Abstract
Fabry disease (FD) is caused by progressive accumulation of neutral glycosphingolipids, including in ganglion neural and vascular endothelial cells, as a result of lysosomal α-galactosidase deficiency. High frequencies progressive sensorineural hearing loss (HL), sudden deafness, tinnitus and dizziness are otological symptoms frequently reported.
A 45-year-old man with FD, on haemodialysis since age 25, complaining of progressive HL, was started on enzyme replacement therapy (ERT) because of cardiac complications. A bilateral sloping sensorineural HL was found at baseline audiological evaluation. Computed tomography of the ears showed enlargement of the intradiploic vascular channels, principally in the petrous bone. The magnetic resonance angiography showed elongation and ectasia of the middle cerebral arteries and the arteries of the Circle of Willis, particularly the internal carotid and the basilar arteries. Follow-up audiological evaluations documented progressive worsening of HL, mainly in the high frequencies range, despite high dose ERT and evidence of cardiac improvement.
The intradiploic vascular abnormalities of the temporal bones reported herein have never been described in association with FD and may have contributed to the pathogenesis of progressive HL, by a ‘stealing’ effect upon the cochlear blood supply (like in cavernous haemangioma of the internal auditory meatus), in addition to the other mechanisms of ischaemic injury to the Organ of Corti described in FD. This clinical observation shows the value of comprehensive neuroimaging investigation of HL in FD and emphasizes the importance of early institution of specific therapy, before the occurrence of irreversible inner ear lesions and hearing damage.
Introduction
Fabry disease (FD) is a rare X-linked metabolic disorder due to accumulation of globotriaosylceramide (GL-3) and other neutral glycosphingolipids (GSL) in many organs and tissues, as a result of deficient activity of the lysosomal enzyme α-galactosidase (Desnick et al. 2001, 2003). Involvement of the microvascular endothelium with progressive luminal narrowing and occlusion leading to tissue ischaemia is regarded as a major pathogenic mechanism of FD (Desnick et al. 2001, 2003).
Life-threatening complications of FD in males include chronic kidney disease (CKD), hypertrophic cardiomyopathy and cerebrovascular disease (Desnick et al. 2001, 2003). Although the age of onset of clinical symptoms and the disease severity may vary widely, CKD invariably progresses to end-stage renal disease (ESRD) in men with the classical phenotype of FD. Heterozygous females show variable organ involvement (Wilcox et al. 2008) but seldom are as severely affected as males (Desnick et al. 2001, 2003).
Otological symptoms of FD include progressive sensorineural hearing loss (HL), sudden deafness, tinnitus, dizziness and vertigo (Germain et al. 2002; Conti and Sergi 2003; Hajioff et al. 2003a, b; Hegemann et al. 2006; Ries et al. 2007; Palla et al. 2007; Keilmann et al. 2009; Sergi et al. 2010). The prevalence of HL and tinnitus associated with FD increases with age, males being usually affected earlier and more severely than females. On pure tone audiometry (PTA), up to 88% of adult males with FD may show evidence of progressive HL (Palla et al. 2007). The HL is more frequent and severe at the high-tone frequencies (Hajioff et al. 2003a, b; Palla et al. 2007). High-frequency HL may occur as an isolated PTA finding in children reporting subjective hearing impairment (Keilmann et al. 2009), as well as in young adults with clinically normal audition (Germain et al. 2002). Residual α-galactosidase activity lowers the risk of HL by about twofold in comparison with patients who have no detectable enzyme activity (Ries et al. 2007). A decreased number of spiral ganglia, atrophy of the stria vascularis and of the spiral ligament in all cochlear turns, loss of hair-cells mainly of the basal turns and GSL accumulation in vascular endothelial and ganglion cells of the ear were the histopathological features described in the temporal bones of two middle-aged FD patients with sensorineural HL (Schachern et al. 1989). These findings can explain most of the otological symptoms associated with FD.
Two genetically engineered human α-galactosidase preparations (agalsidase alfa and agalsidase beta) are available for the treatment of FD by enzyme replacement therapy (ERT) (Eng et al. 2001; Schiffmann et al. 2001). Despite minor glycosylation differences (Lee et al. 2003), the two agalsidases showed similar biodistribution in the mouse model of FD (Lee et al. 2003) and apparently have identical immunogenicity in humans (Linthorst et al. 2004; Vedder et al. 2007). Overall results of ERT for up to 60 months suggest that patients with mild to moderate degrees of HL may slightly improve with ERT, whereas hearing does not change significantly in patients with normal hearing or in those with severe HL, as evaluated by PTA at baseline (Hajioff et al. 2003a, b; Hegemann et al. 2006; Palla et al. 2007).
Herein, we report the significant worsening of the hearing thresholds of a classically affected male with FD while on ERT with agalsidase beta for more than 3 years and the radiological finding of vascular abnormalities in the temporal bones that have never been described in association with FD and may have contributed to the HL in this patient.
Case Report
A 46-year-old male with ESRD and classic FD, hemizygous for the α-galactosidase missense mutation p.C94S, with no detectable residual enzyme activity on the leukocyte and plasma assays, was referred for comprehensive otolaryngological assessment before the beginning of ERT with agalsidase beta. Although the patient complained of bilateral tinnitus and slow progressive impairment in word discrimination for several years, he had refused proper otological assessment 2 months before, when he reported sudden subjective deterioration of hearing. He was dialysis dependent since the age of 25 years. Persistent sinus bradycardia with haemodynamic intolerance to ultrafiltration during the dialysis sessions had been noted for several months. Despite his blood pressure being well controlled with no need for anti-hypertensive medication, the echocardiography showed a mildly dilated left atrium (diameter = 44 mm) and mild left ventricular hypertrophy, with a maximum left ventricular wall thickness of 12 mm at the interventricular septum. There was no past history of transient ischaemic attacks (TIA) or stroke events.
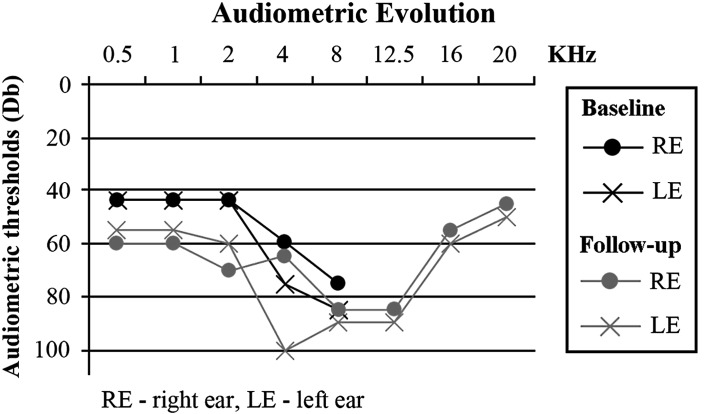
A detailed otolaryngological medical history was obtained at baseline, along with a thorough physical and instrumental examination of the ears, nose and throat (ENT). The patient reported no symptoms of ear fullness, dizziness or instability, and denied previous history of cranial trauma, otological surgery, noise exposure or use of ototoxic drugs. There was no family history of deafness. The only abnormality found on the ENT physical examination was a bilateral inferior turbinates hypertrophy. The baseline audiogram showed bilateral sloping sensorineural HL, most severe at the 4–8 kHz range (Fig. 1). Distortion product and transient-evoked otoacoustic emissions were absent. The tympanogram was normal bilaterally, with absent acoustic reflexes. The patient refused hearing aid.
Fig. 1.
The baseline audiogram showed bilateral sloping sensorineural HL, with a pure tone threshold average for 0.5 kHz, 1 kHz and 2 kHz of 43.3 dB bilaterally and pure tone thresholds of 60 and 75 dB at 4 kHz and of 75 and 85 dB at 8 kHz, respectively on the right and the left ear. In the follow-up audiogram, 40 months later, pure tone threshold averages for 0.5, 1 and 2 kHz were 65 dB in the right ear and 56.7 dB in the left ear; thresholds at 4 and 8 kHz were both 85 dB in the right ear and 100 and 90 dB, respectively, in the left ear. Audiometry of higher frequencies (8 to 20 kHz) showed a HL of 85 dB at the 8 to 12.5 kHz tone range in the right ear and of 90 dB in the left ear, with slightly better results at the 16 and 20 kHz range, bilaterally
Treatment with agalsidase beta was started with 35 mg (~0.8 mg/kg) infused every other week during a haemodialysis session (Kosch et al. 2004). Eleven months later, the unit dose was eventually doubled to 70 mg (~1.6 mg/kg), for lack of cardiac improvement and reversion of plasma GL-3 to pre-ERT levels. The increase in plasma GL-3 levels was coincidental with the first detection of anti-agalsidase IgG antibodies. The evolution of the plasma GL-3 levels, the anti-agalsidase beta antibody titre and the most relevant echocardiographic parameters, up to 30 months after the institution of ERT, are shown in Table 1. During this period, the patient did not miss any of the scheduled agalsidase beta infusions.
Table 1.
Evolution of plasma GL-3 levels, anti-agalsidase beta antibodies titer and echocardiographic parameters while on ERT with agalsidase beta
| Time on enzyme replacement therapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 2 months | 3 months | 6 months | 9 months | 12 months | 24 months | 30 months | |
| Plasma GL-3a | 6.6 | 6.3 | 4.3 | 4.4 | 6.4 | – | 6.5 | 5.7 | – |
| IgG antibodiesb | – | – | – | – | (+) | – | 1/400 | 1/200 | – |
| Echocardiographic parameters | |||||||||
| LVIDD | 48 | – | – | – | – | 50 | – | 54 | 58 |
| PWTD | 10 | – | – | – | – | 11 | – | 11 | 10 |
| IVSTD | 12 | – | – | – | – | 13 | – | 8 | 7 |
| LVMI | 69 | – | – | – | – | 83 | – | 68 | 67 |
Blood samples for GL-3 assay in plasma and for anti-agalsidase antibodies detection in serum were always collected at the start of the corresponding hemodialysis session
aGL-3 plasma levels, normal range: 1.6–3.3 μmol/l (Laboratory Medicine / Clinical Chemistry, Sahlgren University Hospital, Molndal, Sweden)
bAnti-agalsidase beta IgG antibodies (Genzyme Corporation, Cambridge, MA 02142, USA)
Echocardiographic dimensions (mm): LVIDD Left Ventricular Internal Diameter in Diastole, PWTD Posterior Wall Thickness in Diastole, IVSTD Interventricular Septum Thickness in Diastole, LVMI Left Ventricular Mass Index, expressed as g/m^2.7
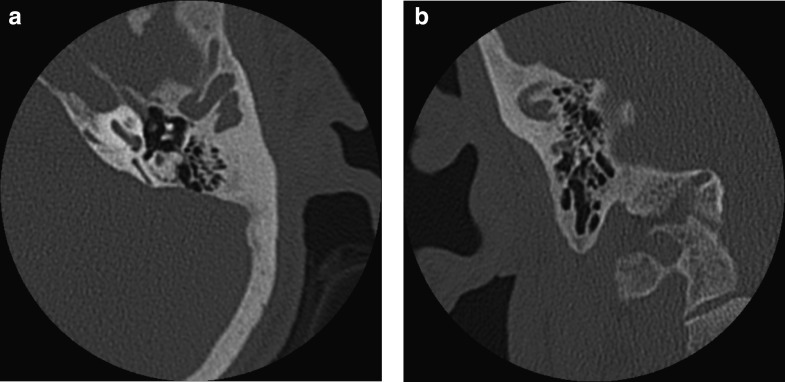
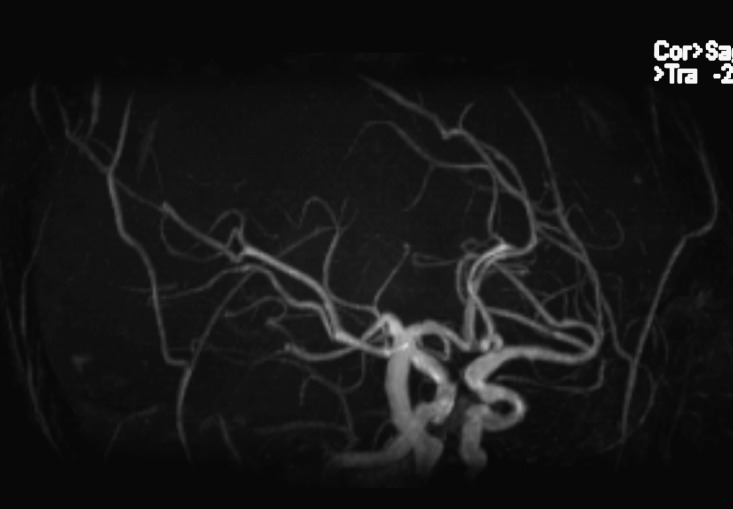
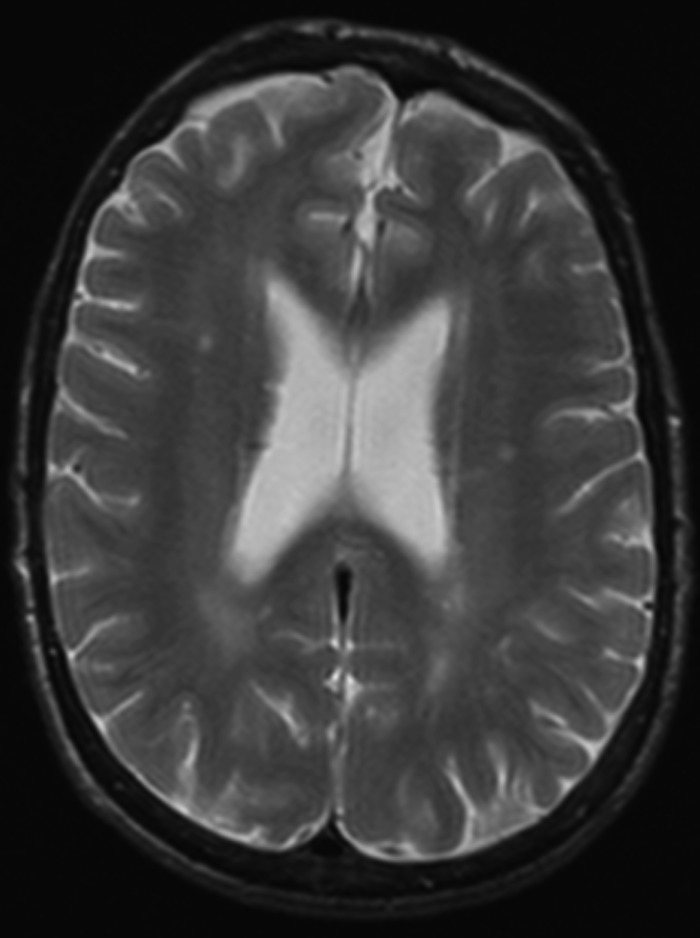
A follow-up audiogram, performed 42 months after the start of ERT, including testing for the 8–20 kHz pure tone frequency range, documented a significant worsening of the hearing impairment (Fig. 1). In addition, comprehensive imaging data of the brain, ears, temporal bones and adjacent skull structures were obtained by computerized tomography (CT) and magnetic resonance (MR) with MR angiography (MRA). Enlargement of intradiploic vascular channels of the temporal bones, particularly in the mastoids, petrous pyramids and adjacent to the temporomandibular joints, was noted on the CT scan of the base of the skull (Fig. 2a, b). Similar lesions were present in the frontal and sphenoid bones. The middle and inner ear on both sides had no abnormalities. There were bilateral atheromatous calcifications of the vertebral, basilar (BA) and internal carotid arteries (ICAs). The MRA (Fig. 3) showed elongation and ectasia of the middle cerebral arteries and the arteries of the Circle of Willis, particularly the ICAs and the BA. The ICAs displayed luminal irregularities. On T2-weighted brain MR imaging scan (Fig. 4) there were multiple focal areas of high signal intensity in the periventricular white matter and corona radiata bilaterally as well as in the right thalamus. At this time, the patient accepted hearing aid.
Fig. 2.
(a, b) The CT scan of the base of the skull showed enlargement of intradiploic vascular channels distributed bilaterally in the temporal bones, particularly in the mastoids, petrous pyramids, and adjacent to the temporomandibular joints
Fig. 3.
The MRA showed elongation and ectasia of the middle cerebral arteries and the arteries of the Circle of Willis, particularly the internal carotid arteries and the basilar artery. The internal carotid arteries displayed luminal irregularities
Fig. 4.
The T2-weighted brain MR imaging scan there were multiple focal areas of high signal intensity in the periventricular white matter and corona radiata bilaterally as well as in the right thalamus
Discussion
Progressive sensorineural HL and sudden deafness, occurring unilaterally or bilaterally, are frequently observed in men with the classic form of FD (Germain et al. 2002; Conti and Sergi 2003; Hajioff et al. 2003a, b; Hegemann et al. 2006; Ries et al. 2007; Palla et al. 2007; Keilmann et al. 2009; Sergi et al. 2010). Although the otological symptoms and the HL are not life-threatening complications of FD, they may have a profound impact on the patients’ quality of life (Mehta 2003). The lack of histopathological evidence of GSL accumulation in the spiral ganglia (Schachern et al. 1989) and the frequent occurrence of sudden deafness are suggestive that the HL in FD is more probably due to ischaemic injury to the organ of Corti rather than to involvement of the neural hearing pathways.
The HL in FD patients correlates with the decline of renal function, as well as with clinical or brain imaging evidence of cerebrovascular disease (Germain et al. 2002). In our patient, the explanation for the HL might be confounded by the long-term haemodialysis treatment, as sensorineural HL, especially in the high frequencies range, is quite prevalent among patients on renal replacement therapies (Quick 1976; Kligerman et al. 1981; Stavroulaki et al. 2001). However, the HL associated with advanced CKD is not clinically relevant in most cases and does not appear to deteriorate with time in long-term haemodialysis patients (Bazzi et al. 1995). Furthermore, a careful review of the patient’s medical records excluded past exposure to the ototoxic drugs that commonly contribute to the risk of HL in the dialysis setting (Quick 1976).
Large-vessel ectasia and large-vessel and small-vessel occlusive disease, predominantly affecting the posterior circulation, are typical brain imaging and angiographic findings in FD (Crutchfield et al. 1998; Fellgiebel et al. 2006), even in patients with no previous history of TIA or stroke. Dolichoectasia of cerebral arteries is a recognized risk factor for ischaemic stroke and small-vessel disease in the general population (Ince et al. 1998; Pico et al. 2005). Blood flow within the dolichoectatic arteries is turbulent and has a retrograde stream component that may compromise the irrigation of distal tissues. Therefore, as the main arterial blood supply of the human cochlea is through the internal auditory artery, which is a branch from the BA, the organ of Corti stands in a high-risk vascular region for ischaemic injury in FD. Our patient had no clinical history of cerebrovascular events but his brain CT, MR and MRA findings were characteristic of those observed in middle-aged classically affected men with FD. Kidney dysfunction is independently related to asymptomatic increase in ICA and BA diameters as evaluated by brain MR imaging in elderly pre-dialysis CKD patients (Ichikawa et al. 2009), but this is of uncertain clinical significance in ESRD patients. Furthermore, we are not aware of any studies showing a clinically significant risk of intracranial dilative arteriopathy in ESRD (with the exception of cerebral aneurysms in patients with Autosomal Dominant Polycystic Kidney Disease), or of case reports of intradiploic dilative arteriopathy in long-term haemodialysis patients.
To the best of our knowledge, enlargement of intradiploic vessels has never been reported in FD patients. The vascular lacunae affecting the temporal, frontal and sphenoid bones observed in our patient resemble those described in intraosseous haemangiomas of the cranium (Bastug et al. 1995). Although it cannot be conclusively demonstrated that the vascular abnormalities identified in our patient are a complication of FD, their multifocality and association with dolichoectasia of the ICAs and BA, and the rarity of cranial intraosseous haemangiomas, suggest that they might be an expression of the dilative arteriopathy of FD. Angiomas of the petrous bone are a cause of rapidly progressive, severe sensorineural HL (Dufour et al. 1994). Stealing of cochlear blood supply by the angiomatous lesion has been suggested as a possible mechanism for ischaemic damage to the organ of Corti in HL associated with a cavernous haemangioma of the internal auditory meatus (Madden and Sirimina 1990). By analogy, we postulate that the vascular malformations of the temporal bones might have contributed to the hearing impairment in our patient. It is also possible that the dilative arteriopathy of the temporal bones is more frequent than so far recognized in FD patients presenting with severe HL, because its diagnostic assessment is not usually done as thoroughly as we did in our case. Furthermore, as the angiectatic bone lesions were observed more than 2 years after treatment with high dose agalsidase beta, which proved effective in reducing the left ventricular mass, most probably they do not regress with ERT.
Footnotes
Competing interests: None declared
Contributor Information
Carla Pinto Moura, Email: cmoura@med.up.pt.
Carlos Soares, Email: csoares@portugalmail.com.
Daniela Seixas, Email: dseixas@med.up.pt.
Margarida Ayres-Bastos, Email: mail.mab@clix.pt.
João Paulo Oliveira, Email: jpo@med.up.pt.
References
- Bastug D, Ortiz O, Schoclet S. Hemangiomas in the calvaria: imaging findings. AJR Am J Roentgenol. 1995;164:683–687. doi: 10.2214/ajr.164.3.7863894. [DOI] [PubMed] [Google Scholar]
- Bazzi C, Venturini CT, Pagani C, Arrigo G, D'Amico G. Hearing loss in short-and long-term haemodialysed patients. Nephrol Dial Transplant. 1995;10:1865–1868. [PubMed] [Google Scholar]
- Conti G, Sergi B. Auditory and vestibular findings in Fabry disease: a study of hemizygous males and heterozygous females. Acta Pediatr Suppl. 2003;92(443):33–37. doi: 10.1111/j.1651-2227.2003.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Crutchfield KE, Patronas NJ, Dambrosia JM, et al. Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology. 1998;50:1746–1749. doi: 10.1212/WNL.50.6.1746. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA, Eng CM. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Kinzler KE, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw Hill; 2001. pp. 3733–3774. [Google Scholar]
- Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–436. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- Dufour JJ, Michaud LA, Mohr G, Pouliot D, Picard C. Intratemporal vascular malformations (angiomas): particular clinical features. J Otolaryngol. 1994;23:250–253. [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, International Collaborative Fabry Disease Study Group et al. Safety and efficacy of recombinant human alpha-galactosidase A-replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Müller MJ, Ginsberg L. CNS manifestations of Fabry’s disease. Lancet Neurol. 2006;5:791–795. doi: 10.1016/S1474-4422(06)70548-8. [DOI] [PubMed] [Google Scholar]
- Germain D, Avan P, Chassaing A, Bonfils P. Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet. 2002;3:10. doi: 10.1186/1471-2350-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajioff D, Goodwin S, Quiney R, Zuckerman J, MacDermot KD, Mehta A. Hearing improvement in patients with Fabry disease treated with agalsidase alfa. Acta Paediatr Suppl. 2003;92(443):28–30. doi: 10.1111/j.1651-2227.2003.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Hajioff D, Enever Y, Quiney R, Zuckerman J, MacDermot K, Mehta A. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. J Inher Metab Dis. 2003;26:787–794. doi: 10.1023/B:BOLI.0000009948.86528.72. [DOI] [PubMed] [Google Scholar]
- Hegemann S, Hajioff D, Conti G, et al. Hearing loss in Fabry disease: data from the Fabry Outcome Survey. Eur J Clin Invest. 2006;36:654–662. doi: 10.1111/j.1365-2362.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Takahashi N, Mukai M, Katoh H, Akizawa T, Kawamura M. Intracranial dilative arteriopathy is associated with chronic kidney disease and small vessel diseases in the elderly. J Stroke Cerebrovasc Dis. 2009;18:435–442. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Ince B, Petty GW, Brown RD, Jr, Chu CP, Sicks JD, Whisnant JP. Dolichoectasia of the intracranial arteries in patients with first ischemic stroke: a population-based study. Neurology. 1998;50:1694–1998. doi: 10.1212/WNL.50.6.1694. [DOI] [PubMed] [Google Scholar]
- Keilmann A, Hajioff D, Ramaswami U, FOS Investigators Ear symptoms in children with Fabry disease: data from the Fabry Outcome Survey. J Inherit Metab Dis. 2009;32:739–744. doi: 10.1007/s10545-009-1290-x. [DOI] [PubMed] [Google Scholar]
- Kligerman AB, Solangi KB, Ventry IM, Goodman AI, Weseley SA. Hearing impairment associated with chronic renal failure. Laryngoscope. 1981;91:583–592. doi: 10.1288/00005537-198104000-00011. [DOI] [PubMed] [Google Scholar]
- Kosch M, Koch HG, Oliveira JP, et al. Enzyme replacement therapy administered during hemodialysis in patients with Fabry disease. Kidney Int. 2004;66:1279–1282. doi: 10.1111/j.1523-1755.2004.00883.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Jin X, Zhang K, et al. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13:305–313. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- Linthorst G, Hollak C, Donker-Koopman W, Strijland A, Aerts J. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004;66:1589–1595. doi: 10.1111/j.1523-1755.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Sirimina KS. Cavernous hemangioma of the internal auditory meatus. J Otolaryngol. 1990;19:288–291. [PubMed] [Google Scholar]
- Mehta A. Commentary. Acta Paediatr Suppl. 2003;92(443):27. [Google Scholar]
- Palla A, Hegemann S, Widmer U, Straumann D. Vestibular and auditory deficits in Fabry disease and their response to enzyme replacement therapy. J Neurol. 2007;254:1433–1442. doi: 10.1007/s00415-007-0575-y. [DOI] [PubMed] [Google Scholar]
- Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol. 2005;57:472–479. doi: 10.1002/ana.20423. [DOI] [PubMed] [Google Scholar]
- Quick C. Hearing loss in patients with dialysis and renal transplants. Ann Otol. 1976;85:776–790. doi: 10.1177/000348947608500607. [DOI] [PubMed] [Google Scholar]
- Ries M, Kim HJ, Zalewski CK, et al. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain. 2007;130:143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachern P, Shea D, Paparella M, Yoon T. Otologic histopathology of Fabry’s disease. Ann Otol Rhinol Laryngol. 1989;98:359–363. doi: 10.1177/000348948909800509. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Kopp JB, Austin HA, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- Sergi B, Conti G, Paludetti G, Interdisciplinary Study Group On Fabry Disease Inner ear involvement in Anderson-Fabry disease: long-term follow-up during enzyme replacement therapy. Acta Otorhinolaryngol Ital. 2010;30:87–93. [PMC free article] [PubMed] [Google Scholar]
- Stavroulaki P, Nikolopoulos TP, Psarommatis I, Apostolopoulos N. Hearing evaluation with distortion-product otoacoustic emissions in young patients undergoing haemodialysis. Clin Otolaryngology. 2001;26:235–242. doi: 10.1046/j.0307-7772.2001.00464.x. [DOI] [PubMed] [Google Scholar]
- Vedder AC, Linthorst GE, Houge G, et al. Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2007;2:e598. doi: 10.1371/journal.pone.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]