Abstract
Risks associated with brain biopsy limit availability of tissues and the role of brain biopsy in diagnosing neurodegeneration is unclear. We developed a simulated brain biopsy paradigm to comprehensively evaluate potential accuracy of detecting neurodegeneration in biopsies. Postmortem tissue from the frontal, temporal and parietal cortices and basal ganglia from 73 cases including Alzheimer’s disease (AD), Lewy body disease (LBD), frontotemporal lobar degeneration-TDP43 (FTLD-TDP), multiple system atrophy (MSA), Pick’s disease (PiD), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) were evaluated using H&E and immunostains. Brain biopsy was simulated in a blinded manner by masking each slide with opaque tape except for an area measuring 10 mm in diameter. Diagnoses obtained from frontal cortex only or all 4-brain regions were then compared with autopsy diagnoses. Diagnostic sensitivity in frontal cortex was highest in FTLD-TDP (88%), AD (80%) and LBD (79%); intermediate for MSA (71%), CBD (66%) and PiD (66%) and lowest for PSP (0%) (average 64%). Specificity was 43%. Sensitivities were enhanced with all 4-brain regions: FTLD-TDP (100%), AD (80%), LBD (100%), MSA (100%), CBD (83%), PiD (100%) and PSP (88%) (average 92%). Specificity was 71%. Simulated brain biopsy addressed limitations of standard brain biopsies such as tissue availability and lack of autopsy confirmation of diagnoses. These data could inform efforts to establish criteria for biopsy diagnosis of neurodegenerative disorders to guide care of individuals who undergo biopsy for enigmatic causes of cognitive impairment or when evidence of an underlying neurodegenerative disease may influence future therapy.
Keywords: Neurodegeneration, Biopsy, Diagnosis, Alzheimer’s disease, Tauopathies, Synucleinopathies
Introduction
Definitive diagnosis of neurodegenerative disorders during life is frequently challenging. Currently, histopathologic analysis of postmortem brains remains the gold standard for definitively diagnosing neurodegenerative diseases. As new therapies emerge, early, accurate diagnosis of neurodegenerative diseases is of paramount importance. Recently, a working group organized by the National Institute on Aging and the Alzheimer’s associations have revised criteria for the diagnosis of Alzheimer’s disease and mild cognitive impairment to include advanced imaging modalities and biomarkers with the goal of early clinical recognition [2, 13, 22, 34]. As newer diagnostic modalities continue to emerge, diagnostic criteria for AD as well as other neurodegenerative disorders will continue to evolve. However, the role of brain biopsy in these evolving diagnostic paradigms is unclear.
Limited data are available regarding brain biopsy in neurodegenerative disorders. This is largely because most brain biopsies are performed in younger patients where clinical and laboratory findings point towards a likelihood of a reversible process (such as vasculitis or an inflammatory process) where biopsy can potentially alter clinical management [33, 36, 37]. The invasive nature and complications associated with the biopsy procedure are also significant issues to be considered before a biopsy is obtained, especially in the elderly [36]. Several studies have examined the utility of brain biopsy in enigmatic neurological diseases including neurodegeneration with diagnostic yields ranging from 20 to 84% [4, 14, 15, 30, 32, 33, 36, 37]. However, once a patient is biopsied, it is often challenging to follow up these patients to autopsy to determine the cause of dementia [18, 33, 36]. Thus definitive autopsy confirmation of biopsy diagnosis of the specific neurodegenerative disease is often not possible and no clear data are available with respect to diagnosing neurodegenerative disorders on biopsy. The ability to accurately diagnose a neurodegenerative disease may become increasingly important as newer therapies emerge targeting specific disease processes and with the growing prevalence of dementia in an increasingly ageing population who may undergo brain biopsy for diverse neurological disorders to guide treatment that could be altered by the detection of an additional concomitant neurodegenerative disorder. Moreover, current advances in imaging and biomarkers have focused on relative more common neurodegenerative diseases like AD, Parkinson’s disease (PD) and FTLD [12, 38]. Thus, brain biopsy to diagnose neurodegenerative disorders may have more clinical value in coming years, but it requires further evaluation, and the current study is an effort to set parameters for how this can be accomplished.
To address these gaps in our knowledge and overcome limitations inherent in current brain biopsy diagnostic practice, we developed a simulated paradigm of brain biopsy using postmortem tissue. This approach overcomes issues of tissue availability, complications associated with brain biopsy and the lack of autopsy confirmation of brain biopsy diagnosis. This paradigm was designed to assist neuropathologists and clinicians involved in the care of patients with neurological disorders requiring brain biopsy to establish specific diagnostic criteria in the future for evaluating biopsy specimens from patients with a neurological disorder the treatment of which may be influenced by the detection of an additional concomitant neurodegenerative disease.
Methods
Cases and simulated biopsy
Archival formalin-fixed brain tissues were obtained from the University of Pennsylvania Center for Neurodegenerative Diseases brain bank and included 10 AD, 9 dementia with Lewy bodies (DLB), 10 PD, 6 CBD, 9 PSP, 6 PiD, 9 FTLD-TDP (Tar DNA binding protein 43), 7 MSA and 7 age-matched control cases (total n = 73). The clinical characteristics of these cases are detailed in Table 1. From each of these cases sections from the mid-frontal cortex, parietal cortex (angular gyrus), superior temporal gyrus and lentiform nucleus were studied.
Table 1.
Case demographics
| Disease
|
Control | |||||||
|---|---|---|---|---|---|---|---|---|
| AD | CBD | FTLD-TDP | LBD | MSA | PSP | PiD | ||
| Number (n) | 10 | 6 | 9 | 19 | 7 | 9 | 6 | 7 |
| Sex (M:F) | 2:8 | 2:4 | 7:2 | 13:6 | 4:3 | 6:3 | 3:3 | 2:5 |
| Age, mean (SD) | 81.4 (8.7) | 66.7 (9.6) | 65.0 (11.9) | 77.8 (7.8) | 68.7 (5.9) | 73.7 (7.4) | 65.5 (8.2) | 78.0 (9.4) |
| Brain weight, mean (SD) Braak stage | 1,055 (140) | 1,093 (90) | 1,117 (166) | 1,293 (178) | 1,363 (188) | 1,205 (106) | 1,046 (194) | 1,261 (107) |
| 0 | - | 4 | 3 | 5 | 2 | 3 | 3 | 1 |
| I–II | - | 2 | 5 | 8 | 4 | 6 | 3 | 5 |
| III–IV | 1 | - | 1 | 6 | 1 | - | - | 1 |
| V–VI | 9 | - | - | - | - | - | - | - |
| Median | V–VI | 0 | I–II | I–II | I–II | I–II | I–II | I–II |
| Ventricular enlargement | ||||||||
| None | 1 | 1 | - | 9 | 4 | 1 | - | 3 |
| Mild | 3 | - | 3 | 6 | 2 | 5 | - | 3 |
| Mod | 2 | 3 | 2 | 4 | 1 | 3 | 3 | 1 |
| Severe | 4 | 2 | 4 | - | - | - | 3 | - |
| Median | Mod | Mod | Mod | Mild | None | Mild | Mod | Mild |
| Atherosclerosis | ||||||||
| None | 1 | 3 | 3 | 4 | 3 | 2 | 5 | 4 |
| Mild | 6 | 3 | 3 | 4 | 1 | 5 | 1 | 1 |
| Mod | 3 | - | 3 | 8 | 2 | 1 | - | 2 |
| Severe | 0 | - | - | 3 | 1 | 1 | - | - |
| Median | Mild | Mild | Mild | Mod | Mild | Mild | None | None |
| Brain atrophy | ||||||||
| None | 2 | - | - | 13 | 3 | 1 | - | 4 |
| Mild | 1 | 2 | 2 | 2 | 2 | 5 | - | 3 |
| Mod | 3 | 2 | 2 | 4 | 1 | 3 | 1 | - |
| Severe | 4 | 2 | 5 | - | 1 | - | 5 | - |
| Median | Mod | Mod | Severe | None | Mild | Mild | Severe | None |
SD standard deviation, M:F male:female, Mod moderate, AD Alzheimer’s disease, LBD Lewy body disease, FTLD-TDP frontotemporal dementia-Tar DNA binding protein 43, MSA multiple system atrophy, PiD Pick’s disease, CBD corticobasal degeneration, PSP progressive supranuclear palsy
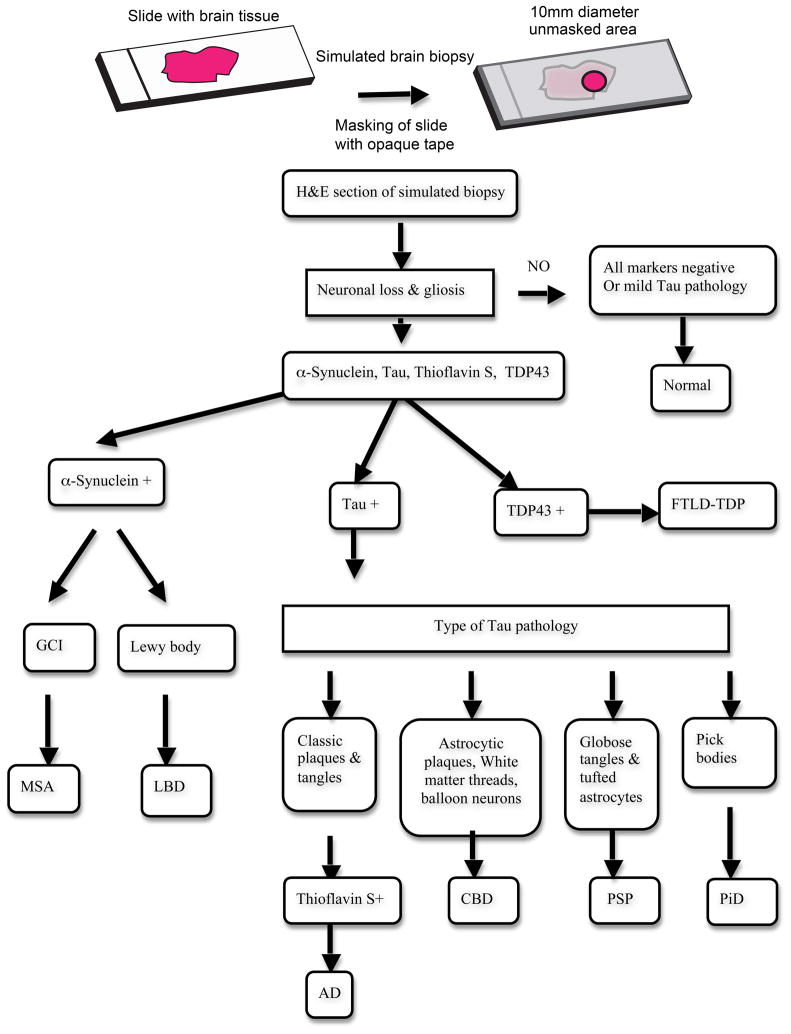
An ideal brain biopsy specimen was simulated on each glass slide by covering the slide with opaque tape to mask the entire slide except for an area of brain tissue measuring 10 mm in diameter. Further, this area of visible tissue contained a portion of cortex and white matter as is usual in brain biopsy specimens, except in the ease of basal ganglia, where a random area was selected. For any given brain region the visible area was identical in the H&E slide and corresponding immunostains and thioflavin-stained sections. The amyloid binding dye thioflavin-S was used to stain sections for evaluating the burden of thioflavin-S positive plaques [23, 24]. All identifying characteristics of the cases were masked and were known only to the person involved in the masking procedure. H&E, phosphorylated tau, α-synuclein, ubiquitin, TDP-43 immunostains and thioflavin-S stains were evaluated for each brain region in all cases. The slides were then sorted into groups containing the H&E and corresponding immunohistochemical and fluorescent sections. Each such group was randomly assigned to three neuropathologists blinded to the diagnoses. Each pathologist assessed a portion of the cases. The neuropathologists then evaluated the specimens in a blinded manner based on previously established criteria used in the diagnoses of neurodegenerative disorders [1, 3, 5, 8, 9, 11, 19–21, 26].
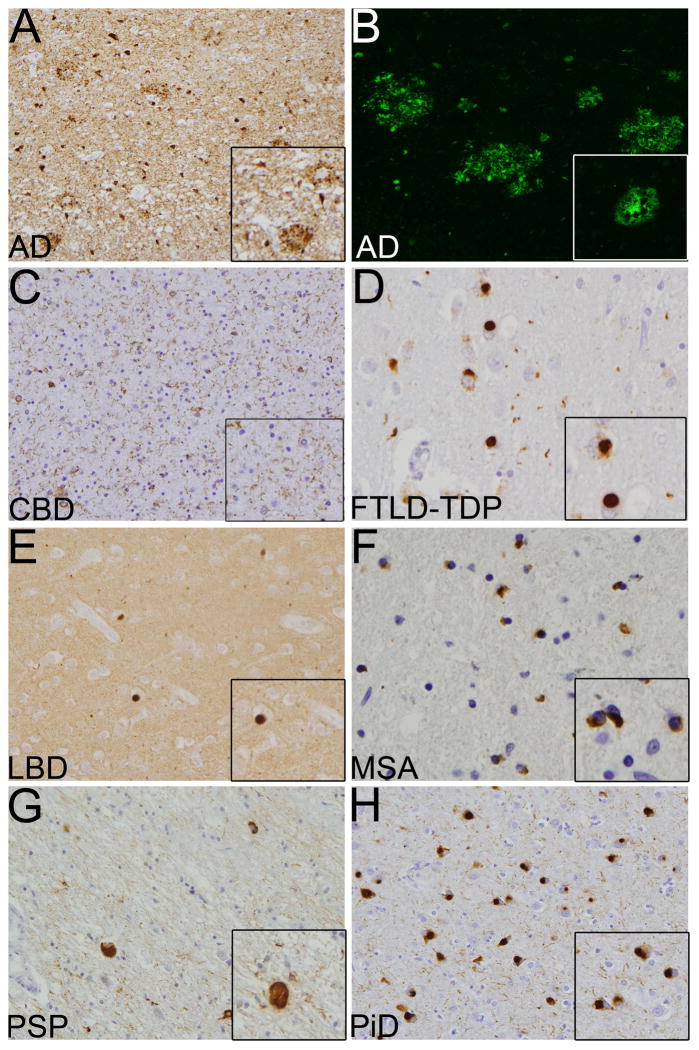
Briefly, these included identification of specific disease characteristics such as thioflavin-S positive plaques and tan-positive tangles in AD; tau-positive Pick bodies in PiD; tau-positive white matter threads, astrocytic plaques and balloon cells in CBD; α-synuclein-positive glial cell inclusions in MSA, TDP43-posittve inclusions in FTLD-TDP and globose tangles and tufted astrocytes in PSP. These histopathologic features and an algorithmic approach to diagnosis modified from previously published guidelines are illustrated in Figs. 1 and 2 [16, 29, 33]. Since the distinction between DLB and PD is not based on histopathology and is largely clinical, both these entities were grouped together under Lewy body disease (LBD) [7, 27]. A diagnosis was established using either the frontal cortex only, which is commonly the site of biopsy for enigmatic causes of cognitive impairment, or all 4-brain regions. Once the diagnoses were established, cases were re-assorted into each known gold standard autopsy-diagnosed disease category.
Fig. 1.
Simulated biopsy and diagnostic algorithm. An ideal brain biopsy specimen was simulated on each glass slide in a blinded manner by covering the slide with opaque tape to mask the entire slide except for an area of brain tissue measuring 10 mm in diameter containing a portion of cortex and white matter (except in the case of basal ganglia). The illustrated algorithm was used in the diagnoses of various neurodegenerative disorders in simulated brain biopsies. H&E, phosphorylated tau, α-synuclein, ubiquitin, TDP43 immunostains and thioflavin-S stains were evaluated for each brain region in all cases. Cases positive for α-synuclein were classified as multiple system atrophy (MSA) when glial cell inclusions (GCI) were detected and as Lewy body disease (LBD) when Lewy bodies were identified. Diagnosis of frontotemporal dementia -Tar DNA binding protein 43 (FTLD-TDP) was made when TDP43-positive inclusions were identified. Tau-positive cases were sub-classified based on the presence of characteristic tau-positive features: Pick bodies in Pick’s disease (PiD), white matter threads, astrocytic plaques and balloon cells in carticobasal degeneration (CBD) and globose tangles and tufted astrocytes in progressive supranuclear palsy (PSP). Thioflavin-S positive plaques and tau-positive tangles were used in diagnosing Alzheimer’s disease (AD)
Fig. 2.
Histological features of various neurodegenerative disorders on simulated biopsy. a, b Alzheimer’s disease (AD) showing tau (a) positive tangles and thioflavin-S (b) positive neuritic plaques, c Corticobasal degeneration (CBD) showing tau-positive white matter threads, d Tar DNA binding protein 43 (TDP43) positive inclusions in frontotemporal dementi-TDP43 (FTLD-TDP). e Lewy bodies disease (LBD) showing α-synuclein-positive Lewy bodies, f Glial cell inclusions (GCI) positive for α-synuclein in multiple system atrophy (MSA), g Tau-positive globose tangles in progressive supranuclear palsy (PSP). h Tau-positive Pick bodies in Pick’s disease (PiD). Images show 20× and 40× (inset) magnifications
Immunohistochemistry
Sections were subjected to immunohistochemistry using the avidin–biotin complex detection method (VECTA-STAIN ABC kit; Vector Laboratories, Burlingame, CA) with ImmPACT DAB peroxidase substrate (Vector Laboratories, Burlingame, CA) as the chromogen using antibodies to phosphorylated tau, α-synuclein, and TDP-43 as previously described [25, 35].
Data analysis and statistical methods
All statistical analyses were performed using SAS software (version 9.2, SAS Institute Inc., Cary, NC). All statistical tests were two-sided. Statistical significance was set at the 0.05 level. Agreement between the gold standard autopsy diagnosis and the 4-brain regions or frontal cortex only diagnoses was assessed with the kappa coefficient [6]. Point estimates and their 95% confidence intervals (CI) were obtained for the Kappa coefficient. Kappa values were interpreted using guidelines proposed by Landis and Koch [17]; kappa values below 0 indicate “poor” agreement, 0–0.20 “slight” agreement, 0.21–0.40 “fair” agreement, 0.41–0.60 “moderate” agreement, 0.61–0.80 “substantial agreement”, and 0.81–1.00 “almost perfect” agreement.
Point estimates and 95% confidence intervals for diagnostic performance measures, including sensitivity, specificity, and positive and negative likelihood ratios, were calculated [28]. Within each disease group, sensitivity is defined as the probability of a positive test result given that the subject has the disease. Specificity is defined as the probability of a negative (normal) test result given that the subject is normal. The positive and negative likelihood ratios (PLR and NLR, respectively) are functions of sensitivity and specificity. The PLR and NLR can be thought of as the ratio of the post-test odds of being diseased or not/pre-test odds of being diseased given a positive or negative test result, respectively [28].
Positive and negative predictive values (PPV and NPV, respectively) arc diagnostic performance measures that depend upon the prevalence of disease. Further, estimates of PPV and NPV using true and false positives and negatives are valid only when the ratio of the number of patients in each disease group and the number of patients in the non-diseased control group used to establish the NPV and PPV are equivalent to the prevalence of the diseases in the studied population. As accurate prevalence for some neurodegenerative diseases such as PSP, CBD, PiD and FTLD-TDP in this study are not well established due to challenges with disease ascertainment, we were unable to detennine their PPV and NPV.
Results
Simulated biopsy versus autopsy diagnoses
The kappa values for the frontal cortex diagnoses indicated moderate to substantial agreement with the gold standard autopsy diagnosis (kappa = 0.602, 95% CI = 0.489–0.715, p < 0.0001). Agreement in diagnoses was observed in 8/10 AD, 4/6 CBD, 8/9 FTLD-TDP, 15/19 LBD, 5/7 MSA, 4/6 PiD and 3/7 aged-matched control cases. We were unable to diagnose PSP (0/9) in the frontal cortex biopsies. Substantial improvement in diagnoses was observed when all 4-brain regions were considered (kappa = 0.904, 95% CI = 0.845–0.986, p < 0.0001). With all 4-brain regions, agreement in diagnoses was observed in 8/10 AD, 5/6 CBD. 9/9 FTLD-TDP, 19/19 LBD. 7/7 MSA, 6/6 PiD, 5/7 aged-matched control cases and 8/9 PSP (Table 2).
Table 2.
Diagnostic measures in simulated brain biopsy
| Disease | AD | CBD | FTLD-TDP | LBD | MSA | PSP | PiD | Control | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Number of subjects | 10 | 6 | 9 | 19 | 7 | 9 | 6 | 7 | 73 |
| Correct diagnosis (n) | |||||||||
| Frontal | 8 | 4 | 8 | 15 | 5 | 0 | 4 | 3 | 47 |
| 4 region | 8 | 5 | 9 | 19 | 7 | 8 | 6 | 5 | 67 |
| Sensitivity (%) | |||||||||
| Frontal | 80 | 66 | 88 | 79 | 71 | 0 | 66 | NA | 64 |
| Lower CI | 55 | 29 | 68 | 61 | 38 | 0 | 28 | NA | 40 |
| Upper CI | 100 | 100 | 100 | 97 | 100 | 0 | 100 | NA | 85 |
| 4 region | 80 | 83 | 100 | 100 | 100 | 88 | 100 | NA | 92 |
| Lower CI | 55 | 53 | 100 | 100 | 100 | 68 | 100 | NA | 82 |
| Upper CI | 100 | 100 | 100 | 100 | 100 | 100 | 100 | NA | 100 |
| Specificity (%) | |||||||||
| Frontal | NA | NA | NA | NA | NA | NA | NA | 43 | 43 |
| Lower CI | NA | NA | NA | NA | NA | NA | NA | 6 | 6 |
| Upper CI | NA | NA | NA | NA | NA | NA | NA | 79 | 79 |
| 4 region | NA | NA | NA | NA | NA | NA | NA | 71 | 71 |
| Lower CI | NA | NA | NA | NA | NA | NA | NA | 38 | 38 |
| Upper CI | NA | NA | NA | NA | NA | NA | NA | 100 | 100 |
| PLR | |||||||||
| Frontal | 1.4 | 1.2 | 1.5 | 1.4 | 1.2 | 0 | 1.2 | NA | 1.1 |
| 4 region | 2.8 | 2.9 | 3.5 | 3.5 | 3.5 | 3.1 | 3.5 | NA | 3.2 |
| NLR | |||||||||
| Frontal | 0.5 | 0.7 | 0.2 | 0.5 | 0.6 | 2.3 | 0.7 | NA | 0.8 |
| 4 region | 0.3 | 0.2 | 0 | 0 | 0 | 0.1 | 0 | NA | 0.1 |
CI confidence interval. Lower CI Lower Limit of CI, Upper CI upper Limit of CI, PLR positive diagnostic likelihood ratio, NLR negative diagnostic likelihood ratio, NA not applicable, AD Alzheimer’s disease, LBD Lewy body disease, FTLD-TDP frontotemporal dementia-Tar DNA binding protein 43, MSA multiple system atrophy, PiD Pick’s disease, CBD corticobasal degeneration, PSP progressive supranuclear palsy
When the correct diagnosis was not reached for the four-region biopsies, we classified these cases as follows: 1/9 PSP cases was classified as tauopathy-insufficient for diagnosis, 1/10 AD cases was misdiagnosed as no significant pathology and no definitive diagnosis was made in 1/10 AD, 1/6 CBD and 2/7 control cases. Similarly, for the frontal cortex biopsies: 8/9 PSP were classified as tauopathy-insufficient for diagnosis; 1/9 PSP cases was misclassified as AD; 1/6 CBD cases was classified as tauopathy-insufficient for diagnosis; 1/6 CBD cases was misdiagnosed as AD and 2/6 PiD cases were classified as tauopathy-insufficient for diagnosis. No definitive diagnosis was made in 2/10 AD, 1/9 FTDP-TDP, 4/19 LB, 2/7 MSA and 4/7 control cases.
Frontal cortex versus 4-brain region diagnoses
Diagnoses from the frontal cortex simulated biopsies showed average sensitivity of 64% and a specificity of 43%. Sensitivities were highest for FTLD-TDP (88%) and AD (80%) and lowest for PSP (0%). Intermediate values were observed for LBD (79%), MSA (71%) and CBD and PiD (both 66%). Diagnostic sensitivities were enhanced when all 4-brain regions were considered with average sensitivity of 92% and a specificity of 71%. Sensitivities for FTLD-TDP, LBD, MSA and PiD were all 100%, 80% for AD, 88% for PSP and 83% for CBD (Table 2).
The average PLRs and NLRs for frontal cortex were 1.1 and 0.8, respectively. PLRs for diagnosis using the frontal cortex were 1.5 (FTLD-TDP), 1.4 (AD and LBD), 1.2 (CBD, MSA and PiD) and 0 (PSP). NLRs for diagnosis using the frontal cortex ranged between 2.3 (PSP) and 0.2 (FTLD-TDP). Similar to the kappa results, improvements in overall PLRs and NLRs were observed for diagnosis using all 4-brain regions and they were 3.2 and 0.1, respectively. PLRs using all 4-brain regions for diagnosis ranged between 3.5 (FTLD-TDP, LBD, MSA and PiD) and 2.8 (AD). NLRs ranged between 0.3 (AD) and 0.1 (PSP) (Table 2).
Discussion
We examined simulated biopsies from various brain regions in cases with a confirmed autopsy diagnosis of AD, PD, DLB, MSA, PiD, FTLD-TDP, CBD and PSP using current histologic and histochemical techniques. Since the non-dominant frontal cortex represents the most biopsied brain region for enigmatic causes of cognitive impairment [36], we chose to evaluate the frontal cortex and compare these results with those obtained using 4-brain regions including the frontal, parietal and temporal cortices and the basal ganglia.
Moderate to substantial agreement was observed between the gold standard autopsy diagnosis and frontal cortex diagnosis (kappa = 0.602) while almost perfect agreement was observed between the gold standard autopsy diagnosis and 4-brain regions diagnosis (kappa = 0.904), Similarly, enhanced average sensitivity and specificity of diagnosis were observed when all 4-brain regions were considered compared to the frontal cortex (Table 2). However, this difference was mainly due to the inability to make a diagnosis of PSP in all frontal cortex biopsies. The sensitivity and PLR for PSP were both 0 using the frontal cortex diagnosis but increased to 88% and 3.1, respectively, when all four regions were considered (Table 2). Similarly, the sensitivity in diagnosing PiD increased from 66 to 100% and that of CBD from 66 to 88% on comparing frontal cortex with 4-brain regions. In contrast, the sensitivity remained 80% for AD in both categories of simulated biopsy. FTLD-TDP, AD and LBD with sensitivities of 88, 80 and 79%, respectively, had the highest diagnostic yield in the frontal cortex category. MSA and PiD showed intermediate sensitivities and PLR values in the frontal cortex, but showed improvement on considering all 4-brain regions (Table 2). These observations reflect the pathologic distribution of disease burden such as PSP involving subcortical regions like the basal ganglia, which showed the highest diagnostic yield. Thus, frontal cortical biopsy may be of more utility in the diagnoses of disease with more cortical distribution such as FTLD-TDP, AD and LBD but of limited value in the diagnosis of PSP. Given the prevalence of AD and LBD in individuals 60 years of age and older, the results of this study may have practical value in guiding treatment decisions for diverse neurological conditions such as brain tumors that commonly require brain biopsy for diagnosis in elderly subjects who are at increased risk for having a concomitant neurodegenerative disease in addition to the one that prompted a diagnostic brain biopsy.
The caveats in this study include potential differences between biopsy material and postmortem tissue and the retrospective nature of our analysis. Moreover, the simulated brain biopsies in this study represent ideal brain biopsies with relatively abundant tissue including both gray and white matter in comparison with more variable brain biopsies encountered in daily practice. The non-dominant frontal cortex is the most frequently biopsied area and hence was our main choice of analysis. Biopsy of four different brain regions, especially the basal ganglia is not realistic in day-to-day clinical practice, but reflects the importance of sampling different brain regions in establishing a definitive diagnosis. However, the clinical scenario will be the ultimate determinant of the choice of brain regions for biopsy. The sample size in this study is limited resulting in wide confidence intervals, future studies with larger sample sizes will address this concern. Further, it will be important to extend these studies in the future to smaller simulated biopsy specimens (such as punch biopsies) to compare diagnostic yields. Our study also does not include other commonly biopsied diseases such as inflammatory, metabolic, prion, infectious, demy-elinating and paraneoplastic disorders and may not be reflective of the cases that are frequently encountered in regular brain biopsies [32, 33, 37]. Since our goal was to detect evidence of a neurodegenerative disorders in a simulated brain biopsy using archival neurodegenerative disease cases, these conditions were not included. Finally, this study focuses on histopathologic diagnostic aspects involving postmortem tissues with pathologists blinded to the clinical scenario. This is often not the case in regular practice wherein clinical aspects weigh heavily in formulating a diagnosis. Indeed, we were unable to histologically differentiate between PD and DLB where the distinction is more clinical [7, 27].
Despite these caveats our study highlights the importance and need for establishing guidelines and standardization in brain biopsy diagnoses of neurodegenerative disorders. The potential importance of brain biopsy in evaluating neurodegeneration is highlighted from recent studies in normal pressure hydrocephalus (NPH). In a retrospective study in 433 patients, the presence of Aβ and hyperphosphorylated tau in frontal lobe biopsies from NPH patients strongly correlated with clinical AD in 22% of patients [18]. Similarly a prospective study in 37 NPH patients showed severe cognitive baseline impairment as well as poor postoperative cognitive improvement in patients with moderate to severe distribution of Aβ, neurofibrillary tangles and neuritic plaques in frontal cortical biopsies [10]. It remains to be seen if similar observations can be made in other neurodegenerative disorders.
Despite potential complications, risk and invasiveness, brain biopsy targeted by advanced imaged techniques may pose a valuable modality in diagnosing various neurodegenerative diseases [31]. In this study we used a simulated biopsy paradigm to comprehensively evaluate various neurodegenerative diseases. This method enabled us overcome limitations of regular brain biopsies and has the potential to be adapted for the standardization of biopsy criteria in diagnosing neurodegenerative disorders. In the idealized situation, in frontal cortical biopsies, we found the highest sensitivities and positive likelihood ratios in diagnosing FTLD-TDP, AD and LBD. CBD and MSA showed intermediate values but were unable to diagnose PSP. When simulated biopsies from the parietal and temporal cortex and basal ganglia were considered along with the frontal cortex, sensitivities and positive likelihood ratios were significantly enhanced.
Our data illustrate the varying ability to diagnose various neurodegenerative disorders in frontal cortical biopsy specimens. Further, multiple brain regions reflecting pathologic distribution of disease such as the basal ganglia in PSP may provide enhanced diagnostic abilities, although we appreciate that the basal ganglia are an unlikely target for biopsy in most diagnostically challenging cases. Moreover, sensitivity of biomarkers is increasing as newer diagnostic tests emerge. However, a tissue-based diagnosis is currently the diagnostic gold standard and may be of utility in evaluating, validating and establishing these novel diagnostic modalities. The main purpose of this study was to evaluate the potential of brain biopsy in establishing a diagnosis in neurodegeneration using existing archival brain tissues. Indeed, it was prompted in part by our recent study examining biopsies done at the time of shunt insertion for normal pressure hydrocephalus which suggested that the presence of AD pathology predicts a poor outcome for shunt treatment [10]. Thus, in special circumstances like this, our findings from this simulated biopsy study may be used to establish guidelines for the biopsy diagnosis of AD and possibly other neurodegenerative diseases. Finally, if a newer therapy or diagnostic modality needs to be compared with the gold standard in the living patient, these guidelines may be of significant importance in reaching a diagnosis. Future studies focused on developing specific criteria to guide the neuropathologist and the attending physician in diagnosing neurodegenerative disorders in biopsy material may be of immense value as newer therapies emerge to combat these devastating diseases. Finally, the ability to diagnose a neurodegenerative disease in a brain biopsy may acquire greater relevance in an increasingly older population who may undergo brain biopsy for diverse neurological disorders, For example, the detection of a neurodegenerative disease in patients who require brain biopsy for brain tumors and other conditions to guide treatment could be altered by the recognition that the patient has an additional concomitant neurodegenerative disorder. Indeed, a meaningful follow-up to this study would be to screen biopsies of individual 60 years of age or older using the methods described here to determine if the presence of neurodegenerative disease pathology influences the outcome of therapeutic interventions for diverse neurological disease as was demonstrated for NPH in a recent report [10].
Contributor Information
Sriram Venneti, Division of Neuropathology, Department of Pathology, and Laboratory Medicine, Hospital of the University of Pennsylvania, Perelman School of Medicine, University of Pennsylvania, Maloney 3rd Floor, 36th and Spruce Streets, Philadelphia, PA 19104-4283, USA.
John L. Robinson, Institute on Aging and Center for Neurodegenerative Disease Research, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
Subhojit Roy, Department of Neurosciences, University of California San Diego, La Jolla, CA, USA.
Matthew T. White, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Jennifer Baccon, Department of Pathology, Penn State Milton S. Hershey Medical Center, Hershey, PA, USA.
Sharon X. Xie, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
John Q. Trojanowski, Email: trojanow@mail.med.upenn.edu, Division of Neuropathology, Department of Pathology, and Laboratory Medicine, Hospital of the University of Pennsylvania, Perelman School of Medicine, University of Pennsylvania, Maloney 3rd Floor, 36th and Spruce Streets, Philadelphia, PA 19104-4283, USA. Institute on Aging and Center for Neurodegenerative Disease Research, Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 2.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Burns JD, Cadigan RO, Russell JA. Evaluation of brain biopsy in the diagnosis of severe neurologic disease of unknown etiology. Clin Neurol Neurosurg. 2009;111:235–239. doi: 10.1016/j.clineuro.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 7.Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:852–856. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibb WR, Lees AJ. The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol. 1989;15:27–44. doi: 10.1111/j.1365-2990.1989.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton R, Patel S, Lee EB, et al. Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol. 2011;68:535–540. doi: 10.1002/ana.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano A. Neuropathology of ALS: an overview. Neurology. 1996;47:S63–S66. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- 12.Hu WT, Chen-Plotkin A, Arnold SE, et al. Biomarker discovery for Alzheimer’s disease, frontotemporal lobar degeneration, and Parkinson’s disease. Acta Neuropathol. 2010;120:385–399. doi: 10.1007/s00401-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javedan SP, Tamargo RJ. Diagnostic yield of brain biopsy in neurodegenerative disorders. Neurosurgery. 1997;41:823–828. doi: 10.1097/00006123-199710000-00011. (discussion 828–830) [DOI] [PubMed] [Google Scholar]
- 15.Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106:72–75. doi: 10.3171/jns.2007.106.1.72. [DOI] [PubMed] [Google Scholar]
- 16.Kleinschmidt-DeMasters BK, Prayson RA. An algorithmic approach to the brain biopsy—part I. Arch Pathol Lab Med. 2006;130:1630–1638. doi: 10.5858/2006-130-1630-AAATTB. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch CG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 18.Leinonen V, Koivisto AM, Savolainen S, et al. Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol. 2010;68:446–453. doi: 10.1002/ana.22100. [DOI] [PubMed] [Google Scholar]
- 19.Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 21.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montine T, Hyman B, Beach T, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer Dement. 2011 doi: 10.1016/j.jalz.2011.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray ME, Graff-Radford NR, Ross OA, et al. Neuro-pathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Comorbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 26.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 27.Noe E, Marder K, Bell KL, et al. Comparison of dementia with Lewy bodies to Alzheimer’s disease and Parkinson’s disease with dementia. Mov Disord. 2004;19:60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 28.Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford University Press; Oxford: 2003. [Google Scholar]
- 29.Prayson RA, Kleinschmidt-DeMasters BK. An algorithmic approach to the brain biopsy—part II. Arch Pathol Lab Med. 2006;130:1639–1648. doi: 10.5858/2006-130-1639-AAATTB. [DOI] [PubMed] [Google Scholar]
- 30.Pulhorn H, Quigley DG, Bosma JJ, et al. Impact of brain biopsy on the management of patients with nonneoplastic undiagnosed neurological disorders. Neurosurgery. 2008;62:833–837. doi: 10.1227/01.neu.0000318168.97966.17. (discussion 837–838) [DOI] [PubMed] [Google Scholar]
- 31.Quinn J, Spiro D, Schulder M. Stereotactic brain biopsy with a low-field intraoperative magnetic resonance imager. Neurosurgery. 2011;68:217–224. doi: 10.1227/NEU.0b013e31820826c2. (discussion 224) [DOI] [PubMed] [Google Scholar]
- 32.Rice CM, Gilkes CE, Teare E, et al. Brain biopsy in cryptogenic neurological disease. Br J Neurosurg. 2011 doi: 10.3109/02688697.2010.551677. [DOI] [PubMed] [Google Scholar]
- 33.Schott JM, Reiniger L, Thom M, et al. Brain biopsy in dementia: clinical indications and diagnostic approach. Acta Neuropathol. 2010;120:327–341. doi: 10.1007/s00401-010-0721-y. [DOI] [PubMed] [Google Scholar]
- 34.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren JD, Schott JM, Fox NC, et al. Brain biopsy in dementia. Brain. 2005;128:2016–2025. doi: 10.1093/brain/awh543. [DOI] [PubMed] [Google Scholar]
- 37.Wong SH, Jenkinson MD, Faragher B, et al. Brain biopsy in the management of neurology patients. Eur Neurol. 2010;64:42–45. doi: 10.1159/000315032. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Le W, Jankovic J. Preclinical biomarkers of Parkinson disease. Arch Neurol. 2011;68:22–30. doi: 10.1001/archneurol.2010.321. [DOI] [PubMed] [Google Scholar]