Abstract
Pompe disease (PD) is a recessive metabolic disorder characterized by acid α-glucosidase (GAA) deficiency, which results in lysosomal accumulation of glycogen in all tissues, especially in skeletal muscles. PD clinical course is mainly determined by the nature of the GAA mutations. Although ~400 distinct GAA sequence variations have been described, the genotype-phenotype correlation is not always evident.
In this study, we describe the first clinical and genetic analysis of Colombian PD patients performed in 11 affected individuals. GAA open reading frame sequencing revealed eight distinct mutations related to PD etiology including two novel missense mutations, c.1106 T > C (p.Leu369Pro) and c.2236 T > C (p.Trp746Arg). In vitro functional studies showed that the structural changes conferred by both mutations did not inhibit the synthesis of the 110 kD GAA precursor form but affected the processing and intracellular transport of GAA. In addition, analysis of previously described variants located at this position (p.Trp746Gly, p.Trp746Cys, p.Trp746Ser, p.Trp746X) revealed new insights in the molecular basis of PD. Notably, we found that p.Trp746Cys mutation, which was previously described as a polymorphism as well as a causal mutation, displayed a mild deleterious effect. Interestingly and by chance, our study argues in favor of a remarkable Afro-American and European ancestry of the Colombian population. Taken together, our report provides valuable information on the PD genotype–phenotype correlation, which is expected to facilitate and improve genetic counseling of affected individuals and their families.
Introduction
Pompe disease (PD) is a rare autosomal recessive metabolic disorder characterized by acid α-glucosidase (GAA) deficiency. This enzyme catalyzes the hydrolysis of the α-1,4 and α-1,6-glucosidic bonds of glycogen, and its deficiency results in lysosomal glycogen storage in all tissues, especially in skeletal muscles. Clinically, PD patients display a broad spectrum of phenotypes with regard to the age of onset, the disease progression rate, and the severity of symptoms (van der Ploeg and Reuser 2008; Raben et al. 2007; Hirschhorn et al. 2001). The infantile form of PD includes severely affected infants (under 1 year of age) who display a combination of generalized skeletal muscle weakness and cardiac hypertrophy that provoke cardio-respiratory failure and death (Kishnani et al. 2006; van den Hout 2003). Conversely, patients with childhood, juvenile, and adult onset of symptoms lack cardiac involvement. These individuals exhibit a less severe skeletal muscle dysfunction with slowly progressive proximal myopathy as well as a marked involvement of respiratory muscles (Hirschhorn et al. 2001; van der Ploeg 2008; Müller-Felber et al. 2007; Laforêt et al. 2000). The GAA gene contains 19 coding exons. At the protein level, human GAA is composed of five distinct regions: trefoil type-P, N-terminal β-sandwich, catalytic (β/α)8 barrel, proximal C-terminal, and distal C-terminal domains. The key catalytic residues are located at Asp518 and Asp616 (Sugawara et al. 2009). GAA is synthesized as an inactive precursor of 110 kD which is subsequently transported to the pre-lysosomal and lysosomal compartments via the mannose 6-phosphate receptor. En route, it is processed to a 95 kD intermediate and subsequently to fully active forms of 76 and 70 kD (Hirschhorn et al. 2001).
From an etiological point of view, PD is caused by GAA mutations that determine the degree of enzyme deficiency and largely the clinical course (Kroos et al. 2008; Reuser et al. 1987). Disease-causing sequence variations have been described along the entire length of the gene including missense and nonsense mutations, splice site variants, and partial insertions/deletions. At present, the Pompe Disease Mutation database (www.pompecenter.nl) lists 393 GAA sequence variations. Among these, 54 are of unknown effect, 75 are considered as nonpathogenic, 2 are probably nonpathogenic, and 257 are confirmed as etiological (Oba-Shinjo et al. 2009; http://www.pompecenter.nl/). Several in vitro studies have permitted to propose distinct molecular mechanisms underlying PD etiopathology (Kroos et al. 2008). The majority of pathogenic missense mutations seem to affect folding, posttranslational processing, and/or intracellular transport of GAA which partially or completely abolishes its function (Pittis et al. 2008; van der Ploeg and Reuser 2008).
Some GAA mutations seem to have spread through a founder effect. African American patients originating from the north of Africa frequently present c.2560 C > T (p.Arg854X) and Asian patients c.1935 C > A (p.Asp645Glu) sequence variants (Becker et al. 1998). Common mutations among Caucasian patients include c.2481 + 102_2646del (delexon18; p.Gly828_Asn882del), c.525del (delT525; p.Glu176fsX45), and c.925 G > A (p.Gly309Arg) (Hirschhorn et al. 2001; Kroos et al. 2008; Raben et al. 1999). The c.-32-13 T > G mutation, which reduces the GAA-mRNA splicing fidelity, is the most common GAA pathogenic sequence variant among Caucasian adults and children with a slowly progressive course of the disease (Boerkoel et al. 1995; Huie et al. 1994). The GAA residual activity in patients presenting the c.-32-13 T > G/null genotype is usually reduced to 5–25% of average normal (van der Ploeg and Reuser 2008; Kroos et al. 2007). Some patients with this genotype manifest symptoms in early childhood, whereas others remain presymptomatic until late adulthood. This demonstrates the role of modifying factors in PD pathophysiology (Pittis et al. 2008; Kroos et al. 2007; Slonim et al. 2007).
In this study, we describe the first clinical and genetic analysis of Colombian PD patients performed in 11 affected individuals who belong to 8 families. Direct sequencing of the complete GAA open reading frame revealed eight distinct mutations related to PD etiology. Two novel missense mutations were investigated for their functional effect along with four previously described mutations to obtain a better understanding of the disease pathophysiology. Interestingly and by chance, our study argues in favor of a remarkable Afro-American and European ancestry of the Colombian population.
Material and Methods
Patients
PD patients (pt) who belong to eight distinct families were included in this study. These individuals originate from five different Colombian cities: Cartagena, Barranquilla, Bucaramanga, Medellín, and Bogotá. As previously described, PD diagnosis was performed by quantifying GAA activity from peripheral blood leukocytes using 4-methylumbelliferyl-α-d-glucoside as substrate (Li et al. 1785). Maltase-glucoamylase activity was inhibited with 120 μmol/L of acarbose. For each patient, GAA activity was assayed in the presence and absence of acarbose at pH 3.8. More than 85% inhibition confirms PD (Palmer et al. 2007). The age at diagnosis ranged from 2 to 47 years and the initial symptoms were mostly related to limb girdle weakness (LW) (Table 1). Ten patients (pt 1 to pt 10) experienced first symptoms in childhood or adulthood. One patient (pt 11) was diagnosed at 4 months of age since he displayed hypotonia and delayed motor development. Pt 4, 5, and 6 belong to one family. The same holds for patients 9 and 10. In all the cases, parents of the patients were included in the study in order to evaluate the segregation of the GAA mutations. All participants in the study provided written informed consent. The Institutional Ethics Committee of each participating institution approved the clinical and experimental aspects of the study.
Table 1.
Phenotypes and Genotypes of Colombian Pompe Disease Patients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical features | |||||||||||
| Gender | M | M | F | F | M | M | M | F | M | M | M |
| First symptoms (years) | 1.1 | 6 | 12 | 20 | 19 | 17 | 27 | 38 | 10 | 15 | 0.3 |
| Age at diagnosis (years) | 5 | 11 | 29 | 33 | 35 | 36 | 31 | 47 | 19 | 20 | 2 |
| Initial clinical signs | Pl | LW | LW | LW | LW | LW | LW | LW | LW | LW | HDMD |
| Muscle weakness | LL | WC | LL | LL | LL | LL | LL | LL | LL | WC | RPMW |
| Respiratory distress | VS | VS | No | No | No | No | O | O and FE | VS/night | VS | VS |
| Cardiomyopathy | No | No | No | No | No | No | No | No | No | No | Yes |
| Hepatomegaly | Yes | No | No | No | No | No | No | No | No | No | No |
| Underweight | Yes | No | No | No | No | No | No | No | No | No | Yes |
| Associated | FD | SDB | No | No | No | No | No | No | SDB | FD | FD |
| CK (IU/L) | 127 | 545 | 332 | No data | No data | No data | 488 | 912 | 977 | 736 | 350 |
| AST/ALT (IU/L) | 142/144 | 187/112 | 66/70 | No data | No data | No data | 56/46 | 46/44 | 125/118 | 213/154 | 226/180 |
| GAA mutations | |||||||||||
| c.1064T>C (p.Leu355 Pro) | c.1064T>C (p.Leu355 Pro) | c.-32-13T>G | c.2481+102_ 2646del (p.Gly828_ Asn882del) | Idem Pt4 | Idem Pt4 | c.2560 C>T (p.Arg 854X) | c.-32-13T>G | c.2560 C>T (p.Arg 854X) | c.2560 C>T (p.Arg 854X) | c.2560C>T (p.Arg854X) | |
| c.1106T >C (p.Leu369 Pro)* | c.525delT (p.Glu176 fsx45) | c.-32-2A>G | Idem Pt4 | Idem Pt4 | c.1581 A>G (p.Arg 527Arg) | c.1551+42A>G | c.1551+42A>G | c.2236 T>C (p.Trp746 Arg)* | |||
| c.-32-13T>G | c.1581A >G (p.Arg527 Arg) | c.1551+42A>G | c.596G >A (p.Arg 199His) | ||||||||
| c.596G >A (p.Arg199 His) | c.596G >A (p.Arg199 His) | c.596G >A (p.Arg199 His) | c.68A >G (p.His223 Arg) | ||||||||
| c.668A >G (p.His223 Arg) | c.668A >G (p.His223 Arg) | c.668A >G (p.His223 Arg) | c.2553A >G (p.Gly851 Gly) | ||||||||
| c.1551+49A>C | c.2338A >G (p.Ile780 Val) | c.2338A >G (p.Ile780 Val) | c.1551+49A>C | ||||||||
| c.2338A >G (p.Ile780Val) | c.2553A >G(p.Gly851 Gly) | c.2553A >G (p.Gly851 Gly) | |||||||||
| Laboratory findings | |||||||||||
| CK (IU/L) | 127 | 545 | 332 | No data | No data | No data | 488 | 912 | 977 | 736 | 350 |
| AST/ALT (IU/L) | 142/144 | 187/112 | 66/70 | No data | No data | No data | 56/46 | 46/44 | 125/118 | 213/154 | 226/180 |
Pl Pulmonary, LW Limbgirdle weakness, HDMD Hypotonia/delayed motor development, LL Lower limbs, WC Wheelchair; O Orthopnea, FE Fatigue on exertion, FD Feeding difficulties, SDB Sleep-disordered breathing, RPMW Rapidly progressive muscle weakness.
Underlined mutations denote homozygous status; mutations in bold are pathogenic; *denotes novel mutations.
GAA Mutational Analysis
Genomic DNA was extracted from whole blood samples using standard procedures. In all patients, the complete GAA open reading frame (19 exons) was amplified by PCR as previously described (Becker et al. 1998). Each amplicon was purified by using shrimp alkaline phosphatase and exonuclease I. PCR primers were used to sequence the coding regions in both sense and antisense directions using an ABI 3730xl sequencer (Ko et al. 1999). The presence of each non-synonymous variant was confirmed by an additional round of PCR and sequencing. Variations at the DNA level were identified using human GAA wild-type mRNA sequence (NM_000152.3). Sequence variations were described according to the international mutation nomenclature guidelines as set forth by the Human Genome Variation Society (http://www.hgvs.org/mutnomen/). Intron mutations were designated by locating its cDNA position and, as described by den Dunnen et al. (den Dunnen and Antonarakis 2000), negative numbers were reported from the starting of the splice acceptor site. GAA mutant protein sequences were aligned and compared with the human wild-type version (NP_000143.2) using ClustalW software. In order to assess conservation during evolution of residues at mutated sites, this program was also used to perform multiple alignments of protein sequences from vertebrate species: Homo sapiens, Pongo abeili, Bos taurus, Mus musculus, and Rattus norvegicus. Data from the Pompe disease mutation database (www.pompecenter.nl) were used to define novel GAA sequence variants as well as to identify which of them were previously related to pathogenic effects. To predict the effect of newly identified missense mutations we also used SIFT and PolyPhen2 software. PolyPhen2 prediction values are the result of an algorithm, which considers distinct features such as comparative analysis of protein sequences from different species, physicochemical characteristics of the exchanged amino acids and mapping of residues replacement to available 3D structures. Results are assessed as a quantitative value (a probability of being deleterious) and as a qualitative feature (benign, possibly damaging, or probably damaging). SIFT program predicts the potential pathogenic effects of amino acid substitutions on the basis of sequence homology and physical properties of the exchanged residues. Scores lower than 0.05 predict a potential deleterious effect.
Functional Analysis of GAA Mutations
An expression vector (pSHAG2), containing the wild-type GAA open reading frame (named GAA-Wild-Type), was used to perform site-directed mutagenesis. We introduced into this plasmid the two novel GAA c.1106 T > C (p.Leu369Pro) or c.2236 T > C (p.Trp746Arg) missense mutations found in Colombian patients. These constructs were named GAA-Leu369Pro and GAA-Trp746Arg, respectively. Similarly, we created four additional constructs (GAA-Trp746X, GAA-Trp746Cys, GAA-Trp746Gly and GAA-Trp746Ser) carrying mutant GAA versions, which represent previously reported mutations located at position 746. The integrity of the resulting mutant constructs was, in each case, confirmed by direct sequencing.
HEK 293 T cells were seeded into 24-well plates and grown overnight in DMEM medium supplemented with 10% of fetal bovine serum, 50U/mL of penicillin and 50 μg/mL of streptomycin, in a 10% carbon dioxide and 90% air humidified incubator. Cells at 80–90% of confluence were transfected with 1.4 μg of GAA-WT or mutant constructs using polyethyleneimine. Mock transfected cells served as negative controls. Seventy-two hours after transfection, cells were washed with PBS and harvested with lysis buffer (50 mM Tris–HCl pH 7.0, 150 mM NaCl, 50 mM NaF, and 1% TritonX-100). After centrifugation (10,000 g for 10 min), the supernatant fraction was recovered. GAA activity was measured in both medium and cell homogenates (Müller-Felber et al. 2007). As described by Kroos et al. (2008), the mutation severity scoring system is based on the assessment of GAA activity levels in the medium and in the cells, and on the quality and quantity of the different molecular species that arise during GAA posttranslational modification (Kroos et al. 2008). To visualize protein biosynthesis and posttranslational processing, cell homogenates and immunoprecipitated GAA from the medium were subjected to SDS-PAGE followed by Western-blotting (Müller-Felber et al. 2007). To visualize GAA on the blots we used GAA-specific polyclonal mouse and rabbit antisera as primary antibodies and goat anti-mouse IRDye 800LT (LI-COR Biosciences) and goat anti-rabbit IRDye 700LT (LI-COR Biosciences) as secondary antibodies. Transfection assays and GAA measurements were performed three times as duplicates.
Results
GAA Mutation Detection and In Silico Analysis
Sequence analysis of the complete coding region of GAA, performed in 11 Colombian PD patients, revealed six sequence variants previously related with PD pathogenesis: c-32-13 T > G, c.-32-2A > G, c.525delT (p.Glu176fsX45), c.1064 T > C (p.Leu355Pro), c.2481 + 102_2646del (p.Gly828_Asn882del), and c.2560 C > T (p.Arg854X) (Table 1). Pt 2 was homozygous for the p.Leu355Pro mutation and pt 3 showed compound heterozygosity for the two pathogenic variants c.-32-13 T > G and c.525delT (p.Glu176fsX45). Three related patients (pt 4, 5, and 6) shared the c.-32-2A > G/c.2481 + 102_2646del (p.Gly828_Asn882del) compound heterozygous genotype. Pt 7 was compound heterozygote for the sequence variants c.2560 C > T (p.Arg854X) and c.-32-13 T > G. Pt 8 displayed c.-32-13 T > G homozygosity. Patients 9 and 10 were also related. They shared the c.2560 C > T (p.Arg854X) heterozygous mutation. Pt 7, 9, and 10 also carried non-synonymous and intronic variants, which were previously described in PD patients but were not related with the disease pathogenesis (Table 1).
Pt 1 and 11 displayed novel c.1106 T > C (p.Leu369Pro) and c.2236 T > C (p.Trp746Arg) heterozygous variants, respectively. These mutations are located in the catalytic (β/α)8 barrel (p.Leu369Pro) and in the proximal C-terminal (p.Trp746Arg) domains of GAA. Both these patients are compound heterozygous for deleterious GAA mutations since they also presented c.1064 T > C (p.Leu355Pro) (pt 1) and c.2560 C > T (p.Arg854X) (pt 11).
At the protein level, comparative in silico analysis of the novel p.Leu369Pro and p.Trp746Arg mutations showed a strict conservation among vertebrate species of both Leucine and Tryptophan residues at positions 369 and 746, respectively (Fig. 1). Polyphen bioinformatic tool predicted that these mutations are probably damaging. Similarly, SIFT software showed probabilistic scores compatible with a potential deleterious effect (p.Leu369Pro =0.00, p.Trp746Arg =0.01).
Fig. 1.
Alignment of the GAA sequences of selected vertebrates: for leucine at position L369 (1A) and for tryptophane at position W746 (1B)
Functional Characterization of GAA Mutations
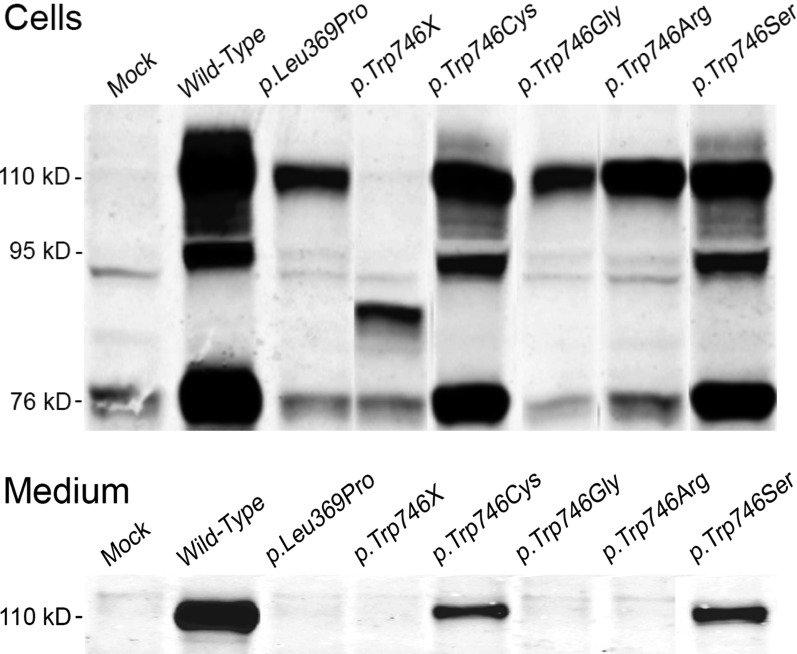
To further investigate the effect of p.Leu369Pro and p.Trp746Arg, both these mutations were introduced in the wild-type GAA cDNA by site directed mutagenesis and transiently expressed in HEK-293 cells. Cells and media were analyzed for GAA content by polyacrylamide gel electrophoresis followed by western blotting. The results are shown in Fig. 2a (cells) and 2b (media). Mock transfected HEK-293 cells showed faint signals in cells and culture medium (Fig. 2a–b). Since HEK-293 cells constitutively express human GAA, these signals can be considered as background staining.
Fig. 2.
Western blot analysis of GAA expression in transiently transfected HEK293 cells. Cells and culture media were harvested 72 h after transfection and the different molecular species representing precursor GAA (110 kD), partially processed precursor (95 kD) and mature GAA (76 kD) were separated by SDS-PAGE and visualized by immunoblotting as described in Materials and Methods. Panel A, cell homogenates; Panel B, media
Cells transfected with the GAA wild-type construct contained three molecular species representing the 110 kD precursor, the 95 kD partially processed intermediate and the 76 kD mature form of GAA. The medium contained only the 110 kD GAA precursor (Fig. 2). Similar results in cells and media were obtained after transfection of HEK-293 cells with the mutated p.Trp746Cys and p.Trp746Ser constructs. Quite different results were obtained after transfection with p.Trp746Arg, p.Trp746Gly, and p.Leu369P: only the intracellular 110 kD GAA precursor and no other forms of GAA were detected in cells or media, except for possibly a little bit of 76 kD mature enzyme after transfection with p.Trp746Arg. Transfection with p.Trp746X resulted in the formation of a unique molecular species with an apparent molecular mass between 76 and 95 kD in the cells. In all cases we also measured the GAA activity in cells and media and used the scoring system as described by Kroos et al. (2008) to evaluate the severity of all the different mutations. The results are summarized in Table 2.
Table 2.
GAA sequence variations analyzed by transient expression in HEK293 cells
| Nucleotide change | Amino acid change | M 110* | C110* | C95* | C76* | M% | C% | Class* |
|---|---|---|---|---|---|---|---|---|
| c.1106 T > C | p.Leu369Pro | 1,1 | 3,4 | 2,4 | ? | 0.2 | 3.1 | B |
| c.2238 G > A | p.Trp746X | 1,1 | 3,2 | 1,1 | ? | –0.2 | –0.14 | A |
| c.2238 G > C | p.Trp746Cys | 3,4 | 3,4 | 3,4 | 3,4 | 5.4 | 29.4 | D |
| c.2236 T > G | p.Trp746Gly | 1,1 | 3,4 | 2,4 | ? | 0.2 | 2.1 | B |
| c.2236 T > C | p.Trp746Arg | 1,1 | 3,4 | 2,4 | ? | –0.1 | 3.5 | B |
| c.2237 G > C | p.Trp746Ser | 3,4 | 4, 4 | 3,4 | 3,4 | 8.2 | 40.1 | E |
The two new missense mutations that we analyzed in this study are shown in bold
*M110, C10, C95, and C76 stand for the various molecular forms of GAA that arise during synthesis and posttranslational modification and that can be visualized by western blotting as illustrated in Fig 2a and b. The numbers refer to the severity rating system as published by Kroos et al. (2008). Class A mutations are very severe, class B mutations are potentially less severe, class D mutations are mild, and class E mutations are probably nonpathogenic. M% stands for the percentage of GAA activity in the culture medium and C% for the percentage of GAA activity in the cells as compared to Wild-Type GAA activity.
The question mark (?) signifies that there remains uncertainty about the formation of the 76 kD form of GAA
Discussion
In an effort to delineate the clinical and molecular features of Colombian PD patients, we identified 11 cases in which we performed GAA genotype analysis. Among the six different mutations that we identified in this study and that were previously related to PD pathogenesis, two affect the GAA mRNA splicing (c.-32-13 T > G and c.-32-2A > G), one is a missense mutation (c.1064 T > C/p.Leu355Pro), one is a single base pair deletion (c.525delT/p.Glu176fsX45), one is a large deletion including exon 18 (c.2481 + 102_2646del/p.gly828_Asn882del), and one is a nonsense mutation (c.2560 C > T/p.Arg854X).
GAA c.-32-13 T > G was most frequently encountered since three patients (pt 3, 7, and 8) (allelic frequency = 0.27) were found to be either homozygous or heterozygous for this mutation. This sequence variation is the most common pathogenic GAA mutation among Caucasian individuals (>70%) affected by slowly progressive PD (Hirschhorn et al. 2001; Ko et al. 1999). Pt 3 and 7, respectively, presented p.Glu179fsX45 and p.Arg854X as second pathogenic mutation. In both patients, limb girdle weakness and orthopnea were recorded as the first PD symptoms, but the age of onset differed substantially (12 vs 27 years). These findings fit with the notion that the clinical picture of patients carrying the c.-32-13 T > G mutation can vary in terms of the age of onset and rate of disease progression due to modifying factors (Huie et al. 1994; Kroos et al. 2007). Pt 8, who displayed c.-32-13 T > G homozygosity, showed first symptoms at the age of 38 years and had mild clinical features. Homozygosity for the c.-32-13 T > G variant is particularly rare as only three cases have been reported so far (Müller-Felber et al. 2007;, Laforêt et al. 2000; Labrousse et al. 2010). Similarly to the patient reported by Laforet et al., this individual was classified as a late-onset case since the first symptoms manifested after the age of 38 years. It has been proposed that the rare finding of affected c.-32-13 T > G homozygotes is probably related to the high level of residual GAA activity that is associated with this genotype. Notably, all our patients having the c.-32-13 T > G mutation shared six additional sequence variants (SNP-IDs: rs17410539, rs11150843, rs7225049, rs3176968, rs1042397, rs1042397), which together mark the most common c.-32-13 T > G haplotype encountered among Caucasian PD patients (Müller-Felber et al. 2007; Kroos et al. 2008).
Pt 2, who was diagnosed at the age of 11 years and homozygous for c.1064 T > C (p.Leu355Pro), confirms the previously established genotype-phenotype correlation for this mutation in that it is associated with an early childhood presentation of PD (Labrousse et al. 2010; Montalvo et al. 2004). Pt 4, 5, and 6, who are related and were diagnosed at ages of 33, 35, and 36 years, appeared to have the c.-32-2A > G/c.2481 + 102_2646del (p.Gly828_Asn882del) genotype. Interestingly, c.-32-2A > G was previously reported to be associated with early-onset PD (Kroos et al. 2008). Thus, our results do not correlate with those previously reported and argue in favor of a relatively mild effect of the c.-32-2A > G mutation or the impact of modifying factors.
The p.Arg854X mutation, which has been reported as the most frequent GAA sequence variation among Afro-American PD patients, was identified in four of our Colombian patients (pt 7, 9, 10, and 11) at heterozygous state (Raben et al. 1999). Homozygotes for this mutation have infantile onset PD (Laforêt et al. 2000; Kroos et al. 2008; Reuser et al. 1689). The patients in our study lacked cardiomyopathy and presented their first clinical signs after 10 years of age. The difference in age of onset must be due to residual GAA activity conferred by a less severe mutation located on the second allele. Indeed, pt 7 carried the c.-32-13 T > G mutation, which displays significant residual activity that apparently compensates the strong deleterious effect of the Arg854X mutation. Unfortunately, we failed to establish a more precise genotype-phenotype correlation in pt 9 and 10 (who are related) since we did not find the second GAA mutation.
Pt 11 with infantile PD had the first clinical signs (hypotonia, delayed motor development) at 3 months of age. Apart from the p.Arg854X amino acid change, this patient displayed the novel p.Trp746Arg mutation, which is located in the proximal C-terminal domain of GAA. Comparative sequence analysis of vertebrate species demonstrated a strict conservation of this tryptophane suggesting its essential functional role. The substitution Trp to Arg implicates a drastic modification in terms of physicochemical properties. Indeed, tryptophane is a nonpolar aromatic amino acid whereas Arg is a small polar hydrophilic residue. These features were reinforced by SIFT and Polyphen2 bioinformatic tools which predicted a potential deleterious effect of the p.Trp746Arg mutation.
In accordance with these predictions, transient expression studies demonstrated that the structural changes conferred by p.Trp746Arg do not inhibit the synthesis of the 110 kD precursor but affect the processing and intracellular transport of GAA (Fig. 2). According to the mutation severity scoring system proposed by Kroos et al. (2008) these results argue in favor of a potentially less severe mutation. Additional analysis of previously described variants revealed similar results for the substitution p.Trp746Gly, which was found during a newborn screening program in a patient with low GAA activity (Labrousse et al. 2010). The p.Trp746Cys and p.Trp746Ser variants can both be classified as relatively mild mutations since the 110 kD GAA precursor as well as processed forms of GAA were detected in both cases, albeit in less than normal amount (Fig. 2a–b, Table 2). Notably, up till now some controversy existed concerning the pathogenicity of p.Trp746Cys since it was described as a polymorphism as well as a causal mutation (Wan et al. 2008; Chien et al. 2011). Our results argue in favor of a mildly deleterious effect.
Transient expression of the p.Trp746X mutation resulted in the appearance of a truncated precursor, which is apparently stable enough to be visualized by western blotting, but lacks catalytic activity. This situation corroborates previous clinical findings in which patients carrying this mutation are affected by infantile PD (Kishnani et al. 2006; Beesley et al. 1998).
Next to p.Trp764Arg, p.Leu369Pro was the second novel mutation identified in our study. It was found in an affected child in combination with p.Leu355Pro. Pulmonary distress was diagnosed at 1 year of age, and the patient required ventilation support at the age of 9 years. The muscle weakness was especially severe. In silico analysis of this mutation suggested a pathogenic effect, similar to p.Trp746Arg, based on the strict conservation of the Leu residue at position 369 among vertebrate species. This prediction was validated by transient expression studies since the amino acid substitution appeared to hamper the posttranslational modification and intracellular transport of GAA (Fig. 2a–b, Table 2).
Finally, from an ethnical point of view, it is interesting that the two most common GAA mutations found in our study are also common in Caucasian (c.-32-13 T > G) and African (p.Arg854X; allele frequency = 0.43) populations (Becker et al. 1998). Notably, all Colombian patients presenting p.Arg854X shared a previously identified haplotype found in black PD patients from the United States, the Ivory Coast, Ghana, and Namibia (Becker et al. 1998; Hermans et al. 1993). These findings evoke a remarkable Afro-American and European ancestry of the Colombian population.
In summary, we investigated the genetics of PD in the Colombian population and identified two novel causative mutations in the GAA gene in addition to other previously reported pathogenic sequence variations. Valuable information on the genotype-phenotype correlation was obtained that is expected to facilitate and improve genetic counseling of affected individuals and their families.
Acknowledgments
This work was supported by the Universidad del Rosario (Grant CS/Genetics) and by Genzyme Corporation, Colombia.
Footnotes
Competing interests: None declared
Authors MónicaYasmín Niño and Heidi Eliana Mateus contributed equally to this work.
References
- Becker JA et al (Apr 1998) The African origin of the common mutation in African American patients with glycogen-storage disease type II. Am J Hum Genet 62(4): 991–994. ISSN 0002-9297. http://www.ncbi.nlm.nih.gov/pubmed/9529346 [DOI] [PMC free article] [PubMed]
- Beesley CE, Child AH, Yacoub MH (1998) The identification of five novel mutations in the lysosomal acid a-(1-4) glucosidase gene from patients with glycogen storage disease type II. Mutations in brief no. 134. Hum Mutat 11(5): 413. ISSN 1059-7794. http://www.ncbi.nlm.nih.gov/pubmed/10206684 [DOI] [PubMed]
- Boerkoel CF et al (Apr 1995) Leaky splicing mutation in the acid maltase gene is associated with delayed onset of glycogenosis type II. Am J Hum Genet 56(4): 887–897. ISSN 0002-9297. http://www.ncbi.nlm.nih.gov/pubmed/7717400 [PMC free article] [PubMed]
- Chien YH et al (Jun 2011) Later-onset Pompe disease: early detection and early treatment initiation enabled by newborn screening. J Pediatr 158(6): 1023–1027.e1. ISSN 1097-6833. http://www.ncbi.nlm.nih.gov/pubmed/21232767 [DOI] [PubMed]
- Den Dunnen JT, Antonarakis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15(1): 7–12. ISSN 1059-7794. http://www.ncbi.nlm.nih.gov/pubmed/10612815 [DOI] [PubMed]
- Hermans MM et al (1993) Two mutations affecting the transport and maturation of lysosomal alpha-glucosidase in an adult case of glycogen storage disease type II. Hum Mutat 2(4): 268–273. ISSN 1059-7794. http://www.ncbi.nlm.nih.gov/pubmed/8401535 [DOI] [PubMed]
- Hirschhorn R, Reuser A. Glycogen storage disease type II (GSDII) In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3389–3420. [Google Scholar]
- Huie ML et al (Dec 1994) Aberrant splicing in adult onset glycogen storage disease type II (GSDII): molecular identification of an IVS1 (-13 T-- > G) mutation in a majority of patients and a novel IVS10 (+1GT-- > CT) mutation. Hum Mol Genet 3(12): 2231–2236. ISSN 0964-6906. http://www.ncbi.nlm.nih.gov/pubmed/7881425 [DOI] [PubMed]
- Kishnani PS et al (May 2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148(5): 671–676. ISSN 0022-3476. http://www.ncbi.nlm.nih.gov/pubmed/16737883 [DOI] [PubMed]
- Ko TM et al (1999) Molecular genetic study of Pompe disease in Chinese patients in Taiwan. Hum Mutat 13(5): 380–384. ISSN 1059-7794. http://www.ncbi.nlm.nih.gov/pubmed/10338092 [DOI] [PubMed]
- Kroos MA et al (Jan 2007) Broad spectrum of Pompe disease in patients with the same c.-32-13 T- > G haplotype. Neurology 68(2): 110–115. ISSN 1526-632X. http://www.ncbi.nlm.nih.gov/pubmed/17210890 [DOI] [PubMed]
- Kroos M et al (Jun 2008) Update of the Pompe disease mutation database with 107 sequence variants and a format for severity rating. Hum Mutat 29(6): E13–26. ISSN 1098-1004. http://www.ncbi.nlm.nih.gov/pubmed/18425781 [DOI] [PubMed]
- Labrousse P et al (Apr 2010) Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab 99(4): 379–383. ISSN 1096-7206. http://www.ncbi.nlm.nih.gov/pubmed/20080426 [DOI] [PubMed]
- Laforêt P et al (Oct 2000) Juvenile and adult-onset acid maltase deficiency in France: genotype-phenotype correlation. Neurology 55(8): 1122–1128. ISSN 0028-3878. http://www.ncbi.nlm.nih.gov/pubmed/11071489 [DOI] [PubMed]
- Li Y et al (Oct 2004) Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem 50(10): 1785–1796. ISSN 0009-9147. http://www.ncbi.nlm.nih.gov/pubmed/15292070 [DOI] [PMC free article] [PubMed]
- Montalvo AL et al (Mar 2004) Glycogenosis type II: identification and expression of three novel mutations in the acid alpha-glucosidase gene causing the infantile form of the disease. Mol Genet Metab 81(3): 203–208. ISSN 1096-7192. http://www.ncbi.nlm.nih.gov/pubmed/14972326 [DOI] [PubMed]
- Müller-Felber W et al (Oct 2007) Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord 17(9–10): 698–706. ISSN 0960-8966. http://www.ncbi.nlm.nih.gov/pubmed/17643989 [DOI] [PubMed]
- Oba-Shinjo SM et al (Nov 2009) Pompe disease in a Brazilian series: clinical and molecular analyses with identification of nine new mutations. J Neurol 256(11): 1881–1890. ISSN 1432-1459. http://www.ncbi.nlm.nih.gov/pubmed/19588081 [DOI] [PubMed]
- Palmer RE et al (Jan 2007) Pompe disease (glycogen storage disease type II) in Argentineans: clinical manifestations and identification of 9 novel mutations. Neuromuscul Disord 17(1): 16–22. ISSN 0960-8966. http://www.ncbi.nlm.nih.gov/pubmed/17056254 [DOI] [PubMed]
- Pittis MG et al (Jun 2008) Molecular and functional characterization of eight novel GAA mutations in Italian infants with Pompe disease. Hum Mutat 29(6): E27–36. ISSN 1098-1004. http://www.ncbi.nlm.nih.gov/pubmed/18429042 [DOI] [PubMed]
- Raben N et al (1999) Novel mutations in African American patients with glycogen storage disease Type II. Mutations in brief no. 209. Hum Mutat 13(1): 83–84. ISSN 1059-7794. http://www.ncbi.nlm.nih.gov/pubmed/10189220 [DOI] [PubMed]
- Raben N et al (Nov–Dec 2007) Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand… Autophagy 3(6):546–552. ISSN 1554-8627. http://www.ncbi.nlm.nih.gov/pubmed/17592248. [DOI] [PubMed]
- Reuser AJ et al (Jun 1987) Clinical diversity in glycogenosis type II. Biosynthesis and in situ localization of acid alpha-glucosidase in mutant fibroblasts. J Clin Invest 79(6): 1689–1699. ISSN 0021-9738. http://www.ncbi.nlm.nih.gov/pubmed/3108320 [DOI] [PMC free article] [PubMed]
- Sharma MC et al (2005) Delayed or late-onset type II glycogenosis with globular inclusions. Acta Neuropathol 110(2): 151–157. ISSN 0001-6322. http://www.ncbi.nlm.nih.gov/pubmed/15986226 [DOI] [PubMed]
- Slonim AE et al (Jan 2007) Modification of the natural history of adult-onset acid maltase deficiency by nutrition and exercise therapy. Muscle Nerve 35(1): 70–77. ISSN 0148-639X. http://www.ncbi.nlm.nih.gov/pubmed/17022069 [DOI] [PubMed]
- Sugawara K et al (Jun 2009) Structural modeling of mutant alpha-glucosidases resulting in a processing/transport defect in Pompe disease. J Hum Genet 54(6): 324–330. ISSN 1435-232X. http://www.ncbi.nlm.nih.gov/pubmed/19343043 [DOI] [PubMed]
- van den Hout HM et al (Aug 2003) The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics 112(2): 332–340. ISSN 1098-4275. http://www.ncbi.nlm.nih.gov/pubmed/12897283 [DOI] [PubMed]
- van der Ploeg AT, Reuser AJ (Oct 2008) Pompe’s disease. Lancet 372(9646): 1342–1353. ISSN 1474-547X. http://www.ncbi.nlm.nih.gov/pubmed/18929906 [DOI] [PubMed]
- van der Ploeg A, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362(15):1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- Wan L et al (Jun 2008) Identification of eight novel mutations of the acid alpha-glucosidase gene causing the infantile or juvenile form of glycogen storage disease type II. J Neurol 255(6): 831–838. ISSN 0340-5354. http://www.ncbi.nlm.nih.gov/pubmed/18458862 [DOI] [PubMed]