme.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Segawa M, Nomura Y, Nishiyama N. Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease) Ann Neurol. 2003;54(Suppl 6):S32–S45. doi: 10.1002/ana.10630. [DOI] [PubMed] [Google Scholar]
- Segawa M. Autosomal dominant GTP cyclohydrolase I (AD GCH 1) deficiency (Segawa disease, dystonia 5; DYT 5) Chang Gung Med J. 2009;32(1):1–11. [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e 57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trender-Gerhard I, Sweeney MG, Schwingenschuh P, et al. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry. 2009;80:839–845. doi: 10.1136/jnnp.2008.155861. [DOI] [PubMed] [Google Scholar]
- Von Mering M, Gabriel H, Opladen T, Hoffmann GF, Storch A. A novel mutation (c.64_65delGGinsAACC[p.G21fsX66]) in the GTP cyclohydrolase 1 gene that causes Segawa disease. J Neurol Neurosurg Psychiatry. 2008;79:229. doi: 10.1136/jnnp.2007.130849. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab. 2002;3:159–173. doi: 10.2174/1389200024605073. [DOI] [PubMed] [Google Scholar]
- Zirn B, Steinberger D, Troidl C, et al. Frequency of GCH1 deletions in Dopa-responsive dystonia. J Neurol Neurosurg Psychiatry. 2008;79:183–186. doi: 10.1136/jnnp.2007.128413. [DOI] [PubMed] [Google Scholar]