Abstract
Aims
To evaluate the effect of topical autologous plasma on nerve morphology in patients with neurotrophic keratopathy (NK) using the confocal microscope.
Methods
Eleven eyes of six patients with neurotrophic keratopathy were evaluated for this study. Corneal fluorescein staining was done, and corneal sensitivity measurements were done with the Cochet–Bonnet and modified Belmonte gas aesthesiometers. The Heidelberg Retina Tomograph 2 Rostock Cornea Module laser scanning confocal microscope was used to image the corneal surface and subepithelial neural plexus. Four images at the level of the subepithelial nerve plexus in the central cornea were randomly selected for analysis of the corneal nerves. Topical autologous plasma was used six to eight times per day.
Results
The best-corrected visual acuity improved significantly after plasma treatment in all patients (p=0.003). The mean corneal fluorescein staining score significantly decreased after treatment (p=0.0003). There was a significant increase in corneal sensitivity measured by Cochet–Bonnet (p<0.0001) and modified Belmonte (p=0.01) aesthesiometers. The mean number, length, width and density of subepithelial nerves increased significantly after plasma treatment (p=0.0002).
Conclusion
In vivo confocal microscopy examination revealed preliminary evidence for improvement of corneal nerve findings suggesting efficacy of autologous plasma treatment in neurotrophic keratopathy.
INTRODUCTION
Neurotrophic keratopathy (NK) is a potentially sight-threatening condition, caused by impairment of trigeminal corneal innervation, leading to a decrease or loss of corneal sensation.1–4 The aetiologies of NK include herpes simplex and herpes zoster keratitis, trigeminal nerve ablation, diabetes mellitus and corneal surgeries, such as laser in situ keratomileusis (LASIK) or penetrating keratoplasty.1–4 NK is characterised by epithelial abnormalities including punctate epithelial keratopathy, recurrent epithelial erosions and persistent epithelial defects. Lack of innervation is accompanied by decreased release of sensory neuromediators such as substance P and calcitonin gene related peptide (CGRP), decreased aqueous tear production and decreased blink rate.5–6 The standard treatment regimen for neurotrophic keratopathy includes topical non-preserved artificial tears, punctal occlusion, patching, bandage contact lenses and tarsorrhaphy.1
Autologous serum drops have been used successfully for treatment of NK and it has been hypothesised that autologous serum may contain neurotrophic factors capable of healing the corneal surface and restoring epithelial integrity.7 In vivo confocal microscopy of the cornea has been used to evaluate nerve density and morphology, and document regeneration of corneal nerve fibres coincident with corneal epithelial healing in a patient with NK.8,9 The purpose of our study was to evaluate corneal nerve morphology and the effect of topical autologous plasma on corneal nerve morphology in NK using the laser scanning confocal microscope.
METHODS
The protocol was approved by the Baylor College of Medicine Institutional Review Board (IRB). The medical records for the 11 eyes of six patients with NK were reviewed in this study. Inclusion criteria for diagnosis of NK included punctate keratopathy or non-healing corneal epithelial defect persisting for at least 4 weeks with no signs of active infection and no improvement following treatment with unpreserved artificial tears, and reduced corneal sensitivity (Cochet–Bonnet aesthesiometer=3.0 cm). Patients were treated with topical autologous plasma six to eight times a day for the first month, tapering to four times a day. Patients were excluded if they were using any topical medications other than non-preserved artifical tears, had ocular surgery in the past year or had any evidence of other ocular surface diseases. Patients completed the Ocular Surface Disease Index (OSDI) symptom severity questionnaire and underwent a complete ocular surface examination of both eyes in the following sequence: visual acuity measured with an ETDRS chart under standard mesopic conditions, Tomey TMS-2N computerised videokeratoscopy (CVK) measuring the Klyce Surface Regularity Index (SRI), measurement of corneal sensation with the Cochet–Bonnet aesthesiometer (Luneau, France), biomicroscopic examination of the lid margins and meibomian glands, fluorescein tear break-up time (TBUT), corneal fluorescein staining, conjunctival lissamine green staining, measurement of corneal sensation with the modified Belmonte gas aesthesiometer (Eye Research and Technology, Sydney, Australia), Schirmer I test and confocal microscopic examination of the corneal surface and subepithelial nerve plexus with the Heidelberg Retina Tomograph 2 Rostock Cornea Module (HRT2-RCM). These tests (except Cochet–Bonnet aesthesiometry and confocal microscopy) were performed as previously described.10
Preparation of autologous plasma
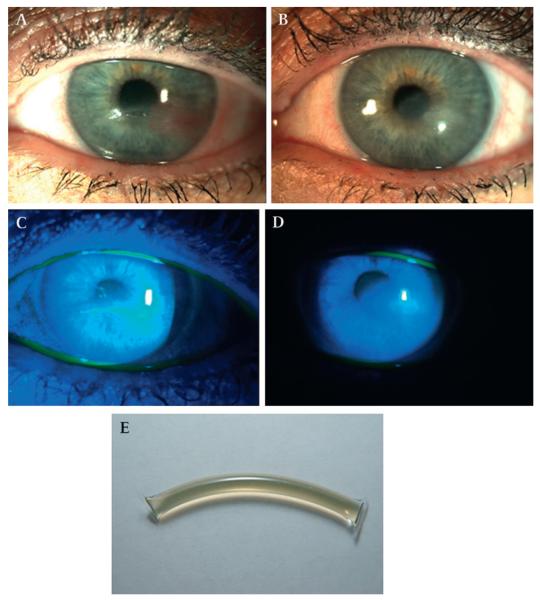
A plasmapheresis machine (Autopheresis-C, Fenwal, Illinois) was used to draw 1 unit of plasma from each patient in to a sterile plasma collection container diluted with 45 cm3 of citrate anticoagulant. The plasma was drawn into sterile intravenous tubing (Hema II Y- type blood set, ICU Medical, San Clements, CA) from the plasma collection container, and the long tubing was crimped and sealed into individual sterile tubes measuring 2.5 to 5 cm (figure 1E). Patients were instructed to store the sterile tubing in their freezer until they were used. The tubes were thawed on a daily basis as required.
Figure 1.
(A) Neurotrophic keratopathy with central corneal ulcer in a 66-year-old female with herpes zoster ophthalmicus (HZO), (table 1, case 1). (B) Central corneal epithelial defect stained with fluorescein dye. (C) The central corneal epithelial defect healed completely with notable decrease in corneal haze and vascularisation after autologous plasma treatment for 6 months. (D) No fluorescein staining seen after plasma treatment. (E) Image of single dose plasma tube.
Measurement of corneal sensitivity
Cochet–Bonnet aesthesiometer
The fully extended tip of the nylon filament was applied perpendicularly to the central surface of the cornea, taking care not to touch the eyelashes, and pushed until the fibre’s first visible bending. The length of the fibre was decreased gradually until a blink reflex was observed and was recorded in centimetres. A corneal sensitivity measurement of 3 was regarded as low corneal sensitivity in this study.
Modified Belmonte aesthesiometer
Subjects were seated in front of the gas aesthesiometer, with their chin placed in a cup and their forehead against a band. Mechanical stimulation consisted of a series of pulses of warmed air (constant temperature of 50°C at the tip of the probe) with flow varying from 0 to 200 ml/min, applied to the central corneal surface. Mechanical stimulation was determined using the method of levels. The lowest degree of air flow that elicited a response was recorded as the mechanical threshold.
Confocal microscopy
Image acquisition
Confocal microscopy of the cornea was performed with the Heidelberg Retina Tomograph 2 Rostock Cornea Module (Heidelberg Engineering GMBH, Germany).11 A drop of topical anaesthesia (proparacaine 0.5%, Alcon, Fort Worth, Texas) was applied to the lower conjunctival fornix of the patient. A drop of optical coupling medium gel (GenTeal, 0.3% hypromellose; Norvatis Ophthalmics, Basel, Switzerland) was applied to the microscope objective, and a single-use contact element (Tomocap) was placed on the microscope objective, advancing it towards the cornea, gently touching the corneal surface. Sequential imaging was performed from the level of the corneal superficial epithelial layer posteriorly to the corneal stroma.
Image analysis
Four images at the level of the subepithelial nerve plexus in the central cornea were randomly selected for analysis. The analysis was done using the freely available Image J analysis software (http://rsb.info.nih.gov/ij/).12 Parameters of corneal nerves analysed in the 400×400 square micrometre image frame included: total length, defined as the sum of the length of all the nerve fibres within a frame, given as micrometres of nerve fibre within a frame; number of nerves, defined as the sum of the nerve fibres observed within a frame; diameter of the nerve fibre, defined as the width of the nerve fibres observed within a frame.
Statistical analysis
Statistical analysis was done using GraphPad Prism (La Jolla, California). The differences in clinical parameters (BCVA, symptom severity, conjunctival and corneal dye staining scores, corneal sensitivity) and confocal nerve parameters, before and after treatment with autologous plasma tears, were calculated using the paired t test.
RESULTS
Demographic data
The demographic data for the 6 patients with neurotrophic keratopathy are presented in table 1. The 39-year-old male patient was 4 years post-LASIK, and the 69-year-old patient was 1.5 years post-LASIK. There were two female patients with diabetic neurotrophic keratopathy, and each had a history of herpes zoster ophthalmicus (HZO) further worsening their keratopathy in one eye. The 66-year-old female with HZO affecting the left eye had a normal asymptomatic right eye. The 54-year-old female with Sjögren syndrome had decreased corneal sensitivity in both eyes. The mean follow-up duration was 5 months (range 3–6 months).
Table 1.
Demographic and clinical features of patients with neurotrophic keratopathy
| Patient no |
Age | Gender | Diagnosis | Duration of plasma therapy (months) |
Resolution of keratopathy (months) |
|---|---|---|---|---|---|
| 1 | 66 | Female | HZO OS | 6 | 4 |
| 2 | 66 | Female | Diabetic neuropathy OU HZO OD |
6 | 3 |
| 3 | 39 | Male | Neuropathy s/p LASIK OU |
6 | 1 |
| 4 | 69 | Female | Neuropathy s/p LASIK OU |
3 | 1 |
| 5 | 67 | Female | Diabetic neuropathy OU HZO OS |
4 | 4 |
| 6 | 54 | Female | Sjögren syndrome | 3 | 3 |
HZO, herpes zoster ophthalmicus; LASIK, laser-assisted keratomileusis; OD, right eye; OS, left eye; OU, both eyes.
Clinical data
The clinical parameters of the patients with neurotrophic keratopathy, before and after treatment, are presented in tables 2, 3. The symptom severity score decreased significantly in all five patients treated with topical plasma from a mean of 39.5±11.2 to 16.8±6.0 (p=0.003). The BCVA improved from a mean of 0.4±0.4 before treatment to 0.16±0.22 after treatment with plasma tears (p=0.003). The patient with BCVA of 1 (20/200) in one eye showed a remarkable improvement of six lines to 0.6 (20/80) after treatment. The three eyes with BCVA of 0.6 (20/80) showed an improvement of four lines, ranging from 0.4 to 0.1 (20/50 to 20/25). There was no conjunctival lissamine green staining seen in any patient after the treatment. Corneal fluorescein staining was significantly lower after treatment than before (p=0.0003) (figure 1). The punctate keratopathy resolved in four patients completely and was markedly reduced in the patients with diabetic neuropathy and HZO and Sjögren syndrome. The mean videokeratoscopic surface regularity index (SRI) improved to 0.3±0.21 from 1.7±0.36 after plasma treatment.
Table 2.
Clinical features of patients with neurotrophic keratopathy pre- and post-treatment with autologous plasma
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS |
| BCVA preplasma | 0.6 | 0.6 | 0.1 | 0.18 | 0.18 | 0.1 | 0.1 | 0.6 | 1 | 0.5 | 0.4 |
| BCVA postplasma | 0.1 | 0.3 | 0 | 0 | 0.0 | 0 | 0 | 0.4 | 0.6 | 0.3 | 0.2 |
| Conjunctival score preplasma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 6 | 3 |
| Conjunctival score postplasma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Corneal staining preplasma | 3, epidefect | 9 | 2 | 4 | 4 | 3 | 3 | 9 | 9 | 15 | 9 |
| Corneal staining postplasma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 | 7 | 4 |
| Corneal sensation preplasma (CB) | 0 | 0 | 2 | 3 | 3 | 0 | 0 | 2 | 0 | 1 | 0 |
| Corneal sensation postplasma (CB) | 4 | 3 | 6 | 6 | 6 | 4 | 4 | 3 | 3 | 3 | 3 |
The scores for 11 eyes for the corneal fluorescein staining, conjunctival dye staining scores, best-corrected visual acuity (BCVA) and corneal sensitivity are presented before and after treatment with autologous plasma.
CB, Cochet–Bonnet aesthesiometer measuring sensitivity (cm); OD, right eye; OS, left eye.
Table 3.
Clinical features of patients with neurotrophic keratopathy pre- and post-treatment with autologous plasma
| Parameter | Symptom severity score |
BCVA (logMAR) |
Conjunctival staining score |
Corneal staining score |
Videokeratoscopic SRI |
Corneal sensitivity (CB) |
Corneal sensitivity (BM) |
|---|---|---|---|---|---|---|---|
| Preplasma | 39.5±10.2 | 0.4±0.4 | 1.2±2.52 | 6.1±4.64 | 1.7±0.36 | 0.9±1.2 | 148±55.8 |
| Postplasma | 16.8±6.0, p=0.003 |
0.16±0.22, p=0.003 |
0±0 | 1.6±4.3, p=0.0003 |
0.3±0.21, p=0.02 |
4.2±1.4, p<0.0001 |
87±19, p=0.01 |
The mean score of 11 eyes for the symptom severity scores, corneal fluorescein staining, conjunctival dye staining scores, videokeratoscopic surface regularity index (SRI), best-corrected visual acuity (BCVA) and corneal sensitivity are presented before and after treatment with autologous plasma. The symptom severity score was measured by ocular surface disease index questionnaire.
BM, Belmonte gas non-contact aesthesiometer measuring mechanical threshold in ml/min (the higher the mechanical threshold, the lower the corneal sensitivity); CB, Cochet–Bonnet aesthesiometer measuring sensitivity in centimeter units.
Corneal sensitivity
Cochet–Bonnet aesthesiometer
The mean pretreatment corneal sensitivity was 0.9±1.24 cm, which increased to 4.2±1.4 after treatment at the last follow-up visit (p<0.0001). All 11 eyes had improvement in corneal sensitivity after treatment, with six eyes attaining normal sensitivity after treatment, three of which had a sensitivity of 6, while the other three showed a sensitivity of 4. Both eyes of the patient with HZO and diabetic neuropathy, the left eye of the patient affected with HZO and both eyes of the patient with Sjögren syndrome showed some improvement in corneal sensitivity.
Modified Belmonte gas aesthesiometer
The mean pretreatment mechanical threshold measured by the modified Belmonte aesthesiometer was 148±55.8, which decreased to 87±19 following plasma therapy, representing a significant improvement in corneal sensitivity (p=0.01).
Confocal microscopy data
Representative images of the corneal nerves before and after autologous plasma treatment are shown in figures 2–5. The nerve measurements are presented in tables 4, 5.
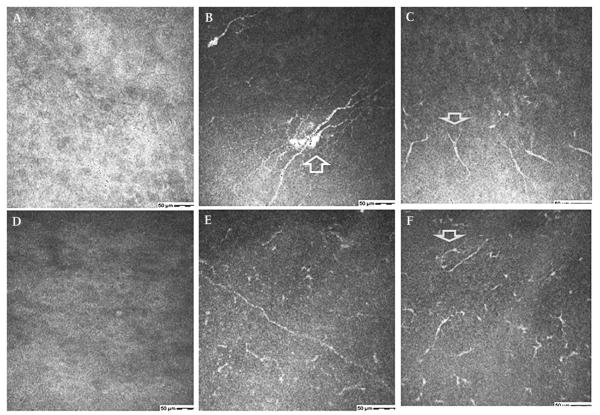
Figure 2.
(A–C) Confocal images of the sub-basal epithelial layer in a 66-year-old female with herpes zoster ophthalmicus (HZO) related neurotrophic keratopathy (table 1, case 1). (A) No nerves seen before treatment. (B) Regeneration of nerves with beading (arrow) and (C) short nerve stumps (arrow) seen after treatment. (D–F) Confocal images at the sub-basal epithelial layer in 67-year-old female with diabetes and HZO related neurotrophic keratopathy (table 1, case 5). (D) Complete absence of nerves before treatment. (E) Regeneration of nerves after 4 months of autologous plasma. (F) Nerve loops (arrow) seen after 4 months of plasma treatment.
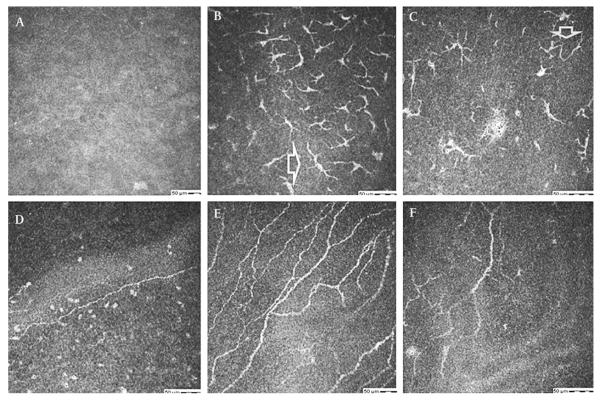
Figure 5.
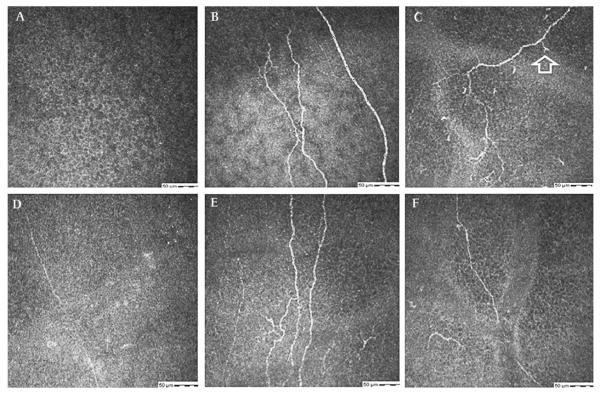
(A–F) Confocal images at the sub-basal epithelial layer in a 39-year-old male with laser-assisted keratomileusis related neurotrophic keratopathy (table 1, case 3). (A, D) Faint, barely visible nerves in right (A) and left (D) corneas, before treatment. (B–F) Regeneration of nerves after treatment with autologous plasma for 6 months.
Table 4.
Corneal nerve measurements in confocal microscopic images of patients with neurotrophic keratopathy pre and post-treatment with autologous plasma
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS |
| No preplasma | 0 | 0.5 | 0.5 | 2 | 1.75 | 0.25 | 0.25 | 1 | 0 | 1 | 1.5 |
| No postplasma | 4.25 | 8 | 6.25 | 6.5 | 5 | 2.5 | 3 | 3 | 2.75 | 4 | 3.5 |
| Length preplasma | 0 | 146.25 | 256.33 | 288 | 324.03 | 83.76 | 123.70 | 195.9 | 0 | 83.34 | 95.45 |
| Length postplasma | 998.2 | 954.13 | 1994.03 | 1760.55 | 1560.84 | 1187.75 | 757.68 | 764 | 704.23 | 567 | 789.56 |
| Width preplasma | 0 | 0.95 | 0.97 | 1.37 | 0.9 | 1.1 | 0.9 | 0.85 | 0 | 0.96 | 0.9 |
| Width postplasma | 2.4 | 2.3 | 2.6 | 3.1 | 2.9 | 2.3 | 2.0 | 2.4 | 2.3 | 2.6 | 2.7 |
The scores of the number, total length and width of nerves in four, 400×400 square micrometre frame are presented before and after treatment with autologous plasma.
Table 5.
Corneal nerve measurements in confocal microscopic images of patients with neurotrophic keratopathy pre and post-treatment with autologous plasma
| Parameter | No of nerves (per frame) | Total length (μm/frame) | Diameter of nerve fibre (μm/frame) |
|---|---|---|---|
| Preplasma tears | 0.75±0.81 | 136.7±127.9 | 0.8±0.6 |
| Postplasma tears | 4.81±1.98, p=0.0004 | 1362.6±559.01, p=0.0002 | 2.0±0.4, p=0.001 |
The mean scores of 11 eyes for number, total length and width of nerves per 400×400 square micrometre frame are presented before and after treatment with autologous plasma.
Number of nerves
The mean number of nerves after treatment increased in all 11 eyes from 0.75±0.81 (range 0–2 per frame) to 4.81±1.98 (range 3–8 per frame) p=0.0004. Two eyes with HZO with no observable nerves in any of the frames before treatment showed an increase to 4.25 and 2.75 nerves per frame, respectively.
Total length of nerve fibres
The total length of nerves increased in all 11 eyes from 136.7±127.9 μm to 1362.6±559.01 μm (p=0.0002). Two eyes with HZO and a total length of zero seen in all frames before treatment showed an increase to 269.45 μm per frame in one patient and an increase to 1088.55 μm per frame in another patient with HZO.
Diameter of nerve fibres
The mean pretreatment nerve diameter was 0.8±0.6 μm, and the mean post-treatment nerve diameter was 2.0±0.4 μm (p=0.006).
Nerve morphology
In three eyes with HZO which had 0–1 nerves before treatment, the regenerated nerves after plasma treatment showed varied morphology ranging from short stumps to long fibres with beadings, loops or flower like sprouts (figures 2, 3). The sprouts of nerves with a flower like appearance resembled the dendritic cells frequently seen in the subbasal layer; however, these nerve sprouts had a mean length of 120.5±20 μm compared with dendritic cells, which have been reported to have a diameter of up to 15 μm.13 The four eyes in patients who had undergone LASIK showed a range of 0–2, thin, barely visible single nerve fibres in some frames before plasma treatment and had a significant increase in the number and width of nerves after treatment (figures 4, 5).
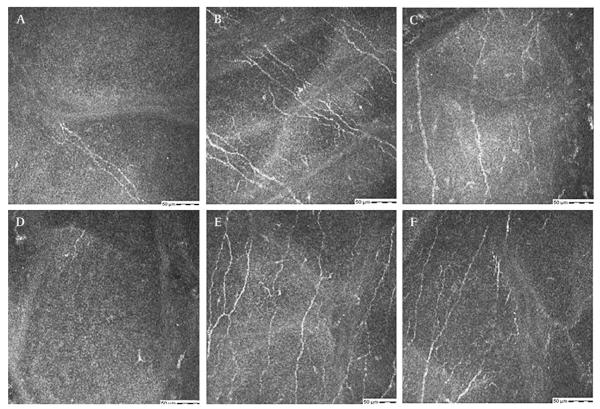
Figure 3.
(A–F) Confocal images at the sub-basal epithelial layer in a 66-year-old female with diabetes and herpes zoster ophthalmicus (HZO) related neurotrophic keratopathy (table 1, case 2). (A) Single short nerve stump in the right eye with previous HZO before treatment. (B) Regeneration of nerves with numerous branching nerves (arrow) and (C) nerve loops (arrow) seen after plasma treatment for 6 months. (D) Single nerve, left eye before treatment. (E, F) Regenerated nerves seen after autologous plasma treatment.
Figure 4.
(A–F) Confocal images at the sub-basal epithelial layer in a 69-year-old female with laser-assisted keratomileusis related neurotrophic keratopathy (table 1, case 4). (A) No nerves right cornea before treatment. (B) Regenerated nerves and (C) nerve sprouts (arrow) seen after 3 months of plasma treatment. (D) Single faint barely visible nerve, left cornea before treatment. (E, F), Regeneration of nerves after treatment with autologous plasma for 3 months.
DISCUSSION
Neurotrophic keratopathy is a common and under-reported cause of corneal blindness worldwide. As the number of corneal refractive surgeries increases, the incidence of neurotrophic keratopathy is likely to increase in the future due to delayed and abnormal nerve renervation.14,15 Furthermore, an increase in neuropathy related corneal disease from diabetes mellitus, herpes zoster and ophthalmic surgery is likely to occur in the ageing population in developed countries.2 The increase in neurotrophic keratopathy will present therapeutic challenges to ophthalmologists, especially when conventional treatment regimens including non-preserved artficial tears, patching and bandage contact lenses fail. Various new treatment modalities such as fibronectin eye-drops, nerve growth factor drops and serum eye-drops have been reported to improve neurotrophic keratopathy.7,8,16 Autologous serum application has been reported to improve corneal sensation and epithelial disease significantly in neurotrophic keratopathy.7 Regeneration of corneal nerves following topical fibronectin therapy in neurotrophic keratopathy has been noted by confocal microscopy in a previously reported case report.8 The purpose of our study was to evaluate the effect of autologous plasma on corneal nerve function and appearance in neurotrophic keratopathy. Autologous plasma was used in our patients because a protocol preparing sterile unit dose phials of plasma has been developed by our blood bank, and plasma retains clotting proteins which may bind higher levels of serum and platelet-derived growth factors.
We observed complete resolution of corneal epithelial disease in seven eyes and a significant improvement in four eyes. There was a significant increase in BCVA after autologous plasma treatment in all the patients. Improvement in the epithelial surface, as well as increased corneal surface regularity measured by the videokeratoscope, appears to have contributed to the improvement in BCVA in our patients. There was a significant improvement in corneal sensitivity in treated eyes, with six eyes attaining sensitivity levels of 4, which is considered to be in the normal range. This correlated with an increase in the number, length, width and density of corneal nerves seen by confocal microscopy after treatment with the autologous plasma. Paradoxically, patients with neurotrophic epitheliopathy complained of eye irritation, despite their decreased sensitivity and nerve disease. This may be due to hypersensitivity of the remaining nerve endings.17 Plasma tears may contain various neurotrophic factors which may help to regenerate the corneal nerves. The mechanism responsible for the actual nerve regeneration however, remains to be determined.
The morphological features of corneal nerve degeneration and regeneration remain to be clearly delineated. Increased corneal nerve tortuosity seen in diabetic neuropathy, has been hypothesised to be a morphological marker of nerve regeneration.18 Nerve sprouting, indicating nerve regeneration in the sub-basal nerve fibre bundles as seen by confocal microscopy, has been reported in Sjögren syndrome.19
In our patients with HZO neuropathy, the nerve morphology noted after treatment was varied; the loop or flower patterns seen in some eyes suggest that actual nerve regeneration may have occurred. The post-LASIK patients showed thin barely visible nerves before treatment with plasma tears. There was a marked increase in the diameter, as well as the number of nerves after plasma treatment in these patients. The sub-basal nerve fibres bundles are composed of several axons surrounded by a Schwann cell sheath.20 It is possible that the extremely thin nerves noted in the corneas of patients with prior LASIK are due to loss of the Schwann cells, which makes them difficult to visualise by confocal microscopy. It is possible that plasma tears speeds regeneration of the Schwann cells in the nerve fibres, thereby increasing their thickness and rendering them easier to visualise by confocal microscopy. The results of this study suggest that autologous plasma therapy is capable of promoting nerve regeneration in neurotrophic keratopathy. However, the limitations of the study are the small sample size and the non-comparative nature of the study. It is possible that the nerve regeneration may not be a direct effect of autologous plasma therapy and may be secondary to a better tissue environment due to improvements in epithelial healing, inflammatory response and tear-film changes. The results of this study suggest that autologous plasma therapy is capable of promoting nerve regeneration in neurotrophic keratopathy either directly or as a secondary effect. Autologous plasma may speed recovery of post-LASIK hypo-aesthesia, and neurotrophic keratopathy due to other causes. Further prospective studies, to assess the potential of autologous plasma in promoting corneal nerve regeneration in neurotrophic keratopathy, are warranted.
Acknowledgments
Funding NIH Grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, The William Stamps Farish Fund, The Hamill Foundation.
Footnotes
Competing interests None.
Ethics approval Ethics approval was provided by the Baylor College of Medicine Institutional Review Board (IRB).
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Lambiase A, Rama P, Aloe L, et al. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10:270–6. doi: 10.1097/00055735-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Goins M. New insights into the diagnosis and treatment of neurotrophic keratopathy. Ocul Sur. 2005;2:96–110. doi: 10.1016/s1542-0124(12)70158-1. [DOI] [PubMed] [Google Scholar]
- 3.Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Sur. 2005;3:194–202. doi: 10.1016/s1542-0124(12)70206-9. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds SA, Kabat AG. Therapeutic options for the management of early neurotrophic keratopathy: a case report and review. Optometry. 2006;77:503–7. doi: 10.1016/j.optm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Nagano T, Nakamura M, Nakata K, et al. Effects of substance P and ICF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:3810–5. doi: 10.1167/iovs.03-0189. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh HD, Colley AM. The molecular basis of neurotrophic keratitis. Acta Ophthalmol Suppl. 1989;192:115–34. doi: 10.1111/j.1755-3768.1989.tb07103.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–20. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Morishige N, Komatsubara T, Chikama T, et al. Direct observation of corneal nerve fibers in neurotrophic keratopathy by confocal biomicroscopy. Lancet. 1999;354:1613–14. doi: 10.1016/s0140-6736(99)04198-7. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 10.De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141:438–45. doi: 10.1016/j.ajo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Eckard A, Stave J, Guthoff RF. In vivo investigations of the corneal epithelium with the confocal Rostock Laser Scanning Microscope (RLSM) Cornea. 2006;25:127–31. doi: 10.1097/01.ico.0000170694.90455.f7. [DOI] [PubMed] [Google Scholar]
- 12.Lysetska M, Knoll A, Boehringer D, et al. UV light-damaged DNA and its interaction with human replication protein A: an atomic force microscopy study. Nucleic Acids Research. 2002;30:2686–91. doi: 10.1093/nar/gkf378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhivov A, Stave J, Vollmar B, et al. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243:1056–61. doi: 10.1007/s00417-004-1075-8. [DOI] [PubMed] [Google Scholar]
- 14.Calvillo MP, McLaren JW, Hodge DO, et al. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–6. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 15.Lee BH, McLaren JW, Erie JC, et al. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–4. [PubMed] [Google Scholar]
- 16.Joo MJ, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122:1338–41. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: Is it real? Ocul Surf. 2009;7:28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 18.Kallinikos P, Berhanu M, O’Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–22. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 19.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–9. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 20.Müller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]