Abstract
Pompe disease (lysosomal acid alpha-glucosidase deficiency) in adolescents and adults presents primarily with muscle weakness. Bone weakness is an under-recognized finding in patients with Pompe disease, but there is emerging evidence that loss of muscle function and mobility can lead to loss of mineral content and a higher risk of fracture. In addition to the mineral content, architecture is also important in determining the overall strength of the bone. We present the results of the longest longitudinal duration study to date using a novel application of high-resolution peripheral quantitative computed tomography (HR-pQCT) in four patients with Pompe disease over 4 years of observation during the normal course of their disease management. The subjects varied in treatment status with recombinant human alpha-glucosidase (rhGAA), use of anti-resorptive therapy (such as bisphosphonates), mobility and weight-bearing status, and the use of side-alternating vibration therapy. Our observations were that HR-pQCT can measure trends in mineral density and architecture over a long period of observation and may be an early indicator of the response to interventional therapies. In addition, a combination of decreased loading forces due to decreased mobility likely contributes to the compromise of bone integrity in Pompe disease. These trends can be reversed by applying increased loading forces such as vibration therapy and maintaining weight-bearing and mobility. We conclude that HR-pQCT can serve as a valuable tool to monitor bone health in patients with Pompe disease.
Introduction
Pompe disease (lysosomal acid alpha-glucosidase deficiency; OMIM 606800) is a rare autosomal recessive disorder that presents with muscle weakness with a natural history of progression to respiratory failure. The overall prevalence is 1:40,000 (Kishnani et al. 2006). The severe presentation, termed “the infantile” form, is accompanied by hypertrophic cardiomyopathy and is fatal in the majority of subjects in their first year of life without treatment (van den Hout et al. 2003). Pompe disease presenting after infancy, termed “the juvenile” or “late-onset” forms, presents at any age with neuromuscular weakness progressing to immobility and need for assisted ventilation (Hagemans et al. 2005). The standard treatment for Pompe disease involves biweekly infusions of recombinant human alpha-glucosidase (Kishnani et al. 2007).
There is emerging evidence that bone mass is compromised in subjects with Pompe disease and can lead to fractures (Papadimas et al. 2011a). There is no evidence currently to suggest alpha-glucosidase deficiency directly affects bone metabolism, but rather a combination of effects such as the degenerative myopathy from Pompe disease, loss of weight-bearing ability, and deconditioning with subsequent unloading of forces on the bone leading to secondary loss of bone mass (van den Berg et al. 2010). Although dual x-ray absorptiometry (DXA) is the standard clinical method of measurement of bone mass and assessment of fracture risk in the general population, the use of DXA to assess bone strength in Pompe disease presents a number of challenges. Subjects can have scoliosis and/or orthopedic hardware which can create artifacts during scanning or subjects may spend a significant portion of their time in a wheelchair because of the proximal myopathy, but still have use of their limbs resulting in regional differences in loading forces on the bone. DXA does not measure a true volumetric bone density, but rather an areal density in g/cm2 calculated by dividing the total bone mineral content by the projected area of the X-ray image of the region of interest. This provides a reasonable estimate of fracture risk in the average-sized older adult, but underestimates true bone density in individuals with smaller bones (e.g., children, subjects with short stature) (Faulkner et al. 1995). Furthermore, bone architecture, and not just mineral density, contributes to the overall strength of bone and is not assessed by DXA. Combining the architectural analysis with densitometry can explain up to 90 % of the mechanical structure-function relationships in bone (Goulet et al. 1994). We present a novel application of a noninvasive, in vivo method of assessing bone microarchitecture using three-dimensional high-resolution peripheral quantitative computed tomography (HR-pQCT) in subjects with Pompe disease (Boutroy et al. 2005; Ruegsegger et al. 1996). Validation of HR-pQCT for densitometry and morphological measurements has been performed (MacNeil and Boyd 2007) and tests in premenopausal women, postmenopausal women, and age-matched controls have been performed (Macdonald et al. 2011a). However, to our knowledge, this technology has not been used to examine osteopenia in lysosomal storage diseases such as Pompe disease (Boutroy et al. 2005; Hasegawa et al. 2000; Cortet et al. 1999).
Methods
Subjects
HR-pQCT scans were performed on an annual basis for 4 years in all of our subjects with Pompe disease during their routine clinical care. During the period of this study, these were all of the adult subjects with Pompe disease monitored in the province of Alberta, Canada. A summary of subject characteristics is provided in Table 1.
Table 1.
Descriptive information for the four Pompe disease subjects
| Age (y) | Sex | Genotype | Mobility | Enzyme replacement therapy duration prior to first pQCT scan (months) |
|---|---|---|---|---|
| 42 | F | IVS 1-13T>G 1912G>T | Ambulatory without aids | 1 |
| 19 | M | c.525delT c.-32-13T>G | Weight-bearing but non-ambulatory | 13 |
| 17 | M | 1927G>A 2040G>A | Non-weight-bearing and non-ambulatory | 36 |
| 34 | F | c.-32-13T>G 2481 + 102_2046 + 31del |
Ambulatory without aids | None |
Subject A is a 42-year-old woman diagnosed at 37 years of age presenting with muscle weakness. She does not have cardiomyopathy, and does not need assistive ventilation. She can walk with difficulty but without walking aids. She does not have a history of fractures. She received 20 mg/kg alglucosidase-alfa (Myozyme®) every other week initiated at the start of HR-pQCT measurements. Her lumbar spine (L1-L4) t-score was 0.3 and left total hip t-score 1.4 at the beginning of this study. Plasma 25-hydroxy vitamin D levels determined on two occasions were 64.5 and 73.9 nmol/L and the subject was prescribed a supplement of 1,000 IU per day of vitamin D.
Subject B is a 19-year-old male who presented with muscular weakness at 5 years of age and was diagnosed with Pompe disease at 15 1/2 years of age. He has not had any fractures. He had received 20 mg/kg alglucosidase-alfa every other week. He can stand but he cannot walk and uses a wheelchair for his mobility. He was started on side alternating vibration therapy as he lost mobility (Vibraflex®, Galileo®, Home Edition, Novotec Medical, Pforzheim, Germany). A steel frame was constructed that allows him to pull himself out of his wheelchair with support, hold his weight using his arms and legs while standing on the vibration platform for 4 minutes at 20 Hz per day three times a week. He maintained this activity during the course of HR-pQCT measurements described below. During the last 6 months of observation, he was treated with vitamin D in addition to his enzyme therapy. He had severe scoliosis, spinal rods and therefore his lumbar spine DXA scores were difficult to interpret. His left total hip z-score ranged from –5.5 to –4.1 and his total body z-score –4.7 to –2.7 during the course of this study. These changes were commensurate with an increase in height as part of his growth during adolescence. The subject’s vitamin D was 31.2 to 98.1 nmol/L during the course of this study and he was prescribed a vitamin D dose of 2,000 IU per day.
Subject C is a 17-year-old male who presented with hypotonia and hypertrophic cardiomyopathy at 11 months of age. He had several long bone fragility fractures secondary to minor trauma (left femur, left humerus, and left radius) beginning while he was under 13 years of age. Treatment with alglucosidase-alfa 20 mg/kg i.v. every 2 weeks was initiated at 14 years of age and a few weeks later he was started on intravenous pamidronate. Pamidronate infusions were started at monthly intervals (due to overlap with his enzyme infusions) at 1 mg/kg/dose for three consecutive days per infusion cycle and were discontinued 1 year before the first HR-pQCT scan. Two years prior to his first HR-pQCT scan, his rhGAA dose was increased from 20 to 40 mg/kg every other week. The higher dose was due to his enrollment in the AGLU03306 study (An Exploratory, Open-Label Study of the Safety and Efficacy of High Dose or High Dosing Frequency Myozyme® (alglucosidase alfa) Treatment in Patients with Pompe Disease Who Do Not Have an Optimal Response to the Standard Dose Regimen). The family elected to continue at this does once the study was completed. He had severe scoliosis and his DXA spinal scores were difficult to interpret. His right total hip z-score was –4.8 to –4.2 over the course of this study. The subject’s vitamin D level ranged from 46.4 to 99.1 nmol/L during the course of this study and the subject was prescribed a dose of 2,000 IU per day of vitamin D. The lower plasma levels of vitamin D reflect periods of decreased compliance at various times. Twenty-four months prior to the first HR-pQCT scan, the subject was started on intravenous pamidronate and continued until 20 months after the first HR-pQCT scan (18 years of age). He was initially started on an “Osteogenesis Protocol” of 0.5 mg/kg on day 1 and then 1 mg/kg on days 2 and 3 followed by 1 mg/kg every month for the remainder of the duration of infusions because of difficulties in traveling for both the enzyme and bisphosphonate infusions.
Subject D is a 34-year-old female who was diagnosed at 30 years of age. She has not had any fractures. She can ambulate but does have proximal muscle weakness, slow walking speed, and difficulty getting from a sitting to standing position without support. She was treated with 20 mg/kg alglucosidase-alfa every other week and alendronate sodium 70 mg plus 5,600 IU vitamin D3 (Fosavance®) during the course of these observations. She started using a vibration platform (Vibraflex®, Galileo®, Home Edition, Novotec Medical, Pforzheim, Germany) after her first scan which added loaded forces to her lower limbs but not her upper limbs since she was able to balance on the platform without the use of her arms (Khan et al. 2009). Her lumbar spine (L1-L4) t-score ranged from 0.2 to 0.4 and her left total hip t-score ranged from –2.4 to –2.2 over the course of this study. The subject’s vitamin D levels ranged from 78.2 to 83.9 nmol/L during the course of the study while taking an additional supplement of 1,000 IU per day (the Fosavance also contained vitamin D).
Measurements
HR-pQCT measurements. Scans were performed by HR-pQCT (XtremeCT, Scanco Medical, Brüttisellen, Switzerland) at two standard skeletal sites including the distal radius and the distal tibia (Boutroy et al. 2005). The subjects’ arm or leg was supported in the scanner by a carbon cast to reduce subject motion. An initial x-ray (“scout view”) was taken providing a basis to precisely manually identify the site for 3D scanning. The total time for each 3D scan was less than 3 min and resulted in 110 slices representing an axial section 9.02 mm long with a nominal isotropic resolution of 82 mm (60 kVp, 1000 μA, 100 ms integration time). The effective patient dose from a single scan is less than 3 μSv.
For each scan, a region of analysis (ROI) was defined at the periosteal surface for each slice using a semiautomated software routine, and subsequently the data was submitted for automated densitometry and morphometry evaluation (IPL v4.3, Scanco Medical). The main results from the analysis reported in equivalent mg/cm3 of hydroxyapetite for the entire scanned region include total density in a scanned region (D100, mg/cm3), cortical density (Dcort, mg/cm3), and trabecular density (Dtrab, mg/cm3). Additionally, morphometric parameters included the trabecular number (Tb. N, mm-1) and cortical thickness (Ct.Th, mm). The trabecular number is determined based on a direct analysis of the 3D data and then trabecular thickness and separation are derived (MacNeil and Boyd 2007). All subjects were scanned every 6 months during the actual course of their treatment.
Each scan was assessed according to manufacturer guidelines for quality on a scale of 0–4 for motion artifacts and verified by a second, independent assessment (0 = no motion artifact, 1 = slight whisping but no cortical discontinuity, 2 = whisping but no cortical discontinuities, 3 = high whisping and minor cortical discontinuities, and 4 = high whisping and major cortical discontinuities), and the entire scan discarded if any one of the images scored 3 or higher (Fig. 1). Individuals performing and reading the scans were blinded to the treatment details of the subjects.
Fig. 1.
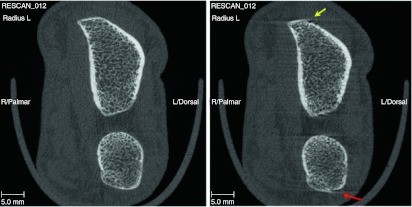
Assessment of scan quality. The scan on the left shows no motion artifacts. The scan on the right shows radial whisping (yellow arrow) and whisping with cortical irregularities of the ulna (right arrow)
Results
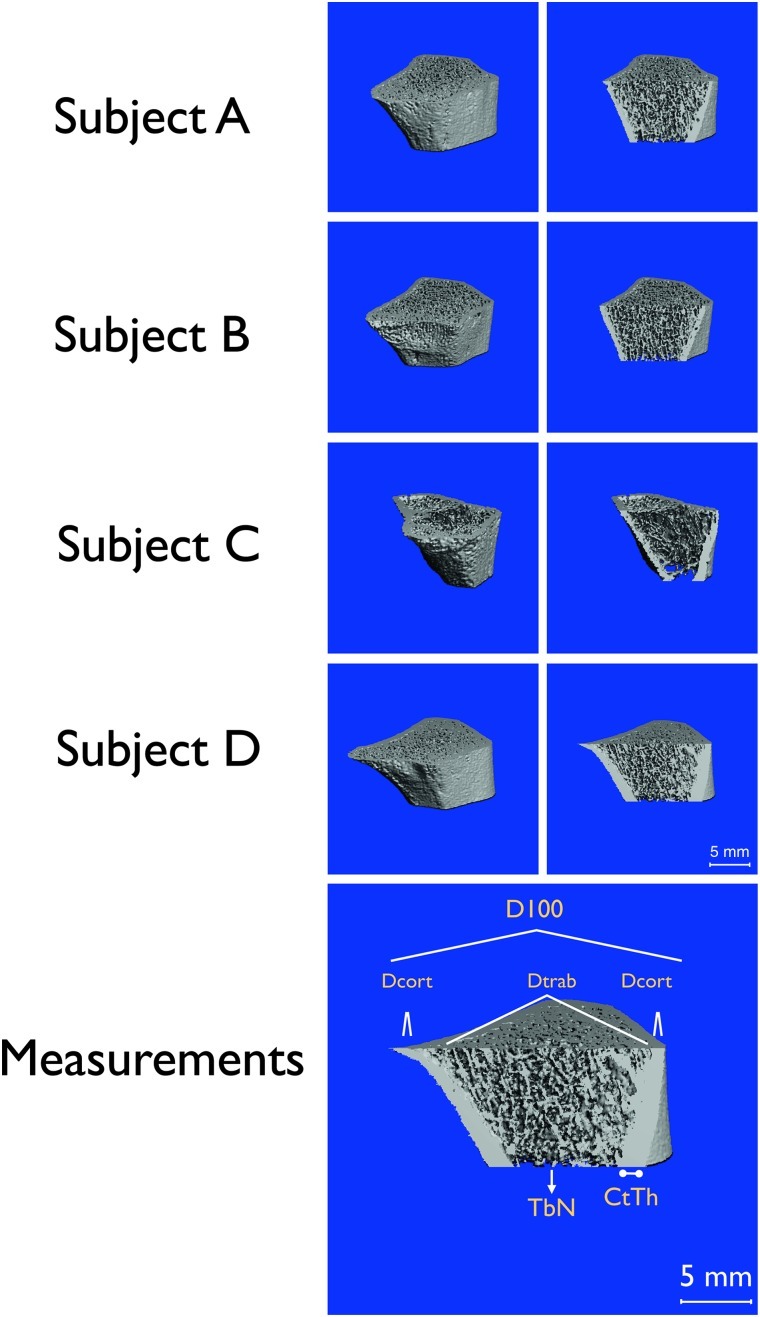
Figure 2 shows representative images of the left distal radius using HR-pQCT. Qualitative inspection of the scans shows how the underlying cortical thickness and trabecular number are reduced in subjects that are non-ambulatory, subjects B and C, compared to the subjects that were ambulatory, subjects A and D.
Fig. 2.
Top. Three-dimensional HR-pQCT imaging of left radius representing a 9.02 mm axial region located 9.5 mm from the distal end of the radius. Left column shows the entire scan and the right column shows the interior microarchitecture from the same scan data. Bottom. Measurements. Graphical representation of average density at the radius (D100), density of compact bone (DComp) and trabecular bone (Dtrab), and bone architecture represented by cortical thickness (CtTh) and trabecular number (TbN)
Analysis of the trends in mineral density and morphologic bone architecture are summarized in Fig. 3. For each subject, a 4-year interval is presented while they were monitored using HR-pQCT. The ordinate uses the same scale for each subject for the representative measurement. These trends were then analyzed with respect to time on standard therapy and differences in loading versus unloading of the limbs as follows:
-
Changes in mineral density in unloaded limbs
In subjects A, C, and D, the upper limbs were not exercised (hence no additional loading forces) and therefore these limbs show the natural course of bone density in patients being treated with rhGAA and vitamin D. Figure 3 shows the change in D100 over time at the radius. Subjects A and D, both ambulatory women on standard therapy, show continuous reductions in D100 over time. In comparison, subject C, treated with bisphosphonates prior to HR-pQCT scanning, showed an attenuated decline in D100. All three of these cases were treated with enzyme replacement therapy, but without any additional loading of the upper limbs. These three cases suggest that drug therapy alone was not sufficient to reverse the loss of bone mineral density in a non-weight-bearing limb.
-
Changes in Mineral Density with Loading of the Limbs
We analyzed compartmental changes in mineral density. Subject B had an external loading force applied using a vibration platform during which he supported his weight with both his hands and legs using a metal frame. Increases in D100 appear to be the result mostly of increases in cortical bone density (Dcomp) and, to a lesser degree, trabecular density (Dtrab), showing a 22.4 % improvement at the left radius in D100 and 19.5 % in Dcomp at the left tibia with comparable changes on the right side as well (26.3 % and 12.3 %, respectively).
-
Changes in Mineral Density and Architecture
Subject B shows that in addition to mineral density, cortical thickness and trabecular number both show increases when loading forces are added.
-
Changes in Mineral Density in Loaded Versus Unloaded Limbs
The two ambulatory subjects are A and D. Both subjects were on alglucosidase-alfa and vitamin D but subject D was started on a vibration platform, standing only, without any extra force applied on her arms. Therefore, subject A did not use any additional loading force and subject D applied a loading force on the lower extremity but not on the upper extremity. Both subjects A and D had normal peripheral limb bone density and the use of vibration on the lower extremity in subject D showed no improvement when compared to subject A. However, in subject B, who is weight-bearing but not ambulatory and had decreased peripheral limb bone density, there was a sustained improvement in mineral density and architecture using vibration in addition to enzyme replacement and vitamin D.
Fig. 3.
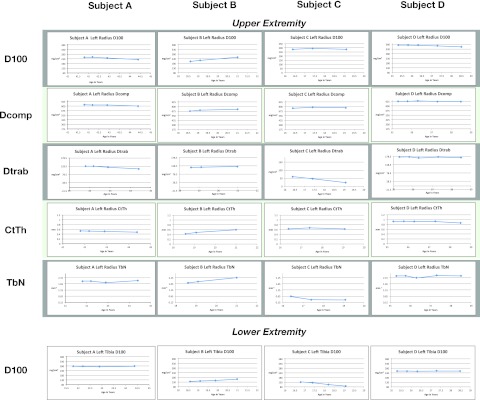
Morphological trends in measurements of the left radius using HR-pQCT. Average density at the radius (D100), density of compact bone (DComp) and trabecular bone (Dtrab), and bone architecture represented by cortical thickness (CtTh) and trabecular number (TbN). Measurements were obtained for each limb distally but only data from the left radius is represented here due to space limitations
Discussion
These results are observations on a convenient sample of subjects under routine clinical care and treatment of their Pompe disease. There are no studies published using HR-pQCT to study bone architecture in subjects with Pompe disease and few longitudinal studies using HR-pQCT from osteopenia due to other causes (Burghardt et al. 2010; Rizzoli et al. 2010; Macdonald et al. 2011b). This study is the longest duration longitudinal study to date by HR-pQCT. This study also includes the first observations after using side alternating vibration therapy on bone architecture using HR-pQCT in two subjects (subjects B and D). The objective of the study was to pilot the use of HR-pQCT in the study of bone density and architecture in subjects with Pompe disease as an adjunct to other measures, such as plain roentgenography and DXA, in assessing their bone quality. HR-pQCT is an emerging technique available only in a small number of academic centers (currently four in Canada: Vancouver, Calgary, Saskatoon, and Toronto). Validation of the use of HR-pQCT on a larger set of subjects with Pompe disease would be valuable. Each center in Canada typically looks after only a few subjects with Pompe disease, therefore, collaboration with larger groups of subjects would provide a broader assessment of bone health in subjects with Pompe disease.
However, despite these limitations, even in this small set of subjects, we note some interesting observations after following them for 4 years: first, whether through a combination of reduced muscle mass, or decreased weight-bearing and mobility, the result of decreasing loading forces leads to continued declines in bone density and architecture in the peripheral limbs of subjects with Pompe disease. This trend did not appear to be reversed with a combination of enzyme replacement therapy, vitamin D and even oral bisphosphonates in some cases. Therefore, despite the benefits of ERT on respiratory function and mobility reported in clinical trials (Vielhaber et al. 2011; Papadimas et al. 2011b; van Capelle et al. 2010; Orlikowski et al. 2011), the treatment of subjects with Pompe disease should include load-bearing activities and physical activity in their management plan. Second, HR-pQCT can measure trends in mineral density and architecture that may be useful to indicate the direction of these changes before a clinical endpoint, such as a fracture is noted. Third, the quality of the images on HR-pQCT should be assessed and trends should be evaluated over a long period of observation. We feel the overall message that needs to be stressed is to keep subjects with Pompe disease as mobile as possible in addition to their standard medical therapy. This is the first application of HR-pQCT to evaluate bone density and structure in subjects with Pompe disease and we feel there may be potential for clinical application of this technique in the future to monitor bone health.
The methods we used in obtaining HR-pQCT scans have been shown to have a precision of 0.7–1.5 % for total, trabecular, and cortical densities and 2.5–4.4 % for trabecular architecture (Boutroy et al. 2005; MacNeil and Boyd 2007). Overall, the changes we observed in our subjects with Pompe disease over the course of 4 years are greater than the error range of HR-pQCT, and the changes in response to applying a loading force to the limbs exceeds that seen in shorter-term studies using alendronate in postmenopausal women (Burghardt et al. 2010). Nevertheless, there can be measurements in individual architectural parameters despite a good quality scan that should be interpreted with caution and verified by longitudinal follow-up. Hence, we feel the results from HR-pQCT should be evaluated using more than just two measurements.
This study was intended to be an observational study to pilot the use of HR-pQCT in subjects with Pompe disease. The treatments are varied since each subject was managed based on their individual clinical needs and therefore it is not intended to directly evaluate the effect of any particular treatment on bone architecture. All of our subjects had scoliosis and/or metallic prostheses that made the DXA scans difficult to interpret – in fact, this reason prompted us to use HR-pQCT to assess their bone. This study was not intended to directly compare DXA versus HR-pQCT in the assessment of bone health in patients with Pompe disease.
An HR-pQCT scan of the distal forearm gives an exposure of 3 μSv compared to a whole body DXA at 4.6 μSv and a background exposure of 7 μSv per day at sea level (Griffith and Genant 2008). Peak bone mass, the amount of bone at the end of skeletal maturation determined by DXA, is a major determinant of fracture risk in subjects who develop osteoporosis (Marshall et al. 1996). For each standard deviation reduction in the DXA T-score, there is an approximate doubling of the fracture risk (Cefalu 2004). However, bone mechanical strength is not dependent on mineral content alone. High-resolution peripheral quantitative computed tomography provides true volumetric density of both cancellous and cortical bone. In some subjects, cancellous bone is more responsive to interventions than cortical bone which appears to be the case in this study with trabecular indices showing more fluctuation than cortical measurements. Furthermore, in subjects with Pompe disease, angular deformities of the axial skeleton, disuse atrophy of the muscle and bone, and loss of ambulation and their effects on mineral density and architecture are not clear. We have demonstrated that HR-pQCT has potential as a noninvasive, in vivo method of evaluating changes in bone in subjects with Pompe disease.
Acknowledgments
Images in Fig. 1 are courtesy of Yves Pauchard, University of Calgary. We would like to thank Ion Robu for his engineering and technical expertise in the vibration training setup. This research was supported by funding from the Alberta Children’s Hospital Research Foundation, Alberta Health Innovates Health Solutions (formerly Alberta Heritage Foundation for Medical Research), and through the support of Alberta Health Services.
Synopsis
High-resolution peripheral computed tomography can be used to monitor peripheral limb bone health in patients with Pompe disease.
Footnotes
Competing interests: None declared
References
- Boutroy S, Bouxsein ML, Munoz F, Delmas PD (Dec 2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab [Comparative Study Evaluation Studies Research Support, Non-U.S. Gov't]90(12):6508–6515 [DOI] [PubMed]
- Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S (Dec 2010) A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: Relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res [Randomized Controlled Trial Research Support, N.I.H., Extramural]25(12):2558–2571 [DOI] [PMC free article] [PubMed]
- Cefalu CA (March 2004) Is bone mineral density predictive of fracture risk reduction? Curr Med Res Opin [Review]20(3):341–349 [DOI] [PubMed]
- Cortet B, Dubois P, Boutry N, Bourel P, Cotten A, Marchandise X. Image analysis of the distal radius trabecular network using computed tomography. Osteoporos Int. 1999;9(5):410–419. doi: 10.1007/s001980050165. [DOI] [PubMed] [Google Scholar]
- Faulkner RA, McCulloch RG, Fyke SL, De Coteau WE, McKay HA, Bailey DA et al (1995) Comparison of areal and estimated volumetric bone mineral density values between older men and women. Osteoporos Int [Comparative Study Research Support, Non-U.S. Gov't]5(4):271–275 [DOI] [PubMed]
- Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA (Apr 1994) The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech [Research Support, U.S. Gov't, P.H.S.]27(4):375–389 [DOI] [PubMed]
- Griffith JF, Genant HK (Oct 2008) Bone mass and architecture determination: state of the art. Best Pract Res Clin Endocrinol Metab [Review]22(5):737–764 [DOI] [PubMed]
- Hagemans ML, Winkel LP, Van Doorn PA, Hop WJ, Loonen MC, Reuser AJ et al (March 2005) Clinical manifestation and natural course of late-onset Pompe's disease in 54 Dutch patients. Brain [Research Support, Non-U.S. Gov't]128(Pt 3):671–677 [DOI] [PubMed]
- Hasegawa Y, Schneider P, Reiners C, Kushida K, Yamazaki K, Hasegawa K et al (2000) Estimation of the architectural properties of cortical bone using peripheral quantitative computed tomography. Osteoporos Int [Research Support, Non-U.S. Gov't]11(1):36–42 [DOI] [PubMed]
- Khan A, Ramage B, Robu I, Benard L. Side-alternating vibration training improves muscle performance in a patient with late-onset pompe disease. Case Report Med. 2009;2009:741087. doi: 10.1155/2009/741087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D (May 2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr [Multicenter Study Research Support, Non-U.S. Gov't]148(5):671–676 [DOI] [PubMed]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL et al (Jan 2007) Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology [Multicenter Study Randomized Controlled Trial]68(2):99–109 [DOI] [PubMed]
- Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK (Jan 2011) Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res [Research Support, Non-U.S. Gov't]26(1):50–62 [DOI] [PubMed]
- Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK (Jan 2011) Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int [Clinical Trial Research Support, Non-U.S. Gov't]22(1):357–362 [DOI] [PubMed]
- MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Marshall D, Johnell O, Wedel H (May 1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ [Meta-Analysis Research Support, Non-U.S. Gov't]312(7041):1254–1259 [DOI] [PMC free article] [PubMed]
- Orlikowski D, Pellegrini N, Prigent H, Laforet P, Carlier R, Carlier P, et al. Recombinant human acid alpha-glucosidase (rhGAA) in adult patients with severe respiratory failure due to Pompe disease. Neuromuscul Disord. 2011;21(7):477–482. doi: 10.1016/j.nmd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Papadimas GK, Terzis G, Spengos K, Methenitis S, Papadopoulos C, Vassilopoulou S et al (Feb 2011) Bone mineral density in adult patients with Pompe disease. Bone [Comment Letter]48(2):417; author reply 8–9 [DOI] [PubMed]
- Papadimas GK, Spengos K, Konstantinopoulou A, Vassilopoulou S, Vontzalidis A, Papadopoulos C, et al. Adult Pompe disease: clinical manifestations and outcome of the first Greek patients receiving enzyme replacement therapy. Clin Neurol Neurosurg. 2011;113(4):303–307. doi: 10.1016/j.clineuro.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Laroche M, Krieg MA, Frieling I, Thomas T, Delmas P et al (Aug 2010) Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int [Randomized Controlled Trial Research Support, Non-U.S. Gov't]30(10):1341–1348 [DOI] [PMC free article] [PubMed]
- Ruegsegger P, Koller B, Muller R (Jan 1996) A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int [Research Support, Non-U.S. Gov't]58(1):24–29 [DOI] [PubMed]
- van Capelle CI, van der Beek NA, Hagemans ML, Arts WF, Hop WC, Lee P et al (Dec 2010) Effect of enzyme therapy in juvenile patients with Pompe disease: a three-year open-label study. Neuromuscul Disord [Clinical Trial, Phase II Research Support, Non-U.S. Gov't]20(12):775–782 [DOI] [PubMed]
- van den Berg LE, Zandbergen AA, van Capelle CI, de Vries JM, Hop WC, van den Hout JM et al (Sept 2010) Low bone mass in Pompe disease: muscular strength as a predictor of bone mineral density. Bone [Research Support, Non-U.S. Gov't]47(3):643–649 [DOI] [PubMed]
- van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT et al (Aug 2003) The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics [Research Support, Non-U.S. Gov't Review]112(2):332–340 [DOI] [PubMed]
- Vielhaber S, Brejova A, Debska-Vielhaber G, Kaufmann J, Feistner H, Schoenfeld MA, et al. 24-months results in two adults with Pompe disease on enzyme replacement therapy. Clin Neurol Neurosurg. 2011;113(5):350–357. doi: 10.1016/j.clineuro.2010.09.016. [DOI] [PubMed] [Google Scholar]