Abstract
Protein–meibum and terpenoids–meibum lipid interactions could be important in the etiology of meibomian gland dysfunction (MGD) and dry eye symptoms. In the current model studies, attenuated total reflectance (ATR) infrared (IR) spectroscopy was used to determine if the terpenoid β-carotene and the major proteins in tears and meibum affect the hydrocarbon chain conformation and carbonyl environment of wax, an abundant component of meibum. The main finding of these studies is that mucin binding to wax disordered slightly the conformation of the hydrocarbon chains of wax and caused the wax carbonyls to become hydrogen bonded or experience a more hydrophilic environment. Lysozyme and lactoglobulin, two proteins shown to bind to monolayers of meibum, did not have such an effect. Keratin and β-carotene did not affect the fluidity (viscosity) or environment of the carbonyl moieties of wax. Based on these results, tetraterpenoids are not likely to influence the structure of meibum in the meibomian glands. In addition, these findings suggest that it is unlikely that keratin blocks meibomian glands by causing the meibum to become more viscous. Among the tear fluid proteins studied, mucin is the most likely to influence the conformation and carbonyl environment of meibum at the tear film surface.
Keywords: attenuated total reflectance, dry eye, infrared spectroscopy, meibum, tears
1. Introduction
Lipid-protein interactions are important to biological function and have been the focus of thousands of studies. A sample of some of the studies and reviews are listed in the following citations: Jing et al., 2003; Killian, 1998; Koenig et al., 1999; Lee, 2003; Marsh, 2008. Proteins can be either imbedded deeply into the lipid ensemble and are surrounded by an annulus of lipids, or they may bind extrinsically to the surface of membranes such as α-crystallin does in lens membranes (Boyle and Takemoto, 1996; Cenedella and Fleschner, 1992; Grami et al., 2005; Tang et al., 1999). Interacting proteins can immobilize select lipid species in its annulus. Upon binding, proteins modulate lipid structure and vice-versa. Depending on the length of the amino acid chains interacting with the lipid and the thickness of the lipid membrane, long protein hydrophobic segments order shorter hydrocarbon lipid chains and disorder longer hydrocarbon lipid chains. In addition to affecting the degree of order/disorder, changes occur in the phase transition temperatures and cooperativity of lipid hydrocarbon chains. Depending on the lipid composition, lipids cause proteins to aggregate and may induce changes in the α-helical tilt angle, and helical pitch. All of the studies mentioned above involve phospholipid, cholesterol and protein interactions. Except for a few studies mentioned below, almost no reports address wax and cholesterol ester interactions with proteins. These interactions could be relevant to tear film stability.
Tears form a bacteriostatic and environmental barrier and keep the cornea clear and hydrated (Tiffany, 2008). Blinking distributes tears over the surface of the cornea (Cruz et al., 2011). Evaporation causes the tear film to break up (King-Smith et al., 2008). Tear break-up time increases with age (Cho and Yap, 1993; Isenberg et al., 2003; Mohidin et al., 2002; Ozdemir and Temizdemir, 2010) and meibomian gland dysfunction (MGD) (Foulks, 2007; Tomlinson and Khanal, 2005; Tsubota et al., 1996; Tsubota, 1998) and leads to dry eye symptoms. Blinking also distributes meibum from the meibomian glands in the eye lids over the tear film surface (Chew et al., 1993; Glonek and Greiner, 1994; Korb et al., 1994) forming a thin lipid layer about 17 molecules thick (Creech et al., 1998; King-Smith et al., 2005; McDonald, 1968; Radke, 2005). Meibum lipid is composed of wax, cholesterylesters, glycerides and diesters (Green-Church et al., 2011). The composition of meibum lipid changes with age (Borchman et al., 2010a,b, 2012a; Joffre et al., 2009; Oshima et al., 2009; Shrestha et al., 2011) and dry eye symptoms (Borchman et al., 2010b, 2012b; Oshima et al., 2009; Shrestha et al., 2011; Shine and McCulley, 1991, 1996, 1998, 2000, 2004). The lipid layer is believed to inhibit the evaporation of tears (Borchman et al., 2009; Craig and Tomlinson, 1997; King-Smith et al., 2010; Maïssa and Guillon, 2010; Van Haeringen, 1981; Wolff, 1946).
The amount of meibum protein also changes, decreasing with age and increasing with MGD (Borchman et al., 2010b). Over 90 proteins have been identified in human meibum (Tsai et al., 2006). Meibum from donors with MGD was found to contain cellular debris (Korb and Henriquez, 1980; Terada et al., 2004). The cellular debris, presumably containing proteins, composed 60% of the meibum (Terada et al., 2004). The protein keratin was found to be 10% higher in meibum from donors with MGD compared to meibum from normal donors and, over 30 years ago, it was suggested that the increase in keratinization leads to the occlusion of the meibomian gland orifice and intiates MGD (Ong et al., 1991). Hyperkeratinization of epithelial cells aggregating in clusters was first noticed by Korb and Henriquez (1980). Sloughing of keratinized cells and elements of keratinization were believed to obstruct and narrow the ducts of the meibomian glands (Gutgesell et al., 1982; Jester et al., 1981).
As mentioned above, meibum lipid and protein content change with age and MGD. It is unknown if changes in lipideprotein interactions contribute to dry eye symptoms. Lipid–protein interactions may occur in meibum itself, or between the meibum lipid on the tear film surface and proteins located in the aqueous region of the tear film. Meibum lipid–protein interactions have recently been reviewed (Green-Churchet et al., 2011). Lipid order (structural stiffness) increased with MGD, and the amount of meibum protein was correlated with lipid order (stiffness/viscosity) (Borchman et al., 2010b). Since keratinization has been suggested to be the cause of some forms of dry eye symptoms (Gutgesell et al., 1982; Jester et al., 1981; Ong et al., 1991), we have used attenuated total reflectance (ATR) infrared (IR) spectroscopy to determine if keratin changes the hydrocarbon chain order of wax, the major component of meibum lipid.
Squalene, a triterpenoid, has been reported in meibum. Krenzer et al. identified squalene with the use of HPLC/MS (Krenzer et al., 2000), and the level of squalene in human meibum has been reported to be between 0 and 7% using thin layer chromatography (Ehlers, 1965; Keith, 1967; Tiffany, 1978). Glyceryl isoprene acetal terpenoids were identified in human tears by electrospray tandem mass spectrometry (Ham et al., 2004) and matrix-assisted laser desorption ionization time-flight mass spectrometry (Ham et al., 2005). However, the possible presence of other terpenoids has yet to be explored and confirmed. Because our recent NMR studies (Borchman et al., 2012a,b) and those reported by others (Robosky et al., 2008) show the presence of multiple resonances between 5 and 5.2 ppm, the spectral region where the HC=R resonance of terpenoids appears, it is possible that other terpenoids or forms of squalene(Architouv et al., 2004), may be present in meibum. Importantly, the data analysis revealed that the contents of these species decrease with MGD (Borchman et al., 2012b; Foulks et al., in press; Oshima et al., 2009). In the current model study, we have used betacarotene, a tetraterpentene, because it is one of the more common terpenoids, but may not be necessarily present in meibum. It has a typical terpenoid structure and due to its length and extended degree of unsaturation, we hypothesized that it may interact with the long-tail waxes present in meibum. For that reason, we carried out the model studies reported in this manuscript.
Lactoglobulin, mucin and lysozyme, which are major proteins found in tears, bind to and change the properties of monolayers of meibum lipids, as measured on a Langmuir trough (Miano et al., 2005; Millar et al., 2006, 2009; Mudgil and Millar, 2008; Mudgil et al., 2006; Tragoulias et al., 2005). The protein–lipid interactions were different for bovine and human meibum (Millar et al., 2009). In this study we tested whether or not mucin, lysozyme or lactoglobulin in the aqueous phase bind to wax and change the hydrocarbon chain conformation or the environment of the carbonyl region of the lipid layer. Water is a strong absorber of infrared light so conventional transmission infrared (IR) spectroscopy is impractical for studying aqueous samples. To circumvent this problem, in this study, we used attenuated total reflectance spectroscopy (ATR). The technique uses an optically dense crystal such as ZnSe onto which the wax sample is first deposited and then the aqueous film is layered. Infrared radiation is internally reflected along the crystal. As a result, an evanescent field is generated at the crystal–sample interface. The infrared radiation that interacts with the adsorbed species has a limited depth of penetration that depends on the wavelength and angle of incidence of the IR radiation, the refractive index of the crystal, and the refractive indices of the layers at the ATR crystal interface (Harrik, 1967). In our system, the depth of penetration (sampling depth) was about 2 μm at 1000 cm−1. The intensity of the evanescent field decreases exponentially with the depth of penetration; therefore, the ATR IR spectra reflect most of the absorption of the wax layer with little interference of the aqueous layer on top of the wax. From the IR spectra of the wax we can determine the hydrocarbon chain order and carbonyl environment of the wax and assess whether or not these parameters are influenced by the binding of proteins present in the aqueous phase above the wax.
2. Methods
2.1. Materials
Silver chloride windows for infrared spectroscopy were obtained from Crystran Limited, Poole, United Kingdom. β-carotene type 1, palmityloleate, stearylpalmitate, oleyloleate, lysozyme from chicken egg white, type 1-S mucin from bovine submaxillary glands and β-lactoglobulin from bovine milk were purchased from Sigma–Aldrich Chemical Company, St Louis, MO. Buffered saline (pH 7.2, 280–320 mOsm/kg, and no calcium or magnesium chloride) was obtained from Invitrogen Corporation, Carlsbad, CA. Keratin was purchased from Spectrum Chemical MFG. Corp., Gardena CA.
2.2. Preparation of palmityloleate (PO) with keratin or β-carotene
Approximately 1 mg of either keratin or β-carotene was weighed exactly (to 0.01 mg) on a AgCl window using a Mettler Toledo (Columbus OH) A7261 analytical balance that was recently cleaned and calibrated. An equal weight of PO was added to the keratin and mixed on the infrared AgCl window (13 × 2 mm) with a glass tube. The window with the mixture was placed in an ultrasonic bath (Branson 1510, Branson Ultrasonics Co., Danbury, CT) for 10 min to further mix the samples constituent.
2.3. Fourier transform infrared spectroscopy of palmityloleate (PO) with keratin or β-carotene
The method used to acquire IR spectra of lipids is similar to that published in 2007 (Borchman et al., 2007). Briefly, IR spectra were measured using a Nicolet 5000 Magna Series Fourier transform infrared spectrometer (Thermo Fisher Scientific, Inc., Waltham MA). The sample was deposited onto a AgCl window that was placed in a temperature-controlled infrared cell jacketed by an insulated water coil connected to a Neslab R-134A (NESLAB Instruments, Newton NH) circulating water bath that enables the control of the sample temperature and the rate of heating or cooling (1°C/15 min). Temperatures were maintained within ±0.01 °C. Each spectrum was obtained from the acquisition and FT of 150 interferograms. Spectral resolution was set to 1.0 cm−1. The infrared spectra were collected independently by four different investigators. The analysis of the spectral data was performed with GRAMS/386 software (Galactic Industries, Salem, NH). The frequency of the CH2 band near 2850 cm−1 was used to estimate the content of trans and gauche rotomers in the hydrocarbon chains. The ṽ was calculated after performing either mathematical spectral subtraction of the background spectrum of buffer or by baseline leveling the OH–CH stretching region between 3500 and 2700 cm−1. The center of mass of the CH2 symmetric stretching band was calculated by integrating the top 10% of the intensity of the band. The baseline for integrating the top 10% of the intensity of the band was parallel to the OH–CH region baseline. Each spectrum was measured by two investigators and again by Professor Borchman. Lipid CH2 groups in the hydrocarbon chains are present as gauche rotomers, prevalent in disordered hydrocarbon chains, or trans rotomers, more abundant in ordered hydrocarbon chains. Thus, lipid hydrocarbon chain order may be evaluated in terms of the relative amount of CH2 trans rotomers. The frequency of the CH2 symmetric stretch, ṽ, depends on the relative amount of trans or gauche rotomers (Kóta et al., 1999) and has been used to characterize lipid phase transitions (Borchman et al., 1991, 1996, 1999, 2004; Casal and Mantsch, 1984; Kóta et al., 1999; Mantsch and McElhaney, 1991; Moore et al., 1993; Popova and Hincha, 2003) and to measure the trans rotomer relative content of lipid hydrocarbon chains with changes in temperature (Borchman et al., 1991, 1996, 1999, 2004, 2007, 2011; Kóta et al., 1999). Since rotomers are either in trans or gauche conformations, phase transitions can be described by a two-state sigmoidal equation as described by Borchman et al. (2007).
2.4. Attenuated total reflectance infrared spectroscopy of wax/tear protein mixtures
A mixture of two waxes, stearylpalmitate and oleyloleate (4:1, mole:mole) was dissolved in tetrahydrofuran/methanol (3:1, v:v) and was used as a model of human meibum. This wax composition was chosen because at 35 °C it is near the center of the phase transition where conformational changes are most sensitive to environment and binding. The wax mixture (1 mg) was layered onto the ATR crystal (1 cm by 7 cm, ZnSe, 45° angle). The solvent was evaporated under a stream of nitrogen gas, and the crystal was placed in a lyophilizer for four hours to remove all traces of solvent. The crystal was then placed in a temperature-controlled IR cell holder (Heated ARK, Thermo Fisher Scientific, Inc. Waltham, MA). The rate of heating or cooling (1 °C/15 min) at the sample was controlled. Temperatures were maintained within ±0.01 °C. The sample temperature was set to 35 °C and the sample was ± allowed to equilibrate at that temperature for over 1 h before spectral acquisition.
To test if water influenced lipid conformation, phosphate buffered saline was applied on top of the wax on the ATR cell and the temperature/equilibration protocol above was followed.
Each protein, either mucin, lysozyme, or lactoglobulin, was mixed separately with buffered saline to reach a concentration of 100 mg/ml. To determine if these proteins change the lipid–lipid interactions, the steps listed in the two paragraphs above were followed but the buffer solution was replaced with a buffer solution containing a given protein. ATR IR spectra were measured using a Nicolet 5000 Magna Series Fourier transform IR spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA). Exactly 50 interferograms were recorded and averaged. Spectral resolution was set to 1.0 cm−1. The spectra were corrected for wavenumber changes due to the use of ATR with software that came with the instrument.
2.5. Statistical analysis
Data are presented as the average ± the standard error of the mean. Significance was determined using the Student’s t test or the correlation coefficient from the linear regression best fit. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Fourier transform IR spectroscopy of palmityloleate (PO) with keratin or β-carotene
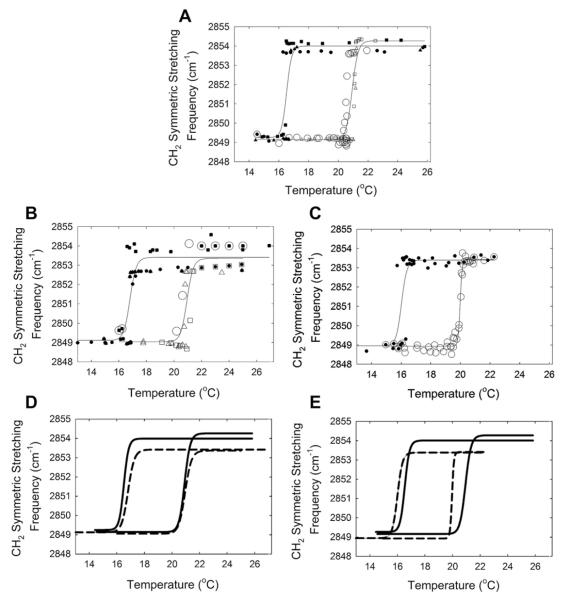
Upon heating, PO changed from an ordered gel phase to a disordered liquid crystalline phase as reflected by the increase of the CH2 symmetric stretching frequency, (Fig. 1A). Hysteresis was observed in the phase transition curves for PO as there was a 4 °C difference in the heating and cooling phase transition temperatures (Tables 1 and 2, Fig. 1A). The PO transition is considered a phase transition and not a melting transition because below the phase transition the wax is not a completely ordered solid, 16% gauche rotomers, and above the phase transition temperature, the wax is not a completely disordered liquid, 83% gauche rotomers. The phase transition temperature was measured with an accuracy of better than ± 0.1 °C. Keratin at 50% by weight had no significant (p > 0.05) effect on the phase transition temperature, minimum or maximum frequency or cooperativity of PO (Tables 1 and 2, Fig. 1B and D). β-carotene at 50% by weight lowered both the heating and cooling the phase transition of PO by a very small but statistically significant (p < 0.001) amount, about 1 °C (Tables 1 and 2, Fig. 1C and E).
Fig. 1.
Lipid phase transitions measured using infrared spectroscopy. Open symbols are heating curves, filled symbols are cooling curves. Symbol types represent separate experiments. Lines are curve fit of a 4-parameter sigmoidal equation to the data. A) palmityloleate. B) palmityloleate and Keratin 1:1, wt:wt. C) palmityloleate and β-carotene 1:1, wt:wt D) (—) fit of palmityloleate curves from A (–) fit of palmityloleate and Keratin curves from B. E) (—) fit of palmityloleate curves from A (–) fit of palmityloleate and β-carotene curves from C.
Table 1.
Lipid phase transition parameters Cooling Curve
| Phase transition parameter | Palmityloleate | Palmityloleate and Keratin | Significance (p) | Palmityloleate & β-carotene | Significance (p) |
|---|---|---|---|---|---|
| Minimum frequency (cm−1) | 2849.3 ± 0.3 | 2849.1 ± 0.4 | 0.70 | 2848.9 ± 0.3 | 0.24 |
| Maximum frequency (cm−1) | 2854.0 ± 0.3 | 2853.4 ± 0.2 | 0.091 | 2853.4 ± 0.1 | 0.60 |
| Cooperativity | −97 ± 41 | −74 ± 31 | 0.65 | 87 ± 36 | 0.66 |
| Phase transition temperature (°C) | 16.52 ± 0.07 | 16.80 ± 0.09 | 0.019* | 16.01 ± 0.09 | >0.0001* |
statistically significant, p < 0.05.
Table 2.
Lipid phase transition parameters Heating Curve
| Phase transition parameter | Palmityloleate | Palmityloleate and Keratin | Significance (p) | Palmityloleate & β-carotene | Significance (p) |
|---|---|---|---|---|---|
| Minimum frequency (cm−1) | 2849.2 ± 0.2 | 2849.0 ± 0.4 | 0.62 | 2848.9 ± 0.5 | 0.53 |
| Maximum frequency (cm−1) | 2854.3 ± 0.5 | 2853.4 ± 0.4 | 0.22 | 2853.42 ± 0.08 | 0.88 |
| Cooperativity | 104 ± 25 | 91 ± 42 | 0.78 | 293 ± 36 | >0.0001* |
| Phase transition temperature (°C) | 20.93 ± 0.06 | 20.94 ± 0.11 | 0.93 | 19.98 ± 0.01 | >0.0001* |
statistically significant p < 0.05.
The cooperativity, a measure of the ability of one lipid to influence the conformation of surrounding lipids, increased significantly (Table 2, p < 0.05) for the PO heating phase transition curve when β-carotene was added.
3.2. Attenuated total reflectance (ATR) IR spectroscopy of wax/tear protein mixtures
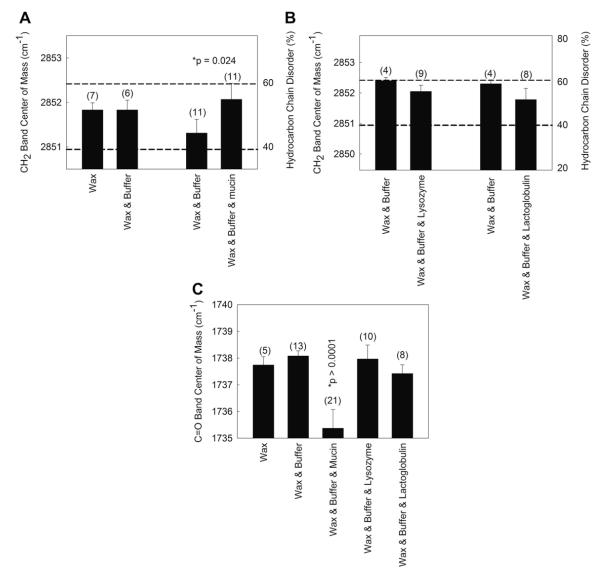
Two major regions are resolved in the ATR IR spectrum of a thin film of wax with buffer, the wax CH2 and CH3 region and the wax carbonyl region (Fig. 2). The two wax regions are shoulders on the large water HOH stretching and bending bands. The center of mass of the bands in the two wax regions may be used to measure changes in the conformation of hydrocarbon CH2 moieties (as discussed in detail in the Methods) and the environment of the carbonyl moiety. Conformation is the orientation of molecules about their bond axes. The addition of aqueous saline buffer did not change the hydrocarbon chain conformation or the environment of the carbonyl region of the wax mixture composed of stearylpalmitate and oleyloleate (4:1, mole:mole, 35 °C) (Figs. 3A and C,4). The wax mixture was 48% ordered at 35 °C. At this temperature, this wax composition goes through the center of the phase transition where conformational changes are most sensitive to environment and binding. The net absorbance of CH stretching bands (after mathematical spectral subtraction of the background, i.e. water) was about 0.50 absorbance units and exhibited signal-to-noise ratios (S/N) greater than 100. The absorbance of the carbonyl band was also quite large, greater than 0.20. Noise in the area was ±0.0007 giving a S/N of nearly 300. The average measurement and standard error of the frequency of the CH2 stretching frequency for the wax mixture/buffer was 2851.7 ± 0.2 cm−1 (n = 21). The standard error for each measurement was due to variations from one wax mixture to another and not due to measurement errors (less than ± 0.1 cm−1).
Fig. 2.
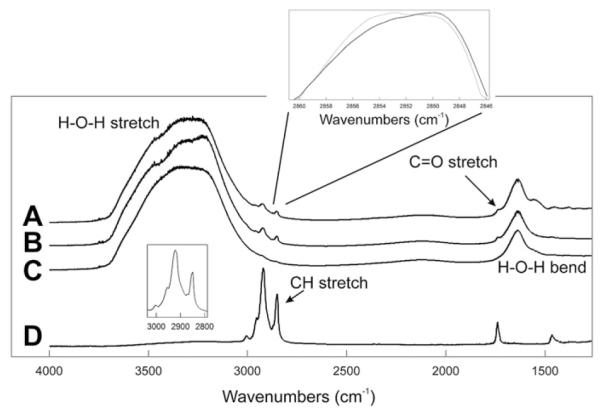
Average ATR IR spectrum of a thin film of the wax mixture oleyloleate and palmitylpalmitate (4:1, wt:wt) A) exposed to buffer B) exposed to buffer containing 100 mg/ml of mucin. C) Buffer containing 100 mg/ml of mucin. D) Wax mixture oleyloleate and palmitylpalmitate (4:1, wt:wt). Top Inset: Dotted line corresponds to a mucin/wax/buffer sample. Solid line corresponds to a wax/buffer sample. Bottom inset: Infrared CH stretching region after subtracting C from B.
Fig. 3.
Band center of mass was calculated from the infrared spectra of a thin film of the wax mixture oleyloleate and stearylpalmitate (4:1, wt:wt) exposed to a buffer containing various proteins found in human tears. A and B) The infrared CH2 symmetric stretching frequency (left y axis) is used to calculate the hydrocarbon order (right y axis) of the wax mixture. C) The wax carbonyl band center of mass can be used to ‘sense’ the environment around the ester bond. A higher carbonyl band center of mass indicates a more hydrophilic environment or hydrogen bonding.
Fig. 4.
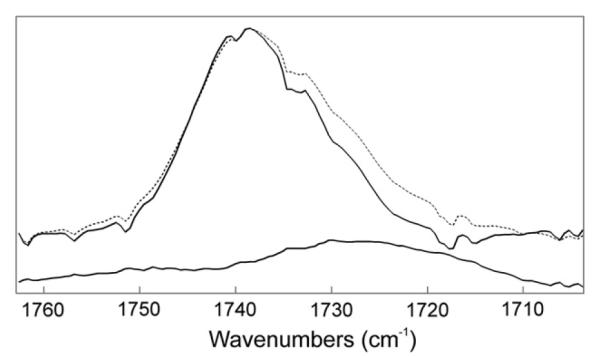
Average ATR IR spectrum of the carbonyl band. (—) Average spectrum of a thin film of the wax mixture oleyloleate and stearylpalmitate (4:1, wt:wt) exposed to buffer. (–) Average spectrum of the wax mixture exposed to 100 mg/ml mucin at 35 °C. Lower spectrum: difference spectrum of average mucin/wax/buffer spectrum minus the average wax/buffer spectrum.
Mucin at 100 mg/ml caused a very small, yet significant (p = 0.025), increase (0.7 ± 0.3 cm−1) in the CH2 symmetric stretching frequency in 9 out of 11 trials (Fig. 3A). This increase corresponds to a change in the hydrocarbon chains of the wax mixture from 49% ordered to 45% ordered (% trans rotomers). A paired Student’s t test was used to test significance. Unpaired t test analysis of the averages was not significant (p > 0.05). Neither lysozyme nor lactoglobulin at 100 mg/ml caused a statistically significant (p > 0.05) change in the conformation of the hydrocarbon chains of the wax mixture (Fig. 3B).
The wax mixture had a carbonyl infrared band center of mass of 1737.7 cm−1 (Fig. 3C). When the carbonyl is in an environment with a relatively low dielectric constant such as that of CCl4 (ε 2.24) the center of mass of the band is 1742 cm−1. As the dielectric constant is increased, such as in CHCl3 (ε 4.8), the carbonyl center of mass decreases to 1732 cm−1 (Blume et al., 1988). The center of mass of this band in the wax mixture is between these two values. The layering of mucin in the buffer lowered the center of mass of the C=O band by an average of 3.3 ± 1 cm−1 in 11 out of 11 determinations. Mucin lowered significantly (p < 0.05) the center of mass of the infrared carbonyl band in our wax mixture to 1735 cm−1 (Fig. 3C), close to that when lipid is dissolved in a solvent with a greater dielectric constant, such as that of CHCl3 (ε 4.8) (Blume et al., 1988). This change suggests that the binding of mucin causes the wax carbonyls to experience a less hydrophobic (more hydrophilic) environment or an increase in hydrogen bonding. In one experiment (data not shown), the change in the CH2 conformation with mucin binding to wax occurred quickly, within the initial 10 min after adding the protein aqueous layer, and no further changes in the CH2 were detected between 10 min and 50 h of equilibration. No protein bands were visible in the ATR IR spectra of the buffer with protein alone (data not shown).
4. Discussion
The major finding of this study is that mucin binding to wax caused a small conformational change in the CH2 moieties of wax and caused the wax carbonyls to experience a more hydrophilic environment. Lysozyme and lactoglobulin, two proteins shown to bind to monolayers of meibum, showed no such change. The change in order of the wax hydrocarbon chains caused by mucin (49%–45%) is small relative to the large difference in order observed between meibum from normal donors, 35%, and meibum from donors with MGD, 47% (Borchman et al., 2011). To influence the carbonyl environment of the wax, mucin must penetrate deeply into the wax layer and place polar moieties in the vicinity of the wax. Nagyová and Tiffany (1999) observed that mucin was unique and lowered the surface tension of saline buffer to the level of human tears whereas lactoglobulin, lactoferrin, or lysozyme caused no such change. In the first reported observation of mucin–protein interactions, Holly (1973) showed that mucin facilitated the spreading of meibum on an aqueous surface. Mucin is highly glycosylated and it is possible that the OH moieties on the glycosylated sites may interact with the carbonyl groups of the wax via H-bonds. As a result, the interactions among neighboring wax molecules would decrease and lead to a slight disordering of the hydrophobic tails. It is difficult to imagine how mucin, a hydrophilic protein could interact with a hydrophobic wax layer. In other systems, mucin has been shown to bind to lipids such as cholesterol and phosphatidylcholine (Nunes et al., 1995; Smith, 1987). Gallbladder mucin contains non-glycosylated domains rich in serine, glutamic acid/glutamine and glycine that bind to hydrophobic lipids (Nunes et al., 1995; Smith, 1987). It is these “lipid coated, non-glycosylated, cysteine rich” hydrophobic domains that are believed to stabilize lactoferrin-lipid emulsions (Singh and Sarkar, 2011). Perhaps mucin interacts with wax in a similar manner.
Changes in meibum composition with MGD have been reported (Borchman et al., 2010b, 2011, 2012b; Joffre et al., 2009; Oshima et al., 2009; Shine and McCulley, 1991, 1996, 1998, 2000, 2004; Shrestha et al., 2011). It is unknown if the reported changes cause changes in lipid–protein interactions. Based on our findings, mucin–meibum interactions may be more significant than those with other proteins. Because of the small changes in the center of mass of the infrared bands, it is not practical to use IR spectroscopy to measure the binding constant Kb of mucin, lysozyme or lactoglobulin with to wax. Fluorescent probe techniques similar to those we used to measure alpha crystalline lens membrane lipid binding could be more effective (Borchman and Tang, 1996, Cenedella et al., 2004; Grami et al., 2005; Tang and Borchman, 1998; Tang et al., 1988a,b, 2003). Using the fluorescent probe N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadeconoyl-sn-3-phosphoethanolamine, triethylammonium salt (NBD-PE) we found that there are moieties in human tears that bound to wax causing the surface of the wax to exclude water (Borchman et al., 2007). An important set of proteins that was not investigated in this study: surfactant proteins are likely to alter the structure of meibum on the tear film surface (Green-Church et al., 2011).
5. Extrapolation of in vitro results to what occurs in vivo
One should always use caution in extrapolating in vitro results to the situation that occurs in vivo. The wax model system used in this study was designed to be near the center of its phase transition at physiological temperature, because at the center of the transition the hydrocarbon chains are most sensitive to conformational changes. Small changes in temperature, the ratio of the lipid mixture or perturbations due to protein binding could cause large changes in conformation at the center of the phase transition. Although the predominant lipid in human meibum is wax (Borchman et al., 2012a; Butovich, 2009; Green-Church et al., 2011), the wax system we used is much less complex than human meibum and may interact with proteins differently than human meibum does. The lipid order of our wax mixture was 49% close to that for human meibum from 1 to 35 year old individuals, which is 40–50% ordered (Borchman et al., 2010c).
Human lipocalin penetrates human meibum better than bovine β-lactoglobulin (Millar et al., 2009). Therefore, one might expect less effect from bovine lactoglobulin than from human lipocalin in the current model wax mixture if the interaction is similar. Similarly, the mucin from bovine submaxillary gland used in the current study, had no surface activity when compared with purified ocular mucin (Millar et al., 2006). Because we detect no changes in the wax carbonyl environment or hydrocarbon chain conformation in the presence of β-lactogobulin, or lysozyme, this does not mean they do not bind to the wax. It could be the case that binding does not cause a change in hydrocarbon conformation.
The protein–wax interactions we have observed are those in which the protein binds to the wax matrix not that described for the binding of lipids in the cavity of lipocalin (Glasgow et al., 1995; Gasymov et al., 1999, 2009). Wax would not be expected to bind in the cavity of lipocalin (Glasgow et al., 1995; Gasymov et al., 1999, 2009). β-lactoglobulin used in this study is in an apo-form (the protein is replete with fatty acids). It has yet to be determined if lipocalin or β-lactoglobulin without lipids bound to them change the conformation of the wax hydrocarbons when they bind to the wax matrix. The adsorption to human meibum of tear lipocalin with or without lipids bound to it is considerably different (Millar et al., 2009).
6. Keratin- and terpenoid–wax interactions
Lipid-protein interactions may be important not only for the structure and function of meibum on the surface of the tear film but also for meibum in the meibomian glands. It has been proposed that keratinization could be responsible for blocking the orifice of the meibomian glands (Korb and Henriquez, 1980; Ong et al., 1991; Terada et al., 2004). The present study indicates that keratin has no major direct effect on the fluidity (viscosity) of wax, the most abundant lipid component in meibum. Based on these results, it is unlikely that keratin itself blocks meibomian glands by causing the meibum to become more viscous. It is more likely that enlarged or sloughed off keratinized epithelial cells are responsible for the blockage of the meibomian gland orifice. We found that in donors with MGD who had some but not all of the meibomian glands blocked, there was 17–53 times enough lipid-containing esters on the lid margin to adequately supply a lipid layer 17-molecules-thick on the surface of tears (Ashraf et al., 2011). For these select donors, patients with dry eye symptoms due to MGD, it is more likely that the quality rather than the quantity of meibum could potentially contribute to MGD.
Terpenoid-like compounds, including carotenoids, may be present in meibum and their content appears to decrease with MGD (See the Introduction). Based on the current study, tetraterpenoids, such as carotenoids, are not likely to influence the structure of meibum. If future studies confirm that terpenoids are found in meibum, their modes of action are more likely to inhibit oxidation of meibum and subsequent inflammation as well as to confer antibacterial properties (DiMascio et al., 1989).
Of the proteins studied, including mucin, lysozyme and lactoglobulin, mucin is the most likely compound to influence the conformation and carbonyl environment of meibum on the tear film surface. Our findings that mucin–meibum interactions may be more significant than those of other proteins support those of Holly (1973); and Nagyová and Tiffany, 1999. Keratin and β-carotene had little effect on the conformation (viscosity) and carbonyl environment of wax.
Acknowledgments
Supported by Public Health Service research grant EY017094-01 (Bethesda, MD., U.S.A.) the Kentucky Lions Eye Foundation and an unrestricted grant from Research to Prevent Blindness Inc.
Dr Foulks is a member of the part-time staff of the Surgical Service, Department of Veteran Affairs Medical Center, Louisville, KY. The opinions expressed are not those of the Department of Veterans Affairs or the US Government.
Abbreviations
- ATR
attenuated total reflectance
- MGD
meibomian gland dysfunction
- PO
palmityloleate
- ṽ
infrared CH2 symmetric stretching band frequency
References
- Architouv E, Metzger P, Rager M, Largeau C. C31–C34 methylated squalenes from a Bolivian strain of Botryococcus braunii. Phytochemistry. 2004;65:3159–3165. doi: 10.1016/j.phytochem.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Ashraf Z, Pasha U, Greenstone V, Akbar J, Apenbrinck E, Foulks GN, Borchman D. Quantification of human sebum on skin and human meibum on the eye lid margin using sebum tape, spectroscopy and chemical analysis. Curr. Eye. Res. 2011;36:553–562. doi: 10.3109/02713683.2011.574331. [DOI] [PubMed] [Google Scholar]
- Blume A, Hübner W, Messner G. Fourier transform infrared spectroscopy of 13CAO-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988;27:8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Borchman D, Tang D. Binding capacity of alpha-crystallin to bovine lens lipids. Exp. Eye. Res. 1996;63:407–410. doi: 10.1006/exer.1996.0130. [DOI] [PubMed] [Google Scholar]
- Borchman D, Yappert MC, Herrell P. Structural characterization of human lens membrane lipid by infrared spectroscopy. Invest. Ophthalmol. Vis. Sci. 1991;32:2404–2416. [PubMed] [Google Scholar]
- Borchman D, Cenedella RI, Lamba OP. Role of cholesterol in the structural order of lens membrane lipids. Exp. Eye. Res. 1996;62:191–197. doi: 10.1006/exer.1996.0023. [DOI] [PubMed] [Google Scholar]
- Borchman D, Tang D, Yappert MC. Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum membranes. Biospectroscopy. 1999;5:151–167. doi: 10.1002/(SICI)1520-6343(1999)5:3<151::AID-BSPY5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Borchman D, Yappert MC, Afzal M. Lens lipids and maximum lifespan. Exp. Eye. Res. 2004;79:761–768. doi: 10.1016/j.exer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers/Biospectroscopy. 2007;87:124–133. doi: 10.1002/bip.20798. [DOI] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Mathews J, Leake K, Bell J. Factors affecting evaporation rates of tear film components measured in vitro. Eye. Contact. Lens. 2009;35:32–37. doi: 10.1097/ICL.0b013e318193f4fc. [DOI] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr. Eye. Res. 2010a;35:778–786. doi: 10.3109/02713683.2010.490895. [DOI] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye. Res. 2010b;91:246–256. doi: 10.1016/j.exer.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Kakar S, Podoll N, Rychwalski P, Schwietz E. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic. Res. 2010c;44:34–42. doi: 10.1159/000283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Milliner SE. Changes in human meibum lipid composition with age using NMR spectroscopy. Invest. Ophthalmol. Vis. Sci. 2012a;53:475–482. doi: 10.1167/iovs.11-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Millinar SE. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR and principal component analysis. Invest. Ophthalmol. Vis. Sci. 2012b;53:337–347. doi: 10.1167/iovs.11-8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Foulks GN, Yappert MC, Bell J, Wells E, Neravetla S, Greenstone V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2011;52:3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle DL, Takemoto L. EM immunolocalization of alphacrystallins: association with the plasma membrane from normal and cataractous human lenses. Curr. Eye. Res. 1996;15:577–582. doi: 10.3109/02713689609000769. [DOI] [PubMed] [Google Scholar]
- Butovich IA. The Meibomian puzzle: combining pieces together. Prog. Retin. Eye. Res. 2009;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal HL, Mantsch HH. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta. 1984;779:381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ, Fleschner CR. Selective association of crystallins with lens ‘native’ membrane during dynamic cataractogenesis. Curr. Eye. Res. 1992;11:801–815. doi: 10.3109/02713689209000753. [DOI] [PubMed] [Google Scholar]
- Cenedella RJ, Jacob R, Borchman D, Tang D, Neely AR, Samadi A, Mason RP, Sexton P. Direct perturbation of lens membrane structure may contribute to cataracts caused by U18666A, an oxidosqualene cyclase inhibitor. J. Lipid. Res. 2004;45:1232–1241. doi: 10.1194/jlr.M300469-JLR200. [DOI] [PubMed] [Google Scholar]
- Chew CKS, Hykin PG, Jansweijer C, Dikstein S, Bron AJ. The casual level of meibomian lipids in humans. Curr. Eye. Res. 1993;12:255–259. doi: 10.3109/02713689308999471. [DOI] [PubMed] [Google Scholar]
- Cho P, Yap M. Age gender, and tear break-up time. Optom. Vis. Sci. 1993;70:828–831. doi: 10.1097/00006324-199310000-00009. [DOI] [PubMed] [Google Scholar]
- Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci. 1997;74:8–13. doi: 10.1097/00006324-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Creech JL, Do LT, Fatt I, Radke CJ. In vivo tear-film thickness determination and implications for tear-film stability. Curr. Eye. Res. 1998;17:1058–1066. doi: 10.1076/ceyr.17.11.1058.5233. [DOI] [PubMed] [Google Scholar]
- Cruz AA, Garcia DM, Pinto CT, Cechetti SP. Spontaneous eyeblink activity. Ocul. Surf. 2011;9:29–41. doi: 10.1016/s1542-0124(11)70007-6. [DOI] [PubMed] [Google Scholar]
- DiMascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Ehlers N. The precorneal film. Biomicroscopical, histological and chemical investigations. Acta. Ophthalmol. 1965;81(Suppl.):81–134. [PubMed] [Google Scholar]
- Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 2007;52:369–374. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Foulks GN, Borchman D, Yappert MC, Kakar S. Topical Azithromycin and Oral Doxycycline Therapy of Meibomian Gland Dysfunction: A Comparative Clinical and Spectroscopic Pilot Study. Cornea: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim. Biophys. Acta. 1999;1433:307–320. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48:7219–7228. doi: 10.1021/bi9005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr. Eye. Res. 1995;14:363–372. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- Glonek T, Greiner JV. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359. doi: 10.1097/00003226-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Grami V, Marrero Y, Huang L, Tang D, Yappert MC, Borchman D. Alpha-crystallin binding in vitro to lipids from clear human lenses. Exp. Eye. Res. 2005;81:138–146. doi: 10.1016/j.exer.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, Glasgow B. Meibomian gland contribution to the tear film. In: The Reportof the International Workshop on Meibomian Gland Dysfunction. Invest. Ophthalmol. Vis. Sci., vol. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am. J. Ophthalmol. 1982;94:383–387. doi: 10.1016/0002-9394(82)90365-8. [DOI] [PubMed] [Google Scholar]
- Ham BM, Jacob JT, Keese MM, Cole RB. Identification, quantification and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J. Mass. Spectrom. 2004;39:1321–1336. doi: 10.1002/jms.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Anal. Chem. 2005;77:4439–4447. doi: 10.1021/ac058000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrik NJ. Internal Reflection Spectroscopy. Wiley; New York: 1967. [Google Scholar]
- Holly FJ. Formation and rupture of the tear film. Exp. Eye. Res. 1973;15:515–525. doi: 10.1016/0014-4835(73)90064-x. [DOI] [PubMed] [Google Scholar]
- Isenberg SJ, Del Signore M, Chen A, Wei J, Guillon J. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology. 2003;110:1408–1411. doi: 10.1016/S0161-6420(03)00451-2. [DOI] [PubMed] [Google Scholar]
- Jing W, Hunter HN, Hagel J, Vogel HJ. The structure of the antimicrobial peptide Ac-RRWRF-NH2 bound to micelles and its interactions with phospholipid bilayers. J. Pept. Res. 2003;61:219–229. doi: 10.1034/j.1399-3011.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest. Ophthalmol. Vis. Sci. 1981;20:537–547. [PubMed] [Google Scholar]
- Joffre C, Souchier M, Leclere L, et al. Branched-chain fatty acids, increased in tears of blepharitis patients, are not toxic for conjunctival cells. Br. J. Ophthalmol. 2009;93:1391–1395. doi: 10.1136/bjo.2008.156356. [DOI] [PubMed] [Google Scholar]
- Keith GC. Seborrhoeic blepharo-kerato-conjunctivitis. Trans. Ophthalmol. Soc. U. K. 1967;87:85–103. [PubMed] [Google Scholar]
- Killian JA. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1998;1376:401–416. doi: 10.1016/s0304-4157(98)00017-3. [DOI] [PubMed] [Google Scholar]
- King-Smith PE, Fink BA, Hill RM, Koelling KW, Tiffany JM. The thickness of the tear film. Curr. Eye. Res. 2005;29:357–368. doi: 10.1080/02713680490516099. [DOI] [PubMed] [Google Scholar]
- King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom. Vis. Sci. 2008;85:623–630. doi: 10.1097/OPX.0b013e318181ae60. [DOI] [PubMed] [Google Scholar]
- King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest. Ophthalmol. Vis. Sci. 2010;51:2418–2423. doi: 10.1167/iovs.09-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig BW, Ferretti JA, Gawrisch K. Site-specific order parameters and membrane-bound behavior of a peptide fragment from the intracellular domain of HIV-1 gp41. Biochemistry. 1999;38:6327–6334. doi: 10.1021/bi982800g. [DOI] [PubMed] [Google Scholar]
- Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J. Am. Optom. Assoc. 1980;51:243–251. [PubMed] [Google Scholar]
- Korb DR, Baron DF, Herman JP, Finnemore VM, Exford JM, Hermosa JL, Leahy CD, Glonek T, Greiner JV. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359. doi: 10.1097/00003226-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Kóta Z, Debreczeny M, Szalontai B. Separable contributions of ordered and disordered lipid fatty acyl chain segments to nuCH2 bands in model and biological membranes: a Fourier transform infrared spectroscopic study. Biospectroscopy. 1999;5:169–178. doi: 10.1002/(SICI)1520-6343(1999)5:3<169::AID-BSPY6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, Sullivan DA. Effect of androgen deficiency on the human meibomian gland and ocular surface. J. Clin. Endocrinol. Metab. 2000;85:4874–4882. doi: 10.1210/jcem.85.12.7072. [DOI] [PubMed] [Google Scholar]
- Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Maïssa C, Guillon M. Tear film dynamics and lipid layer characteristics–effect of age and gender. Cont. Lens. Anterior. Eye. 2010;33:176–182. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Mantsch HH, McElhaney RN. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids. 1991;57:213–226. doi: 10.1016/0009-3084(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim. Biophys. Acta. 2008;1778:1545–1575. doi: 10.1016/j.bbamem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- McDonald JE. Surface phenomena of tear films. Trans. Am. Ophthalmol. Soc. 1968;66:905–939. [PMC free article] [PubMed] [Google Scholar]
- Miano F, Calcara M, Millar TJ, Enea V. Insertion of tear proteins into a meibomian lipids film. Colloids. Surf. B. Biointerfaces. 2005;44:49–55. doi: 10.1016/j.colsurfb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Millar TJ, Tragoulias ST, Anderton PJ, Ball MS, Miano F, Dennis GR, Mudgil P. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest. Ophthalmol. Vis. Sci. 2009;50:140–151. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospholipid and meibomian lipid monolayer films. Colloids. Surf. B. Biointerfaces. 2006;48:128–137. doi: 10.1016/j.colsurfb.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp. Eye. Res. 2008;86:622–628. doi: 10.1016/j.exer.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Mohidin N, Bay TC, Yap M. Non-invasive tear break-up time in normal Malays. Clin. Exp. Optom. 2002;85:37–41. doi: 10.1111/j.1444-0938.2002.tb03070.x. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Wyrwa M, Reboulleau CP, Mendelsohn R. Quantitative IR studies of acyl chain conformational order in fatty acid homogeneous membranes of live cells of Acholeplasma laidlawii B. Biochemistry. 1993;32:6281–6287. doi: 10.1021/bi00075a023. [DOI] [PubMed] [Google Scholar]
- Nagyová B, Tiffany JM. Components responsible for the surface tension of human tears. Curr. Eye. Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- Nunes DP, Keates AC, Afdhal NH, Offner GD. Bovine gall-bladder mucin contains two distinct tandem repeating sequences: evidence for scavenger receptor cysteine-rich repeats. Biochem. J. 1995;310:41–48. doi: 10.1042/bj3100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong BL, Hodson SA, Wigham T, et al. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr. Eye. Res. 1991;10:1113–1119. doi: 10.3109/02713689109024128. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using Raman spectroscopy. Curr. Eye. Res. 2009;34:824–835. doi: 10.3109/02713680903122029. [DOI] [PubMed] [Google Scholar]
- Ozdemir M, Temizdemir H. Age- and gender-related tear function changes in normal population. Eye. 2010;24:79–83. doi: 10.1038/eye.2009.21. [DOI] [PubMed] [Google Scholar]
- Popova AV, Hincha DK. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier transform infrared spectroscopy study. Biophys. J. 2003;85:1682–1690. doi: 10.1016/S0006-3495(03)74598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke CJ. Comments on “the thickness of the tear film”. Curr. Eye. Res. 2005;30:1131–1132. doi: 10.1080/02713680500445815. [DOI] [PubMed] [Google Scholar]
- Robosky LC, Wade K, Woolson D, et al. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J. Lipid. Res. 2008;49:686–692. doi: 10.1194/jlr.D700035-JLR200. [DOI] [PubMed] [Google Scholar]
- Singh H, Sarkar A. Mucin stabilized lactoferrin-lipid emulsions. Adv. Colloid. Interface. Sci. 2011;165:47–57. doi: 10.1016/j.cis.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Shrestha RK, Borchman D, Foulks GN, Yappert MC, Milliner SE. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults and adults with Meibomian gland dysfunction using 1H-NMR spectroscopy. Invest. Ophthalmol. Vis. Sci. 2011;52:7350–7358. doi: 10.1167/iovs.11-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest. Ophthalmol. Vis. Sci. 1991;32:2272–2280. [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346. doi: 10.1097/00003226-199607000-00002. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch. Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74. doi: 10.1097/00003226-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783. doi: 10.1097/01.ico.0000133995.99520.1f. [DOI] [PubMed] [Google Scholar]
- Smith BF. Human gallbladder mucin binds biliary lipids and promotes cholesterol crystal nucleation in model bile. J. Lipid. Res. 1987;28:1088–1097. [PubMed] [Google Scholar]
- Tang D, Borchman D. Temperature induced structural changes of betacrystallin and sphingomyelin binding. Exp. Eye. Res. 1998;67:113–118. doi: 10.1006/exer.1998.0497. [DOI] [PubMed] [Google Scholar]
- Tang D, Borchman D, Harris N, Pierangeli S. Lipid interactions with human antiphospholipid antibody, beta 2-glycoprotein 1, and normal human IgG using the fluorescent probes NBD-PE and DPH. Biochim. Biophys. Acta. 1998a;1372:45–54. doi: 10.1016/s0005-2736(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Tang D, Borchman D, Yappert MC, Cenedella RJ. Influence of cholesterol on the interaction of alpha-crystallin with phospholipids. Exp. Eye. Res. 1998b;66:559–567. doi: 10.1006/exer.1997.0467. [DOI] [PubMed] [Google Scholar]
- Tang D, Borchman D, Yappert MC. Alpha-crystallin/lens lipid interactions using resonance energy transfer. Ophthalmic. Res. 1999;31:452–462. doi: 10.1159/000055571. [DOI] [PubMed] [Google Scholar]
- Tang D, Borchman D, Yappert MC, Vrensen GF, Rasi V. Influence of age, diabetes, and cataract on calcium, lipid–calcium, and protein–calcium relationships in human lenses. Invest. Ophthalmol. Vis. Sci. 2003;44:2059–2066. doi: 10.1167/iovs.02-0345. [DOI] [PubMed] [Google Scholar]
- Terada O, Chiba K, Senoo T, et al. Ocular surface temperature of meibomia gland dysfunction patients and the melting point of meibomian gland secretions. Nippon Ganka Gakkai Zasshi. (J. Jpn. Ophthalmol. Soc.) 2004;108:690–693. [PubMed] [Google Scholar]
- Tiffany JM. Individual variations in human meibomian lipid composition. Exp. Eye. Res. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- Tiffany JM. The normal tear film. Dev. Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul. Surf. 2005;3:81–95. doi: 10.1016/s1542-0124(12)70157-x. [DOI] [PubMed] [Google Scholar]
- Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br. J. Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota K. Tear dynamics and dry eye. Prog. Retin. Eye. Res. 1998;17:565–596. doi: 10.1016/s1350-9462(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Hata S, Okusawa Y, Egami F, Ohtsuki T, Nakamori K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch. Ophthalmol. 1996;14:715–720. doi: 10.1001/archopht.1996.01100130707012. [DOI] [PubMed] [Google Scholar]
- Van Haeringen NJ. Clinical biochemistry of tears. Surv. Ophthalmol. 1981;26:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- Wolff E. Muco-cutaneous function of the lid margin and the distribution of the tear fluid. Trans. Ophthalmol. Soc. U. K. 1946;66:291–308. [Google Scholar]