Abstract
Ipilimumab (MDX-010, Yervoy; Bristol-Myers Squibb), a fully human monoclonal antibody against CTL antigen 4 (CTLA-4), was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastatic melanoma. In both early- and late-phase trials, ipilimumab has shown consistent activity against melanoma. For example, in a randomized phase III trial that enrolled patients with previously treated metastatic disease, ipilimumab, with or without a peptide vaccine, improved overall survival: Median overall survival was 10.1 and 10.0 months in the ipilimumab and ipilimumab plus vaccine arms, respectively, versus 6.4 months in the vaccine-alone group (hazard ratio, 0.68; P ≤ 0.003). Serious (grade 3–5) immune-related adverse events occurred in 10% to 15% of patients. Thus, although it provides a clear survival benefit, ipilimumab administration requires careful patient monitoring and sometimes necessitates treatment with immune-suppressive therapy. Here, we review the mechanism of action, preclinical data, and multiple clinical trials that led to FDA approval of ipilimumab for metastatic melanoma.
Introduction
T cells, both CD4 (helper) and CD8 (cytotoxic), contribute to the adaptive immune response against pathogens and tumors, and activation and recruitment of specific T cells constitute a complex process. For a T cell to become fully activated (and subsequently proliferate and mediate effector function), at least 2 receptor–ligand interactions are required. The first of these occurs when the unique receptor of the T cell recognizes its cognate ligand, a short peptide presented in the context of a MHC molecule. This interaction is exquisitely specific, and if a good fit occurs, T-cell activation is initiated. However, full activation of a CD4 or CD8 T cell requires a second signal transmitted by costimulatory molecules present on the same antigen-presenting cell that expresses the peptide/MHC. This second signal is transmitted from costimulatory molecules (B7.1 and/or B7.2) to a receptor on T cells known as CD28. Only when both signals are received and integrated does a specific T cell proliferate, acquire effector function, and migrate to sites of antigen expression.
CTL antigen 4 (CTLA-4) was first cloned in 1987 (1). Subsequent studies showed this molecule to be a homolog of CD28, suggesting that CTLA-4 might serve, along with CD28, as a costimulatory molecule (2). However, several other studies provided opposing results, and for some time, it was not clear whether CTLA-4 transmitted a stimulatory or inhibitory signal to T cells. The generation of mice lacking CTLA-4 provided a solution for this conundrum: Knockout mice developed a progressive accumulation of activated T cells and died of lymphoproliferative disease ~3 to 4 weeks after birth (3). These and other results (4) suggested that blockade of CTLA-4 with a monoclonal antibody could augment an adaptive immune response to an infectious agent or an evolving tumor. The seminal study in this area (5) showed that CTLA-4 blockade could attenuate the growth of several implanted murine tumors, consistent with the model shown in Fig. 1. On an immunologic basis, this model of T-cell activation and the function of CTLA-4 represents a significant simplification. A more complete description can be found in several relevant reviews (6, 7).
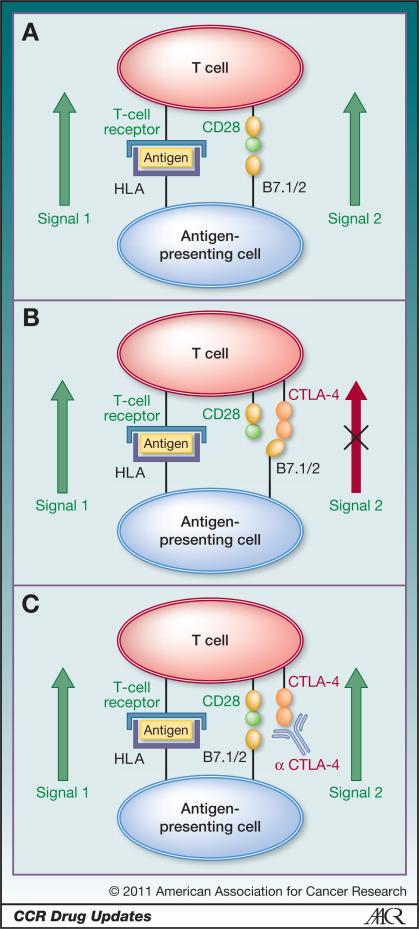
Figure 1.
A, melanoma cells express proteins, such as gp100, MART-1, and tyrosinase, that may be processed and presented by antigen-presenting cells. T-cell recognition of peptide antigens derived from these proteins can potentially drive an antitumor immune response. For such tumor-directed T cells to become activated, 2 signals are required. The first signal (signal 1) occurs when the peptide antigen is recognized by the T-cell receptor on antigen-specific T cells. This recognition is exquisitely specific. However, for full T-cell activation, proliferation, and effector function, a second signal (signal 2) is needed. This signal is typically mediated by the interaction between costimulatory molecules such as B7.1 and B7.2 on antigen-presenting cells and CD28 on T cells. In reality, the situation is more complex, and signal 2 is derived from the integration of several positive and negative events. B, under certain conditions, T cells upregulate the cell surface molecule CTLA-4, which binds to B7.1 and B7.2 with greater affinity than does CD28. This series of events effectively hijacks signal 2, resulting in a situation in which specific T cells cannot be fully activated. C, ipilimumab (and other anti-CTLA-4 antibodies) bind to CTLA-4 on the cell surface, effectively blocking the interaction between CTLA-4 and B7.1/B7.2. This leads to interruption of the negative signal mediated by CTLA-4, the resumption of signal 2, and a relative restoration of T-cell activation.
Early Clinical Development
To translate these findings into a clinical setting, the Medarex Corporation generated a series of monoclonal antibodies using a unique transgenic mouse (HuMAb), in which the endogenous murine immunoglobulin genes have been knocked out and replaced with human loci (8). Immunization of these mice results in fully human monoclonal antibodies devoid of murine sequences that can lead to infusion reactions. The clinical development of MDX-010 was recently reviewed (8); only several selected trials that are most relevant in terms of the current U.S. Food and Drug Administration (FDA) approval for use in melanoma will be outlined here.
Initial phase I studies included both single and repetitive dosing regimens and showed safety and an intriguing suggestion of efficacy (8). In subsequent trials at the National Cancer Institute, investigators administered ipilimumab, first at 3 mg/kg along with a gp100 multipeptide vaccine, and then at doses between 1 and 3 mg/kg (9, 10). They observed sustained responses (>2 years) in several patients. Immune-related adverse events (irAE) were also seen and seemed to correlate with clinical response. An important phase I/II study to determine the pharmacokinetic profile of MDX-010 was conducted in patients with metastatic melanoma (11). A secondary endpoint of this study (MDX-015) was clinical activity, and the study included both single- and multiple-dosing regimens. Escalating single doses of up to 20 mg/kg, as well as every-3-weeks dosing at 10 mg/kg, were examined. Although the study was not adequately powered to compare regimens, the group of patients who received 10 mg/kg every 3 weeks had the highest disease control rate. This study also confirmed the toxicity profile noted in earlier trials, with 19% of patients experiencing grade 3 or 4 adverse events. Many of these events were irAEs, predominantly colitis, rash, and liver function abnormalities. In this study, irAEs appeared to be somewhat correlated with response and were observed in 13 of 14 patients with stable disease, as well as in all 4 patients with an objective response.
Based partially on these findings, a randomized, multi-institution, double-blind, dose-ranging study was performed (12). This study (CA184-022) enrolled 217 patients with previously treated metastatic melanoma, and the patients were randomized to doses of 0.3, 3, or 10 mg/kg MDX-010 administered every 3 weeks. Patients without progressive disease at week 24 entered into a maintenance phase in which MDX-010 continued to be administered every 12 weeks. The primary endpoint of this study was efficacy. The best overall response rates were 0%, 4.2%, and 11.1% in the 0.3-mg/kg, 3-mg/kg, and 10-mg/kg groups, respectively. Survival data were encouraging, with 30% of patients in the 10-mg/kg cohort alive at 2 years, as opposed to 18% in the 0.3-mg/kg cohort. IrAEs were again noted in 0%, 5%, and 18% of patients in the 3 dose cohorts. These findings supported the efficacy of MDX-010 and reinforced the every-3-weeks dosing regimen that was subsequently adapted for phase III investigation.
In terms of combination studies, the safety and efficacy of ipilimumab administered with dacarbazine was first investigated in a phase II trial that randomized 72 chemotherapy-naïve patients to receive ipilimumab at 3 mg/kg every 4 weeks for 4 doses either alone or with dacarbazine (13). The primary endpoint, the objective response rate, was 14.3% [95% confidence interval (CI), 4.8–30.3] with ipilimumab plus dacarbazine and 5.4% (95% CI, 0.7–18.2) with ipilimumab alone; that is, there was a trend toward an increased response rate in the combination treatment group.
Late-Stage Development
Positive results from a pivotal phase III trial of MDX-010 were reported in 2010 (14). This study randomized 676 HLA-A*0201-positive, previously treated melanoma patients 1:3:1 to ipilimumab alone, ipilimumab plus a gp100 vaccine, or gp100 vaccine alone. Mirroring the phase II schedule, ipilimumab was administered every 3 weeks for up to 4 doses at 3 mg/kg. The primary endpoint of this study, overall survival, was significantly better in the groups receiving ipilimumab than in the group receiving gp100 vaccine alone: Median overall survival was 10.1 and 10.0 months in the ipilimumab and ipilimumab plus vaccine arms, respectively, versus 6.4 months in the vaccine-alone group [hazard ratio (HR), 0.68; P ≤ 0.003]. The best overall response rate was 10.9% in the ipilimumab-alone cohort, compared with 5.7% in the ipilimumab plus vaccine group and 1.5% for vaccine alone. The longitudinal survival benefit suggested by phase II studies was confirmed, with 22% of ipilimumab-alone patients alive at 24 months, versus 24% for ipilimumab plus vaccine and 14% for vaccine alone. The rate of grade 3/4 irAEs was also comparable with that observed in previous studies: 10% to 15% in the ipilimumab groups compared with 3% in the gp100 group. This study was especially significant in that it was the first randomized study ever to show a survival benefit in patients with metastatic melanoma.
Similar positive results were seen when ipilimumab was administered as first-line therapy (15). In a randomized phase III study, 502 patients with previously untreated metastatic melanoma were randomized 1:1 to ipilimumab (10 mg/kg) plus dacarbazine (850 mg/m2) or dacarbazine plus placebo, given at weeks 1, 4, 7, and 10, followed by dacarbazine alone every 3 weeks through week 22. Patients with an objective response or stable disease and no dose-limiting adverse effects received maintenance ipilimumab or placebo every 12 weeks thereafter. Overall survival (the primary endpoint of the trial) at 1 year in the ipilimumab–dacarbazine group was 47%, compared with 36% in the dacarbazine–placebo group. Grade 3/4 adverse events were noted more frequently than in trials not involving chemotherapy (56% ipilimumab plus dacarbazine, 28% dacarbazine plus placebo). Of interest, the pattern of irAEs was different, with more frequent hepatotoxicity (~20%) and less frequent colitis (2%). Overall, this study played an important role in bringing ipilimumab into the first-line setting for metastatic melanoma and in showing that the patterns of toxicity of this agent can vary depending on the context in which it is administered. It should be noted that although this study provided information about the safety and tolerability of ipilimumab dosed at 10 mg/kg, the FDA approved the drug at a dosage of 3 mg/kg.
Integration of Ipilimumab into the Melanoma Treatment Paradigm
Currently, few therapeutic options are available for patients with unresectable American Joint Committee on Cancer stage III or stage IV melanoma. Chemotherapy regimens include paclitaxel alone or with a platinum agent, temozolomide, or dacarbazine. Immune therapies include dacarbazine- or temozolomide-based biochemotherapy and high-dose interleukin-2 (IL-2), which result in clinical responses in ~15% to 20% of patients, about one third of whom experience durable, complete responses (16, 17). Administration of IL-2, however, is limited to patients with an excellent performance status who have no evidence of cardiac dysfunction or active central nervous system metastases. Given these constraints, the addition of ipilimumab to the therapeutic armamentarium is particularly welcome because it expands the population of patients who can receive immunotherapy. Indeed, the National Comprehensive Cancer Network (NCCN) has added ipilimumab as a category 1 recommendation in its guidelines of systemic therapy options for advanced or metastatic melanoma (18).
In clinical practice, the order in which a patient receives different therapies may become relevant, particularly if irAEs are encountered. Although high-dose IL-2 administration may be complicated by side effects around the time of drug delivery, such side effects will generally resolve completely within several weeks. The same is not true with certain of the irAEs seen with ipilimumab (19). Some patients who develop ipilimumab-related pan-hypopituitarism, for example, will require long-term replacement with multiple steroid hormones, potentially disqualifying them from receiving IL-2. It may make sense, then, for patients who could tolerate either IL-2 or ipilimumab as initial therapy to consider beginning treatment with IL-2.
The irAEs associated with ipilimumab treatment (Table 1) necessitate careful patient selection as well as thorough and frequent patient monitoring. The NCCN guidelines note “Ipilimumab should be used with extreme caution, if at all, in patients with serious underlying autoimmune disorders.” Bristol-Myers Squibb, in collaboration with the FDA, has developed a Risk Evaluation and Mitigation Strategy program designed to facilitate early identification and appropriate management of patients with moderate or severe irAEs. In addition, comprehensive toxicity management algorithms have been developed and are included in the ipilimumab package insert. It is important to note that the vast majority of these irAEs are reversible with early intervention. Moreover, it appears that the majority of antitumor responses persist despite corticosteroid therapy (11).
Table 1.
Presentation of immune-related adverse events
| Area affected | Symptoms at presentation |
|---|---|
| Gastrointestinal system | Diarrhea, abdominal pain, blood in stools, increased frequency of stools, nausea, vomiting, or constipation, with or without fever; complications may include intense bleeding, bowel perforations, intense diarrhea, or need for colectomy |
| Skin | Rash, usually maculopapular and often accompanied by significant generalized pruritus |
| Liver | Right upper quadrant abdominal pain, nausea, vomiting, elevated aspartate aminotransferase/alanine aminotransferase, or hyperbilirubinemia in the absence of clinical symptoms |
| Nervous system | Muscle weakness or sensory neuropathies, Guillain-Barré syndrome |
| Endocrine system | Nonspecific symptoms, including headache, visual-field defects, behavioral changes, decreased libido, fatigue, new onset of atrial fibrillation, weakness, asthenia, anorexia, nausea and vomiting, lethargy, impotence, amenorrhea, fever, coma, hypotension, hypoglycemia, hyponatremia, and eosinophilia |
A further unresolved question involves the concept of ipilimumab maintenance therapy. It should be noted that in the ipilimumab plus dacarbazine trial, patients who had stable disease or an objective response and no dose-limiting toxic effects after receiving 4 doses of ipilimumab (10 mg/kg) at weeks 1, 4, 7, and 10 received ipilimumab or placebo every 12 weeks thereafter as maintenance therapy. This treatment was administered until disease progression or development of toxicities occurred, or until conclusion of the study. This design was similar to that used in the aforementioned phase II trial CA184-022. Likewise, Hodi and colleagues (14) offered additional courses of a participant's assigned treatment (reinduction) to patients with stable disease of 3 months’ duration after week 12 or a confirmed partial or complete response. In that study, ipilimumab was dosed at 3 mg/kg. FDA approval for ipilimumab does not include maintenance dosing. Although maintenance or reinduction therapy was included in the above studies and both approaches are currently being examined in several nonmelanoma trials (e.g., CA184-043 and CA184-095 for prostate cancer), the role of these therapies is still unclear.
Future Directions
In addition to its documented activity in metastatic melanoma, ipilimumab has shown early evidence of clinical activity in several additional tumor types, including renal cell, lung, and prostate cancers. Two ongoing randomized phase III studies are evaluating ipilimumab in patients with metastatic castrate-resistant prostate cancer either before (CA184-095) or after (CA184-043) administration of docetaxel. In addition, phase II studies combining ipilimumab with standard chemotherapy or other immunotherapy modalities are ongoing in a number of tumor types (NCT01331525, NCT01194271).
Combining ipilimumab with targeted therapy, such as the BRAF inhibitor vemurafenib, will almost certainly become an area of active investigation in the near future. A recent report by Chapman and colleagues (20) showed improved survival for patients with stage IV melanoma treated with vemurafenib compared with standard chemotherapy with dacarbazine. At 6 months, overall survival was 84% in the vemurafenib group and 64% in the dacarbazine group. Vemurafenib has a remarkably high response rate (48% for vemurafenib vs. 5% for dacarbazine) in patients with V600E mutated melanoma, and thus it may be well suited to decrease or mitigate a tolerogenic tumor burden. Additionally, in vitro data suggesting that vemurafenib does not lead to decreased adaptive immunity (21) lend further support to the upcoming trial of vemurafenib plus ipilimumab for patients with BRAF mutation–positive melanoma (NCT01400451).
Another interesting issue regarding the future development of ipilimumab involves its combination with cancer vaccines. Indeed, many of the preclinical studies of this agent showed remarkable enhancement of activity when it was combined with anticancer vaccines, particularly cell-based vaccines that secrete granulocyte macrophage colony-stimulating factor. Some of the earliest published data in this regard showed that the combination of anti-CTLA-4 plus GVAX was able to cure established, poorly immunogenic B16 melanomas, a result that was not achievable with either agent alone (22). However, these results were not reflected in the pivotal phase III trial discussed above (14). This apparent discrepancy most likely stems from the nature of the gp100 vaccine used (2 epitopes administered s.c. in incomplete Freund's adjuvant). Comparative data supporting the clinical efficacy of this particular gp100 vaccine were not striking. In a randomized phase III trial, in combination with IL-2, the vaccine showed a small but statistically significant improvement in progression-free survival (2.2 vs. 1.6 months) but no significant improvement in overall survival (23). It is critically important to note that negative data derived from one particular vaccine should not be misinterpreted as evidence that other ipilimumab/vaccine combinations could not confer additive or synergistic efficacy.
With the best overall response rates in the 10% to 15% range in most published studies, it appears that ipilimumab is not effective in the vast majority of patients treated. In addition, the significant rate of irAEs and other adverse events compels the development and prospective validation of a predictive biomarker that would allow pretreatment selection of patients likely to benefit from treatment. Unfortunately, no such marker appears imminent, despite the ~3,500 patients treated with this agent to date (8). Some insight in this area was provided by the recent observation that patients with an absolute lymphocyte count ≥1,000/μL after 2 ipilimumab treatments had a significantly improved clinical benefit rate and median overall survival compared with patients with a count of <1,000/μL (24). Future studies, perhaps involving paired tumor biopsy analysis or serum antibody profiling, are clearly needed to identify those patients whose disease is most likely to respond to ipilimumab.
Conclusions
The development and FDA approval of ipilimumab represent a major step forward in cancer immunotherapy. Although multiple phase III trials have confirmed a survival benefit for this agent, perhaps the most compelling data are those showing a small but significant proportion of patients with metastatic melanoma surviving 2 and even 3 years from the time of treatment initiation. Given the significant rate of irAEs, clinical application of ipilimumab is challenging and requires careful monitoring and prompt attention to potential adverse events. Future clinical research will likely address the development and verification of predictive biomarkers, permitting the identification of a subgroup of patients most likely to derive clinical benefit. Perhaps more exciting, though, is the potential for additive and/or synergistic efficacy, which may be achieved by combining ipilimumab with targeted agents such as vemurafenib, with radiotherapy, or perhaps with cancer vaccines that optimally prime a tumor-specific T-cell response.
Acknowledgments
Grant Support
NIH (R01 CA127153 and 1P50CA58236-15), Patrick C. Walsh Fund, OneInSix Foundation, and Prostate Cancer Foundation. E.J. Lipson was supported by the John P. Hussman Foundation and an NIH/NCI T32 training grant.
Footnotes
Disclosure of Potential Conflicts of Interest
C.G. Drake is a clinical investigator for Damon Runyon-Lilly; serves as a consultant for Dendreon and Pfizer; has served as a paid consultant for Bristol-Myers Squibb and, through Johns Hopkins University, has licensed patents to that entity; and has an ownership interest in Amplimmune and Bristol-Myers Squibb. E.J. Lipson disclosed no potential conflicts of interest.
References
- 1.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 4.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 5.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 6.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–65. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 7.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–46. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 13.Hersh EM, O'Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489–98. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 16.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–19. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network [2011 Oct 21]; Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.
- 19.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16:6040–8. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]