Abstract
Alternative and innovative targeted strategies hold relevance in improving the current treatments for ischemic heart disease (IHD). One potential treatment modality, gene targeting, may provide a unique alternative to current IHD therapies. The principal function of gene targeting in IHD is to augment the expression of an endogenous gene through amplification of an exogenous gene, delivered by a plasmid or a viral vector to enhance myocardial perfusion, and limit the long-term sequelae. The initial clinical studies of gene targeting in IHD were focused upon induction of angiogenic factors and the outcomes were equivocal. Nevertheless, significant advancements have been made in viral vectors, mode of delivery, and potentially relevant targets for IHD. Several of these advancements, particularly with a focus on translational large animal studies, are the focus of this review. The development of novel vectors with prolonged transduction efficiency and minimal inflammation, coupled with hybrid perfusion-mapping delivery devices, and improving the safety of vector use and efficacy of gene systems are but a few of the exciting progresses that are likely to proceed to clinical studies in the near future.
Keywords: gene therapy, myocardial infarction, ischemia, plasmid, adenovirus, adeno-associated virus
1. Introduction
Ischemic heart disease (IHD) can be defined as that which arises from inadequate myocardial perfusion, which can be acute and transient in nature, such as that associated with an acute coronary syndrome and myocardial infarction (MI), or chronic suboptimal perfusion, which can occur with disease states such as ischemic dilated cardiomyopathy. In either case, IHD is associated with significant changes in the structure and function of both the vascular and myocardial compartments, which is continuous and progressive. While there has been significant and appropriate focus upon the vascular compartment with IHD in terms of thrombolytics, coronary stents, and revascularization, as well as antiplatelet and lipid lowering therapies, much less progress has been made in pharmacological treatments, which directly affect myocardial structure in this context. IHD is invariably associated with changes in LV geometry and myocardial composition, generically termed LV remodeling, and is a significant and independent predictor for morbidity and mortality. [1–4] For example, while early recovery following an MI is excellent, LV remodeling often occurs and is a structural milestone in the progression to heart failure (HF). [4] Current pharmacological therapy in the post-MI setting is primarily focused upon the interruption of neurohormonal pathways, which includes the systemic delivery of beta-adrenergic and angiotensin-II receptor system inhibitors. [5] However, the post-MI remodeling process can continue unabated in patients receiving current standard of care pharmacotherapy. One potential therapeutic approach would be to target the remodeling myocardium directly, such as that of gene targeting. In this approach, targeting specific biological systems that are relevant to adverse LV remodeling and progressive dysfunction can be achieved and therefore provide a unique alternative or adjunct to current IHD therapies. Gene targeting involves the overexpression or inhibition of a specific gene or translational modifier in order to address the underlying mechanisms that cause LV remodeling and/or dysfunction in IHD. There have been significant advancements in the approaches for expressing a specific gene of interest into the myocardium, many of which have progressed to early clinical trials. The overall goal of this review is to examine some issues regarding gene targeting relevant to myocardial delivery and examine specific examples of pathways and systems that have been manipulated by these delivery approaches in the context of LV remodeling and HF. There is a large body of translational and clinical research regarding gene targeting and vascular remodeling, particularly as it applies to vascular stents, grafts, and restenosis. [6–8 ] The focus of this review will not be on these applications per se, but rather on those delivery systems and targets that have been used to affect LV myocardial structure and/or function, with a particular emphasis on those studies targeting LV remodeling and dysfunction with IHD.
2. Expression vectors in gene targeting and relevance to myocardial delivery
To effectively promote successful gene transfer and subsequent expression in a host cell, careful consideration must be given to the choice of DNA delivery vector. The two vector types most commonly examined for translational and clinical studies are naked DNA plasmids and viral vectors. In choosing a plasmid or viral vector, it is important to consider the type of cell that is being altered and the longevity of expression needed to induce an effect in the targeted host cells while limiting the side effects that can occur. In terms of LV remodeling and IHD, the targeted cells may be of a transient nature, such as inflammatory cells, but most often are targeting two distinct cell types: the cardiocyte and the fibroblast. It must be emphasized that significant efforts are underway to modify the vascular smooth muscle cell and endothelial cells of the coronary and systemic vasculature, but this will not be the focus of this review. Rather, potential delivery systems and vectors that would allow for targeting the LV myocardial cells (cardiocytes, fibroblasts) will be briefly reviewed in terms of advantages and disadvantages, with a summary provided in Table 1.
Table 1.
Vectors and systems for gene targeting and myocardial delivery
|
|
|||
|---|---|---|---|
| Advantages | Disadvantages | ||
| Non-Viral | Plasmids | Simplified construction, versatility of expression cassette size, favorable biosafety risk profiles | Low transduction efficiency, transient expression |
|
|
|||
| Liposomes | Allow for targeting at the cell surface, ultrasound manipulation possible, favorable biosafety risk profiles | More complex construction, usually requires competent blood flow, transient expression | |
|
| |||
| Viral | Replication Deficient Adenoviruses | Large DNA packaging possible, enters both dividing and non-dividing cells, well suited for myocardial delivery | Serotype immunogenicity, potential cytotoxicity, transient expression |
|
|
|||
| Adeno-associated Virus | Greater stability than Adenovirus, enters both dividing and non- dividing cells, well suited for myocardial delivery, longer periods of expression, reduced immunogenicity | DNA insertion possible causing oncogenic potential, smaller DNA packaging | |
|
|
|||
| Lentivirus | Integrates into host DNA for long term expression, minimal immunogenicity | Smaller DNA packaging, high tropism for dividing cells, insertional mutagenesis | |
2.1 Plasmid gene delivery
Gene targeting using plasmid vectors was primarily developed to diminish the immunogenic side effects that exist with viral mediated gene expression. [9,10] Plasmids are circular, double-stranded, naked DNA molecules that vary in size from 1 kilobase (kb) to greater than 1,000 kb. A plasmid can hold 1–10 kb of the targeted gene of interest, but the longer the foreign DNA is in kb, the more difficult the plasmid is to transfect efficaciously into a host cell. To effectively deliver exogenous genes using plasmid DNA, multiple extracellular and cellular obstacles must be overcome, which include avoiding the degradation by nucleases in the extracellular space, [11] difficulty crossing the cell membrane, [9] and degradation by nucleases and lysosomes in the cytoplasm of the host cell. [10] All of these impediments contribute to low transfection efficiency and must be factored into vector choice. One advancement in the non-viral approach is the use of cationic liposomes. When mixed with the negatively charged plasmid DNA, the cationic liposome surrounds the plasmid DNA and facilitates interaction with the negatively charged glycoproteins and proteoglycans on the host cell surface. [12] The liposome complexed with the plasmid DNA can then be internalized through the active process of endocytosis, but it can also enter the host cell through passive processes, such as pinocytosis. Typically, plasmids remain extrachromosomal within the nucleus and do not integrate into the genome of the host cell, and therefore have a short period of transduction.
2.2 Viral gene delivery
The major advantage of viral vectors over plasmid DNA alone is the higher transduction efficiency because of the ability of a virus to actively deliver DNA across the host cell membrane and into the nucleus. A previous large animal study compared gene expression of LacZ in the myocardium delivered by a viral vector versus a plasmid-liposome complex. [13] The study demonstrated a 100-fold increase in gene expression when using the virus. [13] Thus, the understanding of the complex structural features and mechanisms of action of various viral vectors remains an important consideration when trying to overexpress exogenous genes. Of the viral vectors that have been studied previously, the most widely used in gene targeting are the replication-deficient adenoviruses (AdV), the adeno-associated viruses (AAV), and the retroviruses.
2.2.1 Adenovirus
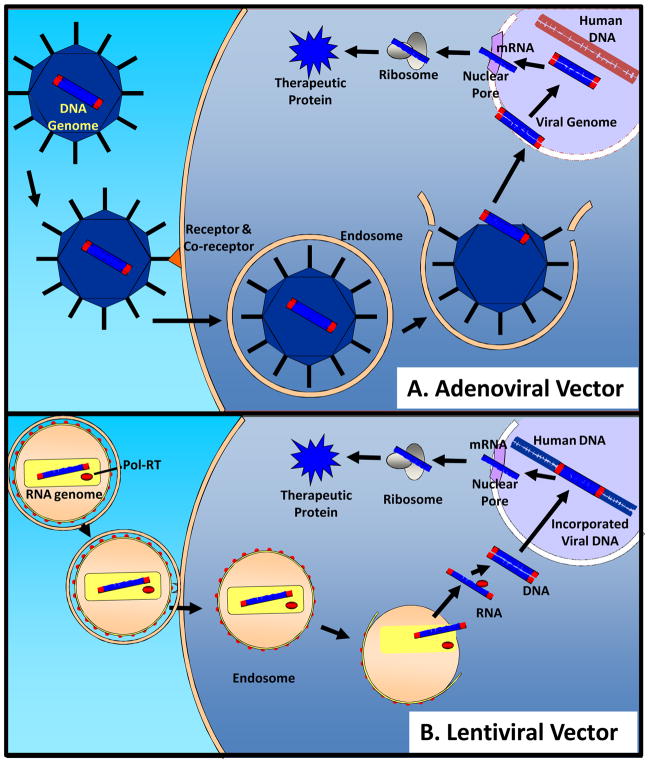
The AdV is a non-enveloped virus with an icosahedral capsid that surrounds a linear, double-stranded DNA genome. The capsid has 12 capsomers, which consist of a spherical base and a long fiber that may help in binding host cell membrane receptors. The genome ranges from 26 to 40kb in length, and it is comprised of two major transcription regions flanked by inverted terminal repeats. One of these major transcription regions contains four transcription units, E1, E2, E3, and E4, which are important for transcription of the viral genome and viral replication. In creating AdV vectors for gene targeting, the E1 and portions of the E3 regions are deleted to render the virus replication-deficient. Importantly, these deletions, along with other non-essential viral DNA components, provide space to allow up to approximately 36 kb of foreign exogenous DNA to be incorporated into the AdV vector. [14] When transducing host cells (Figure 1A), the AdV interacts with the coxsackie and adenovirus receptor (CAR), which in combination with secondary receptors, such as the αv integrin, induces clathrin-mediated endocytosis. Once the endosome is internalized, the viral DNA is released into the cytoplasm and shuttled to the nucleus where it enters through the nuclear pore complex. Thus, a recombinant AdV is capable of infecting both dividing and non-dividing cells. In the nucleus, the viral DNA is maintained extrachromosomally and is stabilized by the adenoviral terminal protein, which binds covalently to the ends of the viral DNA, protecting it from nuclease degradation and directing viral DNA transcription using host cell machinery.
Figure 1.
A) When transducing a host cell, the adenoviral vector (AdV) encoding an exogenous gene within the viral DNA genome binds to receptors and co-receptors to promote clathrin-mediated endocytosis. Once the AdV is incorporated into an endosome within the host cell’s cytoplasm, the viral DNA is released into the cytoplasm. During cellular division, the nuclear membrane is degraded, and the viral DNA enters the nucleus where it is maintained extrachromosomally. The viral DNA, including the exogenous gene, can then undergo transcriptional and translational processing to form a therapeutic protein. B) The envelope of the lentivirus vector encoding an exogenous gene within the viral RNA genome fuses with the cell membrane of the host cell. This causes the release of the lentiviral capsid into the cytoplasm where the viral RNA is subsequently released and reverse transcribed into DNA. The viral DNA is then shuttled into the nucleus where it permanently incorporates into the host cell’s genome. The viral DNA with the exogenous gene can then undergo transcription and translation with the host cell’s genome. (Figure Adapted from references [14–20]
There are two primary disadvantages to using AdV technology. First, in dividing cells, the AdV DNA episome segregates with a single daughter cell, effectively “diluting” out the desired structural or functional change derived from the overexpression of exogenous gene over time. In non-dividing cells, the AdV genome can be stably expressed for many months. Second, and most importantly, AdV expressing cells are often limited due to the host immune response. Viral encoded proteins of the AdV vector stimulate immune reactions, which include the activation of antibodies, inflammation, and elimination of the AdV and host cell death. Because the majority of the population has been exposed to serotype 2 and 5 adenoviruses, overcoming this immune response remains a problematic issue.
2.2.2 Adeno-associated virus
The wild-type AAV was initially discovered as a contaminant within AdV preparations. It is a unique vector because it lacks all the genes necessary for viral replication. In fact, wild type AAVs require co-transduction by a helper virus in order for infection to occur. [15,16] The wild type AAV is a stable, non-pathogenic, non-enveloped, icosahedral DNA virus that is 22 nanometers (nm) in diameter. It contains a single-stranded linear genome, which encodes four replication genes and three capsid genes that are flanked by inverted terminal repeats. [15,16] Currently, there are six described AAV serotypes: AAV-1 and AAV-4 have been isolated from primates; AAV-2, AAV-3, and AAV-5 have been isolated from humans; and AAV-6 has been isolated from an adenoviral preparation. [17] In creating the AAV vector for gene targeting, the genes for replication and the genes for capsid formation are removed from the viral genome to create a “gutted” AAV molecule that can accommodate 4.7–5 kb of foreign DNA. The primary cellular receptors for AAV are heparan sulfate proteoglycans, but like AdV, cellular interaction is enhanced by secondary cell membrane receptors. The recombinant AAV then undergoes clathrin-mediated endocytosis and internalization in a similar manner to the AdV vector. In contrast to the AdV vector, there is evidence to suggest that several mechanisms may exist in promoting the release of viral DNA from the recombinant AAV-endosome complex. Either the intact viral capsid is released from the endosome, or upon fusion of the endosome with the lysosome, the viral DNA is released alone. [18,19] Once the viral DNA is shuttled to the nucleus, the single stranded DNA genome is converted to a double stranded DNA molecule. The double stranded DNA molecule can then either exist extrachromosomally or it can be incorporated into the host cell’s genome where it will remain stable over time, even in dividing cells. [17] For example, delivery of an AAV vector via an intracoronary arterial infusion in pigs resulted in expression of the viral DNA for over 6 months with minimal side effects. [21] While genome-integrating viral DNAs are advantageous in the longevity of expression, there is a potential risk of oncogenesis and gene mutation due to random insertion of the AAV viral DNA.
2.2.3 Retrovirus
Another unique class of viral vectors currently being studied is the retroviral vectors, of which Lentivirus is the prototypical example. The retrovirus is a membrane-enveloped virus that also contains a specific retroviral capsid measuring approximately 80–100 nm in diameter. [22] Like the AdV and AAV, the recombinant retrovirus is packaged with the assistance of multiple co-transfected plasmids encoding the necessary replication and capsid genes. The viral envelope fuses with the cell membrane, which releases the recombinant retroviral vector into the cytoplasm of the host cell (Figure 1B). [22] Next, the viral reverse transcriptase transcribes the single stranded RNA genome into a double stranded cDNA molecule that is incorporated into the host genome. [23,24] Similar to the AAV, one concern that is difficult to address is the lack of specificity of lentiviral genome integration in the host genome. For instance, the viral genome can be incorporated in the middle of a host gene, which can cause insertional mutagenesis. Nevertheless, clinical studies of Parkinson’s disease, sickle cell anemia, and cancer immunotherapy have utilized the lentiviral vector and demonstrated its efficacy and safe use in patients. [25–27]
3. Modes of delivery and expression vectors in translational IHD studies
While significant insight has been obtained from using rodent models of IHD in terms of identification of mechanisms and potential targets for interrupting the progression of LV remodeling and dysfunction, these studies can hold limitations. For example, differences exist with regards to cardiac characteristics, such as heart rate and oxygen consumption between mice and humans. As a result, extrapolation of murine systems of IHD can become problematic when making pathophysiological interpretations. Moreover, DNA delivery in rodent systems may not necessarily recapitulate modes of delivery that would be advanced clinically. Therefore, for the purposes of this review, an emphasis will be on large animal model studies that have provided some of the foundation for advancing gene targeting strategies for LV remodeling and dysfunction. One important advantage of these large animal studies is that it not only provided the platform by which to examine the relative efficacy of the different vectors (i.e. plasmid versus viral), but also another critical consideration –mode of delivery. Thus, the next section first provides a brief review on the different vector delivery methods that have been utilized, and then examines a specific application relevant to IHD.
4. Modes of Vector Delivery
The delivery methods of a non-viral or viral vector for gene targeting can be performed either by direct administration into the myocardium or by utilizing a transvascular approach, through intra-arterial or intra-venous routes.
4.1 Intramyocardial administration
The majority of large animal pre-clinical studies utilize the administration of a vector directly to the myocardium, [28–33] which allows for a higher concentration of gene expression at the targeted injection sites. Intramyocardial injections can be performed either under direct visualization during an open operation or by endocardial injections achieved through percutaneous coronary catheterization. While direct visualization of the targeted injection site provides for precise control, this is highly invasive and would require a significant surgical procedure. Accordingly, endocardial injections via coronary catheterization have been evaluated in the setting of gene targeting for IHD by utilizing non-fluoroscopic electromechanical mapping (NOGA). [34,35] The system is designed so that it can integrate intracardiac electrograms at multiple endocardial locations to create an electroanatomical map that generates a geometric map of the LV. Ischemic myocardium is detected by calculating localized fractional shortening from endocardial potentials, and thereby provides a target for precise endocardial injections. [35]
4.2 Intracoronary Artery/Vein Administration
Minimally invasive approaches to gene targeting in IHD, such as intracoronary artery administration through coronary catheterization, are attractive administration techniques because of the potential for translation to clinical coronary catheterization procedures. Either the vector can be administered through one specific coronary artery or to multiple coronary arteries. [36–38] Continued refinements in the catheter design will likely allow for more efficient and robust delivery from the coronary vasculature. As just one example, an infusion-perfusion catheter was developed to precisely control the infusion of the vector this issue. [39] When inflated, the catheter has an outer lumen allowing prolonged viral contact with the targeted arterial wall, while permitting blood flow through the center of the catheter, maintaining myocardial perfusion. [39] A second commonly used viral delivery mode is by retrograde infusion within a coronary vein. [40,41] For example, in a pig model, retrograde coronary venous infusion of an AdV reporter construct demonstrated significant transduction in a pig. [41] This approach appeared to be as effective as open intramyocardial injections. As detailed in subsequent sections, hybrid coronary vascular injection systems are likely to provide optimal delivery.
There are several considerations regarding the direct or vascular mode of delivery that can be particularly relevant to IHD. Firstly, myocardial injections allow for more precise control and in principle, avoid vascular contamination. With coronary vascular injections, either through a coronary or venous delivery approach, “spillover” to the systemic vasculature can occur, possibly resulting in transduction to off-target sites. For example, in a past report, AdV mediated delivery of fibroblast growth factor (FGF) to the myocardium by intracoronary administration was successful; measurable expression of FGF in distant organs from the target site, including the lungs, liver, and spleen also occurred. [36] Infusion of a vector within the coronary vasculature may also result in more transient exposure to the target cells and sites as opposed to direct myocardial injection. However, myocardial injection can result in more heterogeneous distribution of the vector, whereby coronary vascular delivery may allow for a more uniform delivery pattern. One important consideration of these approaches in terms of IHD is that a vasculature capable of intravascular perfusion and delivery must be present, which may not necessarily exist, particularly with permanently occluded coronary vessels. Thus, the first set of studies utilizing gene targeting in the setting of IHD employed the direct myocardial injection method, and following which, these studies advanced to catheter based delivery approaches. These studies are outlined in the next section.
4.3 Gene targeting in large animal models of IHD
The development of plasmid vectors for use in gene targeting was originally driven by the potential benefits of diminished immunogenicity when compared to viral vectors, and a number of studies used this approach in the context of IHD, particularly for delivery of angiogenic factors. [31,33–38,42–44] For example, in a canine model of myocardial ischemia, a plasmid encoding vascular endothelial growth factor (VEGF) has been utilized via direct myocardial injection or through intracoronary delivery. [31,42–44] One of the main response variables was collateral blood flow, and this appeared to be increased with either delivery approach. However, chronic ischemia in the canine model can be associated with significant collateral growth in and of itself, which would make interpretation of the gene delivery approach of angiogenic factors, such as VEGF, difficult to infer. Thus, subsequent studies utilized a porcine model where, like humans, minimal collateral blood flow develops with chronic ischemia. [43,44] For example, Tio and colleagues demonstrated improvements in myocardial blood flow three weeks post-MI, as measured by coronary angiography. [44] Choi and colleagues demonstrated increased capillary density within the MI region, as well as improved regional segmental perfusion and wall motion on SPECT imaging 60 days after plasmid injection. [43] However, it should be noted that while relative improvements in blood flow or capillary growth were documented, a significant change in indices of LV function were not readily apparent. This may have been due to several factors, which included the short time of follow-up intervals, reduced overall transfection efficiency, and duration of VEGF expression, or that VEGF induction in and of itself is insufficient to significantly improve myocardial function in the context of IHD. As detailed in a subsequent section, clinical trials have provided additional insight into each of these possible factors in terms of the efficacy of VEGF induction in IHD.
In regards to viral delivery approaches, the most studied have been the serotype-2 and serotype-5 human AdV vectors, primarily due to the higher transfection efficiency achieved with these vectors. In studies using an AdV reporter construct, both intracoronary and intramyocardial injections achieved nearly 100 times greater transfection efficiency when compared to that of an analogous plasmid vector. [13,45] The efficacy of AdV-mediated delivery of a reporter gene was compared to plasmid-mediated gene delivery in normal porcine myocardium, utilizing both direct intramyocardial injection and intracoronary arterial infusion. Independent of delivery method, AdV-mediated gene delivery was more than 100 times more efficient in transferring the reporter gene as compared to a plasmid vector. [13,45] Using a porcine model of chronic ischemia, injection of an AdV construct encoding for proline/arginine-rich peptide (PR39) was utilized. [32] The rationale for selecting PR39 was predicated upon the fact that this peptide would inhibit the degradation of hypoxia-inducible factor-1α (HIF-1α), resulting in the upregulation of HIF-1α-dependent genes, including VEGF and FGF. [32] This study reported measurable improvements in myocardial blood flow as well as regional radial wall motion, which was accompanied by increased levels of VEGF, VEGF receptor-1 and receptor-2, and the FGF receptor. In a study by Mack and colleagues using a model of chronic ischemia in pigs, AdV mediated delivery of VEGF also improved indices of myocardial function, which notably was associated with minimal AdV mediated changes in inflammation. [30] Using a single intracoronary administration of an AdV encoding FGF in a porcine ischemia model, Giordano et al reported minimal changes in markers of inflammation, such as lymphocyte-cell specific staining. [47] Following myocardial ischemia in pigs, AdV vector encoding FGF was administered by isolated intracoronary artery injection, and increased protein levels of FGF were measurable within the myocardium 18 days after transduction. Thus, while concerns regarding long term myocardial inflammation and clearance of the AdV construct remain, these studies would suggest that a single administration of AdV, either through intracoronary or myocardial injection routes, were associated with minimal inflammatory response and can yield physiologically meaningful target protein concentrations. However, it is likely that a more robust inflammatory response would be encountered with repeated delivery of these AdV constructs within the same subject. The use of AAV vectors in terms of myocardial delivery has been evaluated in several different animal models in relation to AdV. [29,42] In general, duration of transduction is greatly improved with AAV, whereby persistence of expression and the downstream myocardial effects can be identified for as long as 1–2 months following the initial AAV transduction. [29,42] For example, in a canine model of MI, AAV encoding VEGF was injected into the ischemic region and the area of viable myocardium immediately surrounding it, known as the border zone. [42] Indices of myocardial function, arteriole density, and expression of VEGF receptors demonstrated persistently higher levels in the AAV-VEGF treated group. In a porcine model of myocardial ischemia, direct injection of AAV encoding VEGF yielded measurable improvements in myocardial function by MRI and vascular growth by histology. [29] Thus, AAV mediated delivery VEGF provided prolonged transduction and also associated with a lower risk of immune response. While these AAV strategies may improve VEGF delivery in the post-MI context, it remains unclear whether and to what degree a single angiogenic factor locally delivered to the targeted myocardial region is sufficient in and of itself to provide measureable beneficial effects. In a recent study by Lin and colleagues [48], self-assembling nanofibers were co-administered with the full length VEGF peptide in a porcine MI model. This study demonstrated that an artificial matrix scaffold, along with VEGF delivery, dramatically increased arteriole density and improved indices of the wound healing response. While this past investigation did not utilize gene delivery to augment localized VEGF per se, this investigation demonstrated that the local myocardial environment (i.e. matrix stability) likely plays a contributory role in the overall biological response that will be achieved through a targeted gene delivery strategy. Moreover, this past study as well as others [43–45,48] underscore how these large animal models of IHD can provide relevant preclinical information on viral vector strategies, delivery methods, and novel strategies, which may enhance the relative efficacy of the gene product.
As outlined in the preceding paragraph, the use of these large animal models provide the necessary platform for evaluating the effects of different AdV and AAV constructs in terms of myocardial structure, function, and inflammation in the context of IHD. For example, the efficacy of the NOGA system was evaluated by injecting different doses of AdV encoding VEGF into the porcine model. [33] Six days after injection, VEGF protein levels were detectable in a dose-dependent manner, and the microvessel area was significantly increased in the zone of direct endocardial injections, demonstrating the feasibility of the NOGA system as a delivery method for gene targeting to the myocardium. [33] In fact, several clinical trials have been performed using the NOGA system and targeted vector injections, which are presented in the next section. While these large animal studies can provide an invaluable platform for the development and evaluation of novel delivery strategies for gene targeting, the translation of these preclinical studies, particularly in terms of functional endpoints that would be relevant to regulatory entities can be problematic. For example, primary response variables in preclinical studies are often driven by measures of regional myocardial structure or function. In contrast, the primary response variable in large clinical trials is often driven by events such as morbidity and mortality. Thus, the functional and biological outcomes of these preclinical studies, as well as the short term clinical studies detailed in the following section, must be interpreted within this long term regulatory context. Nevertheless, large animal clinical studies provided the platform and rationale to move forward to initial clinical studies of safety and functional measures of efficacy.
5. Clinical Studies of Gene Targeting in IHD
While the previous large animal studies highlighted a number of unanswered questions regarding vector of choice, delivery method, and angiogenic target(s), optimism regarding the therapeutic potential for IHD was considerable. This can be demonstrated by the significant number of clinical proof of concept and efficacy studies through the transduction of genes involved in angiogenesis, such as VEGF and FGF, [46,47,49–61] summarized in Table 2 and 3.
Table 2.
Clinical studies of gene targeting in Ischemic Heart Disease utilizing plasmid vectors
| Reference | Trial Design | Number of Patients | Therapeutic Agent | Delivery | Endpoints | Outcomes | Major Adverse Events |
|---|---|---|---|---|---|---|---|
| [46] | Phase I | 5 | VEGF-A165 | Intramyocardial injection—mini-thoracotomy | Myocardial perfusion (SPECT) | Improved | None |
| [47] | Phase I | 20 | VEGF-A165 | Intramyocardial injection—mini-thoracotomy | Myocardial perfusion (SPECT); Angina/week | Improved; Improved | Arrhythmia (n=1) |
| [39] | Phase I | 15 | VEGF | Intracoronary injection | Restenosis at 6 months | No change | None |
| [49] | Phase I/II | 6 | VEGF-A165 | Intramyocardial injection—mini-thoracotomy | Maximal systolic velocities; CCS class; NTG/week | Improved; Improved; Improved | None |
| [50] | Open-Label Study | 7 | VEGF-A165 | Intramyocardial injection—mini-thoracotomy | Myocardial perfusion; CCS class; NTG/week | Improved; Improved; Improved | None |
| [51] | Phase I | 30 | VEGF-A2 | Intramyocardial injection—mini-thoracotomy | CCS; Angina/week; NTG/week | Improved; Improved; Improved | Death (n=1) |
| [35] | Pre-clinical | 6 | VEGF-A2 | NOGA—percutaneous endocardial injections | Myocardial perfusion (NOGA and SPECT) | Improved; Improved | None |
| [52] | PhaseI/II— RCT | 27 | VEGF-A2 | NOGA—percutaneous endocardial injections | Myocardial perfusion (NOGA and SPECT); CCS | Improved and Improvement trend; improved | CVA (n=2, placebo group) |
| [53] | Euroinject One Phase II | 80 | VEGF-A165 | NOGA—percutaneous endocardial injections | Myocardial perfusion (NOGA and SPECT); Regional wall motion; CCS; Angina/week | No change; Improved; Improved; Improved; No change | Cardiac tamponade (n=1), 3rd degree AV block (n=1), MI (n=1) |
| [54] | Phase I | VEGF-A165 | NOGA—percutaneous endocardial injections | Myocardial perfusion (SPECT and MRI); EDV (MRI); ESV (MRI) | No change and no change; No change; Improved | Death (n=1), MI (n=2) | |
| [55] | NORTHERN Phase II/III | 93 | VEGF-A165 | NOGA—percutaneous endocardial injections | Myocardial perfusion (SPECT); CCS | No change; Improved | Death (n=2), MI (n=6) |
VEGF, vascular endothelial growth factor; SPECT, single photon emission computerized tomography; CCS, Canadian Cardiovascular Society functional classification of angina; NTG, nitroglycerine tablets; NOGA, non-fluoroscopic electromechanical mapping; RCT, randomized controlled trial; CVA, cerebrovascular accident; MI, myocardial infarction; MRI, magnetic resonance imaging; EDV, end diastolic volume; ESV, end systolic volume
Table 3.
Clinical studies of gene targeting in Ischemic Heart Disease utilizing viral vectors
| Reference | Trial/Design | Number of Patients | Therapeutic Agent | Delivery | Endpoints | Outcomes | Major Adverse Events |
|---|---|---|---|---|---|---|---|
| [56] | Phase I | 21 | AdV-VEGF-A121 | Intramyocardial injections | CCS; NTG/week | Improvement trend; Improvement trend | None |
|
| |||||||
| [57] | AGENT RCT | 79 | AdV-FGF-4 | Intracoronary infusion | Stress-induced Wall Motion; | No change; | None |
| ETT | Improved | ||||||
|
| |||||||
| [58] | AGENT-2 RCT | 52 | AdV-FGF-4 | Intracoronary infusion | Perfusion Defect Size; | No change | None |
| Angina/week; | No change | ||||||
| NTG/week | No change | ||||||
|
| |||||||
| [59] | KAT Phase II | 103 | AdV-VEGF-A165 vs VEGF-A165 (plasmid) | Intracoronary infusion | Myocardial perfusion (SPECT); CCS | Improved (AdV), No change (plasmid); No change (AdV & plasmid) | Death (n=1), Transient fever (n=19, Adv; n=11, plasmid) |
|
| |||||||
| [60] | REVASC Phase II | 67 | AdV-VEGF-A121 | Intramyocardial injection—mini-thoracotomy | Myocardial perfusion (SPECT); | No change; | Death (n=1) |
| CCS | Improved | ||||||
|
| |||||||
| [61] | AGENT-3 & AGENT-4 RCT | 532 | AdV-FGF-4 | Intracoronary infusion | ETT; CCS; | No change; Improved(gender bias); | None |
| Angina/week; NTG/week | No change; No change | ||||||
AdV, adenoviral vector; VEGF, vascular endothelial growth factor; CCS, Canadian Cardiovascular Society functional classification of angina; NTG, nitroglycerine tablets; RCT, randomized controlled trial; FGF, fibroblast growth factor; ETT, exercise tolerance time; CK, creatine kinase; SPECT, single photon emission computerized tomography
5.1 Clinical trials using plasmid vectors
The first clinical study suggesting a positive outcome could be achieved was with plasmid injections encoding VEGF in patients, refractory to maximum medical therapy, and evidence of multi-vessel occlusive disease. [46] Through a small anterior thoracotomy incision, patients received direct intramyocardial injections of a plasmid encoding VEGF-A165. When comparing SPECT images from 60 days post-transfection to pre-treatment, myocardial perfusion was significantly improved. [46] The same group built upon these results and delivered the same plasmid vector encoding VEGF-A165 to a similar patient population using a percutaneous approach with the NOGA system of catheter-based mapping and navigation. [35,52] Myocardial perfusion was evaluated by SPECT imaging and mapped with the NOGA system. The results demonstrated increased myocardial perfusion at 90 days after VEGF-A165 transduction. [35,52]
With the success of the minimally invasive approach and these proof of concept studies, the NOGA system was utilized in subsequent placebo control studies, such as the Euroinject One trial and the NORTHERN trial. [53,60] The Euroinject One trial compared the use of a plasmid encoding VEGF-A165 to the use of a placebo in patients not eligible for conventional revascularization procedures. [53] With SPECT imaging, global perfusion defects under stress showed no improvement in the VEGF-A165 treatment group when compared to placebo injections at 90 days after transfection. [53] A calculation of regional systolic function was improved in the area of targeted VEGF-A165 delivery, but LV global systolic function and peak exercise tolerance time (ETT) remained unchanged. [53] The NORTHERN trial also compared a plasmid incorporating VEGF-A165 to placebo injections administered by the NOGA subendocardial injection system to patients with IHD. [60] Similar to the Euroinject One, SPECT imaging demonstrated no changes in blood flow at three and six months. Likewise, there were no changes in ETT or improvements in functional class when comparing VEGF-A165 treatment to placebo. [60] Alarmingly, the incidence of MI increased five-fold post transfection in the VEGF-A165 treatment group as compared to controls.
5.2 Clinical trials using viral vectors
Some examples of clinical studies using gene targeting by viral vector delivery in IHD are summarized in Table 3. The KAT trial compared AdV vector to plasmid vector encoding VEGF-A165 in IHD patients with greater than 60% re-stenosis in a coronary artery suitable for coronary angioplasty. [59] The vectors were delivered to the affected coronary artery using an infusion-perfusion catheter described in a previous section. By SPECT imaging and perfusion scoring, myocardial perfusion appeared to be qualitatively improved in those patients receiving AdV encoding VEGF-A165, but there were no improvements in coronary restenosis rates, minimal coronary artery lumen diameter, or clinical outcomes such as ETT. Interestingly, higher incidence of transient fever and elevated markers of systemic inflammation were observed in those patients treated with the AdV. [59]. Using a different VEGF isoform, the REVASC trial evaluated an AdV vector encoding VEGF121 in patients with severe angina secondary to coronary artery disease not amenable to conventional revascularization procedures. [59] Myocardial perfusion evaluated by SPECT imaging remained unchanged between the VEGF121 treatment group and patients receiving maximal medical management. However, ETT were improved with VEGF121 treatment at 26 weeks post-transduction when compared to patients on maximal medical management. [55] In alternative approaches, the initial AGENT and AGENT-1 studies administered an AdV encoding FGF-4 via intracoronary infusion to IHD patients, and suggested an early but not persistent improvement in ETT. [57,58] Subsequently larger AGENT-3 and AGENT-4 trials were stopped early when interim analysis demonstrated no improvement in ETT, angina class, or several other clinical outcomes when comparing patients treated with AdV encoding FGF-4 to placebo. [61]
The results from these clinical studies with equivocal results regarding the use of different vectors and delivery approaches for the expression of VEGF and FGF underscore several important issues. First, the choice of targeting one angiogenic factor for inducing meaningful vascular myocardial growth and blood flow may be problematic. Since, angiogenesis or arteriogenesis requires the complex interactions of a number of signaling molecules and proteases in a time and context specific manner, gene mediated induction of one biologically active molecule, such as VEGF, may be inadequate. Second, while coronary vascular delivery of either a plasmid or viral vector holds tremendous clinical potential, particularly when coupled with NOGA, there are likely perfusion related issues in the context of IHD that must be addressed. Third, these clinical studies proceeded without fully developing the viral vector and concentration that would be optimal, which would also have contributed to the variability of the results reported in Tables 2 and 3. However, the outcomes from these initial clinical studies do not imply that DNA targeting of the myocardium does not hold potential. As outlined in the next section, a stepwise approach using relevant animal models has achieved early clinical success in terms of myocardial delivery and efficacy in the context of heart failure.
6. Targets and Approaches for Myocardial Gene Expression – Future Directions
6.1 Developing targets
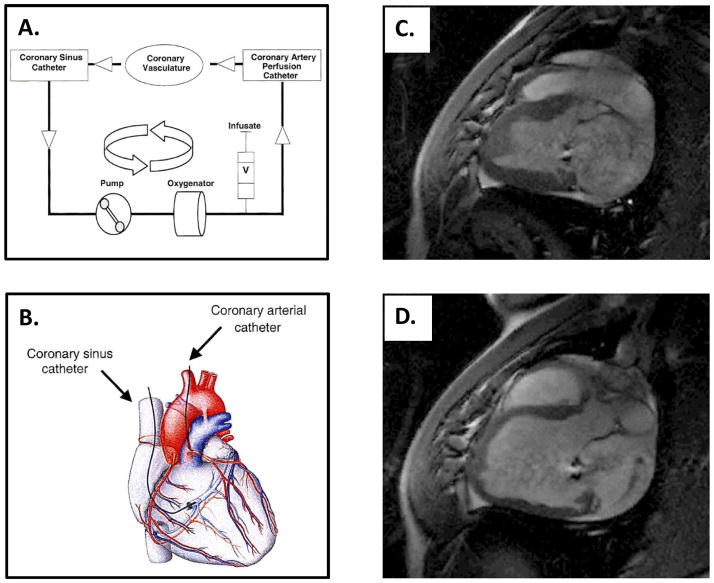
While not advanced for the purposes of IHD specifically, significant progress has been made in terms of gene targeted delivery of a calcium handling gene relevant to HF – the sarcoplasmic reticulum (SR) calcium ATPase (SERCA2a). [62–69] In several animal models of HF, SERCA2a activity is decreased in relationship to phospholamban, and this change in relative ratio has also been observed in human HF patients. AdV-mediated SERCA2a overexpression in primary cardiomyocytes cultured from patients with HF was initially shown to be associated with improved contractility and relaxation. [63] Using a large animal model of pacing induced HF, SERCA2a transduction was evaluated using a recombinant AAV (AAV2/1) administered by a recirculation technique. [62,68] Using this technique, the AAV2/1 encoding SERCA2a was infused into the left coronary and allowed to perfuse through the coronary arterial and venous circulations until the blood entered the coronary sinus. Here, a second catheter collected the blood containing the infusate and recirculated it back through the left coronary artery using a roller pump (Figure 2A and 2B). At 6 weeks post transduction, SERCA2a myocardial expression was significantly increased with minimal to no increase in markers of inflammation. [62] On histology, lymphocytes were noted to be increased at 4 weeks, but there were significantly less lymphocytes at 6 weeks when compared to control. To further build upon these results, a porcine model of volume overload HF was induced by rupture of the chordae tendineae of the mitral valve (Figure 2C and 2D). [67] This model was further evaluated for efficacy and safety of SERCA2a overexpression using an AAV-1 vector administered by the recirculation technique. At 2 months, SERCA2a levels were increased as were indices of LV ejection performance. Based upon these pre-clinical large animal results, the first Phase I/II trial using SERCA2a transduction as a treatment for HF was initiated. Designated as the CUPID studies [65,66], patients were enrolled initially as AAV-1 antibody negative, with significant LV systolic dysfunction. The AAV-1 vector encoding SERCA2a administered by percutaneous infusion into the dominant coronary artery using the recirculation technique was employed, and this approach was not associated with adverse events. [65–67] Initial outcomes revealed acceptable safety profiles and improved HF clinical indices, such as frequency of hospitalization and 6-minute walk tests. [69] However, while these early clinical outcomes are promising, these are not the event driven endpoints necessary to demonstrate clinical efficacy as defined by current regulatory standards. This initial clinical study does provide the basis for a larger scale clinical trial, which would encompass these necessary clinical endpoints. These studies did demonstrate however, that a stepwise translational research approach in gene targeting can provide critical insight in terms of the vector and mode of vector delivery that are relevant to clinical application.
Figure 2.
A) and B) The percutaneous approach to recirculating cardiac gene delivery, with the catheters positioned in the coronary artery circulation and the coronary sinus, has been extensively studied in large animal models of heart failure (HF) for the delivery of an adeno-associated viral (AAV) vector encoding SERCA2a and applied to the CUPID trial. SERCA2a is infused into the coronary artery perfusion catheter, allowed to perfuse through the coronary vasculature, and recovered by the coronary sinus catheter. The venous recovery system within the coronary sinus is connected to an extracorporeal pump-oxygenator circuit, with return to the myocardium by the coronary artery perfusion catheter. C) and D) One of the large animal models used to evaluate the safety and efficacy of the recirculating cardiac gene delivery prior to the CUPID trial is a porcine model of volume overload HF secondary to chordae tendineae rupture of the mitral valve.[72] C) The T1 weighted MRI image depicts the porcine heart prior to chordae tendineae rupture, and D) four months after rupture, confirms mitral regurgitation by the jet from the left ventricle (LV) back into the left atrium during diastole. With 4 months of volume overload, the LV has become severely dilated. (MRI images kindly supplied by Dr. Roger Hajjar whereby the images collected were part of a preclinical feasibility study described in reference 72; Images from A, B adapted from reference 68).
While the studies outlined above have targeted one specific molecular defect in the context of HF, this is by no means the only potential target. Since abnormal calcium handling is a ubiquitous finding in severe HF, other potential targets to modify this process through viral vectors have been evaluated. One approach has been to induce the calcium binding protein S100A1, which interacts with a number of calcium handling proteins and signaling molecules – notably, SERCA2. [70–72] Using an AdV mediated delivery approach, induction of S100A1 in isolated human HF cardiocytes demonstrated improved calcium handling and contractility. [70] In a porcine myocardial ischemia model, retrograde coronary venous delivery of AdV encoding S100A1 resulted in improved LV function and determinants of calcium handling. [71] Another molecular defect that is a commonality in severe HF is a desensitization in G protein coupled receptor signaling, notably the beta adrenergic receptor pathway. [73–77] Using AdV and AAV approaches, the introduction of targets that augment the effects of this signaling pathway is an area of active investigation. [73–78] For example, intracoronary delivery of AdV encoding a specific isoform of adenylyl cyclase, which would putatively increase beta adrenergic mediated phosphorylation, has been performed in a pacing model of HF. [77] This past study documented improved LV function and cyclic AMP levels with AdV mediated adenylyl cyclase induction. Indeed, this AdV mediated approach for delivery of adenylate cyclase has advanced to initial clinical safety trials. [79] Thus, these studies provide critical proof of concept that gene targeting, primarily through viral mediated approaches, can alter critical signaling pathways in HF.
6.2 Alternative Approaches for Myocardial Gene Targeting
Viral mediated gene transfer has been the method of choice for delivery to the myocardium, particularly in the context of myocardial injury. However, as outlined previously, this approach can be time-consuming and cost prohibitive in terms of production of large quantities of competent virus for delivery to the myocardium. Further, this method can induce a localized inflammatory response, can be limited in terms of cargo size, and finally, random insertion can give rise to mutagenesis. Thus, alternative approaches for gene transfer is an active area of research, and several of these alternative approaches likely hold relevance to myocardial delivery. These include the transposon systems, electrical field stimulation (electroporation), and lipid microbubbles. [80–86] The transposon systems have been the most developed and include the Sleeping Beauty and PiggyBac systems. [80–83,86–89] The transposon system utilizes an entirely different method for expressing a gene of interest when compared to the classical viral vector delivery approach. Specifically, this system entails two plasmids, where one plasmid contains the gene of interest and the second expresses the enzyme for the insertion – the transposase. [80–83] The most commonly studied transposon system, which was initially discovered from a prehistoric fish and termed Sleeping Beauty, exhibited relatively low transposase activity. [83] However, second generations of this system have been developed, which increase the relative transposase activity of Sleeping Beauty by 100-fold (SB100X), and this system has been utilized in a number of isolated cell systems. [80–82,89–92] The distinct advantage of the transposon system is a more controlled and targeted integration of the gene of interest, and thereby obviates some of the inherent limitations of viral mediated gene transfer. Specifically, Sleeping Beauty demonstrates an insertion site sequence preference that may allow for more specificity in terms of targeting the host genome. [80–83] A system such as Sleeping Beauty must be transported into the cell, and therefore, a carrier or means of transfer is required. Additional limitations to the transposon system include those similar to a viral mediated approach, comprising of the size of the payload, gene silencing, and disruption of transcription of nearby endogenous genes.
With respect to myocardial applications, possibly the most rate-limiting step of the transposon system is high efficiency transfer to the cell targets of interest. When using the transposon system in ex-vivo cell preparations, the electrical field stimulation or cationic polymer can facilitate transfer. [80–82,88,89,92] For example, a transposon system was delivered into human embryonic stem cells using a cationic polymer formulation, which allowed for transcriptional profiling and the identification of a subpopulation of cells expressing a set of cardiac myocyte related genes. [92] Using a lipid-cationic polymer delivery vehicle, it has been demonstrated that a transposon system can be delivered to pulmonary endothelial cells in-vivo. [90,91] Other potential approaches include electroporation – this technique has been described for gene delivery in a rodent heart, [84] but issues surrounding tissue injury, arrhythmogenesis, and selective targeting remain significant barriers to this approach. The use of lipid vesicles, such as lipid microbubbles sensitive to ultrasound, have been reported in terms of gene delivery, [85] but the specificity of this approach in terms of targeting a relatively low blood flow area, like the ischemic myocardium, and whether and to what degree the transposon system can be packaged in this formulation, remains unknown. In other studies, a hydro-dynamic based approach was utilized to globally deliver a Sleeping Beauty construct, encoding the luciferase gene. [87] In this study, high perfusion pressures were used to transfer the transposon system, but poor retention and persistence of expression were observed following 10 days of injection. From this past report, it is not readily apparent how this hydro-dynamic approach could be utilized in a large animal/clinical for delivery to myocardial cells beyond the vasculature, and particularly in the context of myocardial ischemia/injury. Interestingly and somewhat paradoxically, it has been demonstrated that one potential approach is for this transposon system to be packaged into adenovirus for in-vivo delivery. [93] Thus, gene delivery through the use of a transposon system for the purposes of cell based therapeutics may hold promise. However, mechanisms for specific and targeted delivery of the transposon system, such as Sleeping Beauty to the myocardium, particularly in terms of targeted delivery to the ischemic myocardium, will require further research and development.
7. Summary
The area of gene targeting and viral vectors is an ever growing and expanding field, and thus, this brief review was far from comprehensive. In particular, issues surrounding gene dosing and timing of delivery in the context of IHD and HF were not fully discussed. Nevertheless, it is clear that initial expectations regarding the beneficial effects of gene targeting with IHD in terms of angiogenesis and improvements in myocardial structure with delivery of a single growth factor have been met with disappointment. On the other hand, targeting specific molecular defects such as calcium handling processes or receptor signaling pathways as well as using more effective coronary vascular delivery approaches have yielded promising results in terms of severe HF. The use of large animal models that can properly recapitulate the clinical context in terms of the disease process under study, such as ischemic heart disease, as well as examine the effects of different vectors and delivery methods will continue to be a critical translational research path for myocardial gene targeting.
Acknowledgments
This work was supported by National Institute of Health grants HL057952, HL059165, HL095608, and a Merit Award from the Veterans’ Affairs Health Administration. SRE was supported by National Institute of Health grant T32 HL007260. The authors wish to thank Dr. Roger Hajjar for the images in Figure 2 and Ashley Sapp for editorial assistance.
Bibliography and References Cited
- 1.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011 Jan;4(1):98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Quiñones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000 Apr;35(5):1237–44. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 3.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987 Jul;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006 May 22;97(10A):13F–25F. doi: 10.1016/j.amjcard.2006.03.005. Epub 2006 Apr 21. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009 Apr 14;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. Epub 2009 Mar 26. [DOI] [PubMed] [Google Scholar]
- 6.Gaffney MM, Hynes SO, Barry F, O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol. 2007 Sep;152(2):175–88. doi: 10.1038/sj.bjp.0707315. Epub 2007 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young JJ. Neointimal formation following drug-eluting stents: physiology, timeline, and the influence of drug delivery systems. Rev Cardiovasc Med. 2007;8(Suppl 1):S3–10. [PubMed] [Google Scholar]
- 8.Mitra AK, Agrawal DK. Gene therapy of fibroproliferative vasculopathies: current ideas in molecular mechanisms and biomedical technology. Pharmacogenomics. 2006 Dec;7(8):1185–98. doi: 10.2217/14622416.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 9.Wolff JA, Budker V. The mechanism of naked DNA uptake and expression. Adv Genet. 2005;54:3–20. doi: 10.1016/S0065-2660(05)54001-X. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5:930–7. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 11.Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–30. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 12.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–81. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–24. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 14.Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- 15.Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754–6. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 16.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–12. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 17.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type. 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–99. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–45. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 21.Kaplitt MG, Xiao X, Samulski RJ, Li J, Ojamaa K, Klein IL, et al. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann Thorac Surg. 1996;62:1669–76. doi: 10.1016/s0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev. 2006;58:515–34. doi: 10.1016/j.addr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- 24.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, et al. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994;91:7311–5. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–16. doi: 10.1196/annals.1345.007. [DOI] [PubMed] [Google Scholar]
- 26.Chhabra A, Yang L, Wang P, Comin-Anduix B, Das R, Chakraborty NG, et al. CD4+CD25- T cells transduced to express MHC class I-restricted epitope-specific TCR synthesize Th1 cytokines and exhibit MHC class I-restricted cytolytic effector function in a human melanoma model. J Immunol. 2008;181:1063–70. doi: 10.4049/jimmunol.181.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams DA. Progress reported in two studies of clinical gene transfer into the retina. Mol Ther. 2008;16:1181. doi: 10.1038/mt.2008.123. [DOI] [PubMed] [Google Scholar]
- 28.Horvath KA, Doukas J, Lu C-YJ, Belkind N, Greene R, Pierce GF, et al. Myocardial functional recovery after fibroblast growth factor 2 gene therapy as assessed by echocardiography and magnetic resonance imaging. The Annals of Thoracic Surgery. 2002;74:481–7. doi: 10.1016/s0003-4975(02)03736-0. [DOI] [PubMed] [Google Scholar]
- 29.Jacquier A, Higgins CB, Martin AJ, Do L, Saloner D, Saeed M. Injection of adeno-associated viral vector encoding vascular endothelial growth factor gene in infarcted swine myocardium: MR measurements of left ventricular function and strain. Radiology. 2007;245:196–205. doi: 10.1148/radiol.2451061077. [DOI] [PubMed] [Google Scholar]
- 30.Mack CA, Patel SR, Schwarz EA, Zanzonico P, Hahn RT, Ilercil A, et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. The Journal of Thoracic and Cardiovascular Surgery. 1998;115:168–77. doi: 10.1016/s0022-5223(98)70455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pätilä T, Ikonen T, Rutanen J, Ahonen A, Lommi J, Lappalainen K, et al. Vascular Endothelial Growth Factor C-induced Collateral Formation in a Model of Myocardial Ischemia. The Journal of Heart and Lung Transplantation. 2006;25:206–13. doi: 10.1016/j.healun.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Post MJ, Sato K, Murakami M, Bao J, Tirziu D, Pearlman JD, et al. Adenoviral PR39 improves blood flow and myocardial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am J Physiol Regul Integr Comp Physiol. 2006;290:R494–500. doi: 10.1152/ajpregu.00460.2005. [DOI] [PubMed] [Google Scholar]
- 33.Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivela A, et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation. 1029;109:1029–35. doi: 10.1161/01.CIR.0000115519.03688.A2. [DOI] [PubMed] [Google Scholar]
- 34.Vale PR, Losordo DW, Tkebuchava T, Chen D, Milliken CE, Isner JM. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J Am Coll Cardiol. 1999;34:246–54. doi: 10.1016/s0735-1097(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 35.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–43. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 36.Gao MH, Lai NC, McKirnan MD, Roth DA, Rubanyi GM, Dalton N, et al. Increased regional function and perfusion after intracoronary delivery of adenovirus encoding fibroblast growth factor 4: report of preclinical data. Hum Gene Ther. 2004;15:574–87. doi: 10.1089/104303404323142024. [DOI] [PubMed] [Google Scholar]
- 37.Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nature Medicine. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circulation Research. 2005;96:767–75. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- 39.Laitinen M, Hartikainen J, Hiltunen MO, Eranen J, Kiviniemi M, Narvanen O, et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum Gene Ther. 2000;11:263–70. doi: 10.1089/10430340050016003. [DOI] [PubMed] [Google Scholar]
- 40.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–40. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 41.Raake P, von Degenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol. 2004;44:1124–9. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X, et al. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circulation Research. 2006;98:954–61. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 43.Choi JS, Kim KB, Han W, Kim DS, Park JS, Lee JJ, et al. Efficacy of therapeutic angiogenesis by intramyocardial injection of pCK-VEGF165 in pigs. Ann Thorac Surg. 2006;82:679–86. doi: 10.1016/j.athoracsur.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Tio RA, Tkebuchava T, Scheuermann TH, Lebherz C, Magner M, Kearny M, et al. Intramyocardial gene therapy with naked DNA encoding vascular endothelial growth factor improves collateral flow to ischemic myocardium. Hum Gene Ther. 1999;10:2953–60. doi: 10.1089/10430349950016366. [DOI] [PubMed] [Google Scholar]
- 45.French BA, Mazur W, Ali NM, Geske RS, Finnigan JP, Rodgers GP, et al. Percutaneous transluminal in vivo gene transfer by recombinant adenovirus in normal porcine coronary arteries, atherosclerotic arteries, and two models of coronary restenosis. Circulation. 1994;90:2402–13. doi: 10.1161/01.cir.90.5.2402. [DOI] [PubMed] [Google Scholar]
- 46.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 47.Symes JF, Losordo DW, Vale PR, Lathi KG, Esakof DD, Mayskiy M, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–6. doi: 10.1016/s0003-4975(99)00807-3. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 48.Lin YD, Luo CY, Hu YN, Yeh ML, Hsueh YC, Chang MY, Tsai DC, Wang JN, Tang MJ, Wei EI, Springer ML, Hsieh PC. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012 Aug 8;4(146):146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
- 49.Sylven C, Sarkar N, Ruck A, Drvota V, Hassan SY, Lind B, et al. Myocardial Doppler tissue velocity improves following myocardial gene therapy with VEGF-A165 plasmid in patients with inoperable angina pectoris. Coron Artery Dis. 2001;12:239–43. doi: 10.1097/00019501-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar N, Ruck A, Kallner G, SYH, Blomberg P, Islam KB, et al. Effects of intramyocardial injection of phVEGF-A165 as sole therapy in patients with refractory coronary artery disease--12-month follow-up: angiogenic gene therapy. J Intern Med. 2001;250:373–81. doi: 10.1046/j.1365-2796.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- 51.Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436–9. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- 52.Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–8. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 53.Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: A randomized double-blind placebo-controlled study: The Euroinject One trial. Journal of the American College of Cardiology. 2005;45:982–8. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 54.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–92. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 55.Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–15. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Annals of Surgery. 1999;230:466–70. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 58.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. Journal of the American College of Cardiology. 2003;42:1339–47. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 59.Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 60.Stewart DJ, Hilton JD, Arnold JMO, Gregoire J, Rivard A, Archer SL, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF121 (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–11. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 61.Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50:1038–46. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 63.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–11. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–7. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–67. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–60. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 69.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators . Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011 Jul 19;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. Epub 2011 Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmüller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011 Aug 23;58(9):966–73. doi: 10.1016/j.jacc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Müller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011 Jul 20;3(92):92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2010 Oct;3(5):525–37. doi: 10.1007/s12265-010-9211-9. Epub 2010 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Front Biosci. 2011 Jun 1;17:3047–60. doi: 10.2741/3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reinkober J, Tscheschner H, Pleger ST, Most P, Katus HA, Koch WJ, Raake PW. Targeting GRK2 by gene therapy for heart failure: benefits above β-blockade. Gene Ther. 2012 Jun;19(6):686–93. doi: 10.1038/gt.2012.9. Epub 2012 Feb 16. [DOI] [PubMed] [Google Scholar]
- 75.Rengo G, Lymperopoulos A, Leosco D, Koch WJ. GRK2 as a novel gene therapy target in heart failure. J Mol Cell Cardiol. 2011 May;50(5):785–92. doi: 10.1016/j.yjmcc.2010.08.014. Epub 2010 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phan HM, Gao MH, Lai NC, Tang T, Hammond HK. New signaling pathways associated with increased cardiac adenylyl cyclase 6 expression: implications for possible congestive heart failure therapy. Trends Cardiovasc Med. 2007 Oct;17(7):215–21. doi: 10.1016/j.tcm.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004 Jul 20;110(3):330–6. doi: 10.1161/01.CIR.0000136033.21777.4D. Epub 2004 Jul 12. [DOI] [PubMed] [Google Scholar]
- 78.Ly H, Kawase Y, Yoneyama R, Hajjar RJ. Gene therapy in the treatment of heart failure. Physiology (Bethesda) 2007 Apr;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 79.Kammond HK, Perry W, Henry TD. Clinical Trial: Ad5.hAC6 Gene Transfer for CHF. 2011. Nov 28, NCT00787059. [Google Scholar]
- 80.Meir YJ, Wu SC. Transposon-based vector systems for gene therapy clinical trials: challenges and considerations. Chang Gung Med J. 2011 Nov-Dec;34(6):565–79. [PubMed] [Google Scholar]
- 81.Ivics Z, Li MA, Mátés L, Boeke JD, Nagy A, Bradley A, Izsvák Z. Transposon-mediated genome manipulation in vertebrates. Nat Methods. 2009 Jun;6(6):415–22. doi: 10.1038/nmeth.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 2010 Apr;18(4):674–83. doi: 10.1038/mt.2010.2. Epub 2010 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivics Z, Hackett PB, Plasterk RH, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997 Nov 14;91(4):501–10. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 84.Ayuni EL, Gazdhar A, Giraud MN, Kadner A, Gugger M, Cecchini M, Caus T, Carrel TP, Schmid RA, Tevaearai HT. In vivo electroporation mediated gene delivery to the beating heart. PLoS One. 2010 Dec 30;5(12):e14467. doi: 10.1371/journal.pone.0014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000 Jun 6;101(22):2554–6. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 86.Aronovich EL, McIvor RS, Hackett PB. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum Mol Genet. 2011 Apr 15;20(R1):R14–20. doi: 10.1093/hmg/ddr140. Epub 2011 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakanishi H, Higuchi Y, Kawakami S, Yamashita F, Hashida M. piggyBac transposon-mediated long-term gene expression in mice. Mol Ther. 2010 Apr;18(4):707–14. doi: 10.1038/mt.2009.302. Epub 2010 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belay E, Mátrai J, Acosta-Sanchez A, Ma L, Quattrocelli M, Mátés L, Sancho-Bru P, Geraerts M, Yan B, Vermeesch J, Rincón MY, Samara-Kuko E, Ivics Z, Verfaillie C, Sampaolesi M, Izsvák Z, Vandendriessche T, Chuah MK. Novel hyperactive transposons for genetic modification of induced pluripotent and adult stem cells: a nonviral paradigm for coaxed differentiation. Stem Cells. 2010 Oct;28(10):1760–71. doi: 10.1002/stem.501. [DOI] [PubMed] [Google Scholar]
- 89.VandenDriessche T, Ivics Z, Izsvák Z, Chuah MK. Emerging potential of transposons for gene therapy and generation of induced pluripotent stem cells. Blood. 2009 Aug 20;114(8):1461–8. doi: 10.1182/blood-2009-04-210427. Epub 2009 May 26. [DOI] [PubMed] [Google Scholar]
- 90.Liu L, Sanz S, Heggestad AD, Antharam V, Notterpek L, Fletcher BS. Endothelial targeting of the Sleeping Beauty transposon within lung. Mol Ther. 2004 Jul;10(1):97–105. doi: 10.1016/j.ymthe.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Liu L, Liu H, Visner G, Fletcher BS. Sleeping Beauty-mediated eNOS gene therapy attenuates monocrotaline-induced pulmonary hypertension in rats. FASEB J. 2006 Dec;20(14):2594–6. doi: 10.1096/fj.06-6254fje. Epub 2006 Oct 25. [DOI] [PubMed] [Google Scholar]
- 92.Orbán TI, Apáti A, Németh A, Varga N, Krizsik V, Schamberger A, Szebényi K, Erdei Z, Várady G, Karászi E, Homolya L, Német K, Gócza E, Miskey C, Mátés L, Ivics Z, Izsvák Z, Sarkadi B. Applying a “double-feature” promoter to identify cardiomyocytes differentiated from human embryonic stem cells following transposon-based gene delivery. Stem Cells. 2009 May;27(5):1077–87. doi: 10.1002/stem.45. [DOI] [PubMed] [Google Scholar]
- 93.Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T, Kay MA. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat Biotechnol. 2002 Oct;20(10):999–1005. doi: 10.1038/nbt738. Epub 2002 Sep 16. [DOI] [PubMed] [Google Scholar]