Abstract
Objective
Sleep quality and ethnicity are related to a host of general health outcomes including the experience of pain, yet it remains unclear whether poor sleep quality and ethnicity might interactively affect pain catastrophizing and laboratory-evoked acute pain reports. The current study examined the cross-sectional associations of subjective sleep quality, ethnicity, and their interaction with pain catastrophizing and pain reports.
Design
Healthy (N = 149), ethnically diverse (58% Caucasian American, 23% Asian American, 19% African American) young adults were subjected to a cold pressor task (CPT). Prior to CPT, participants completed the Pittsburgh Sleep Quality Index (PSQI) and a standard version of the Pain Catastrophizing Scale (PCS). Following CPT, participants completed a situation-specific version of the PCS.
Results
Adjusted analyses revealed a significant sleep quality by ethnicity interaction for standard catastrophizing reports. Particularly, African Americans with poor overall sleep quality reported the greatest level of catastrophizing on the standard PCS relative to their Caucasian and Asian American counterparts. Furthermore, African Americans with poorer sleep efficiency reported greater catastrophizing on the situation-specific PCS compared to Caucasian and Asian Americans. Catastrophizing was significantly correlated with pain reports.
Conclusions
These results suggest that African Americans with poorer sleep quality may be at greater risk for catastrophizing, a known contributor to more intense pain and increased pain-related emotional distress. Whether interventions that improve the sleep quality of ethnic minorities affect pain catastrophizing is in need of investigation.
Keywords: Ethnicity, sleep quality, pain catastrophizing, cold pressor, disparities
INTRODUCTION
Catastrophizing, a negative emotional and cognitive response to pain, is a passive and maladaptive coping strategy that has previously been shown to exert a potent influence on individuals’ experiences of pain and accompanying emotional distress [see 1 for review]. Pain catastrophizing has been shown to predict important clinical outcomes such as greater chronic pain severity and related disability [1,2]. Similarly, in experimental studies involving healthy volunteers, catastrophizing has been reliably associated with increased pain sensitivity [3] and with diminished endogenous pain inhibitory controls [4]. Most of the research to date has examined catastrophizing as an independent predictor of pain-related outcomes. Accordingly, it is now generally well accepted that catastrophizing is a consistent risk factor for a host of negative pain experiences and reduced quality of life. Despite the established evidence base linking catastrophizing with negative pain outcomes, far less research has been dedicated to the examination of factors that might contribute to an individual’s propensity for engaging in passive coping strategies such as pain catastrophizing.
Over recent years it has become increasingly evident that insufficient sleep and poor sleep quality are highly predictive of individuals’ pain experiences (e.g., poor sleep quality is related to greater pain severity) as demonstrated in laboratory and clinical studies of sleep and pain [see 5 for review]. Although sleep has garnered increased attention for its relation to acute and chronic pain, far less attention has been given to the explanatory mechanisms that may underlie the sleep and pain relation. Psychosocial factors represent one potential domain explaining how sleep relates with pain, particularly pain catastrophizing. To date, no published study has examined the relation between sleep quality and pain catastrophizing. However, the possibility that sleep quality exerts an effect on pain catastrophizing can be gleaned from other studies which have demonstrated that poor sleep quality is related to increased trait and state anxiety and increased emotional distress [6,7]. Given that anxiety and emotional distress are constructs that overlap with pain catastrophizing, and are related to poor sleep quality, it seems plausible that poor sleep quality may also be related to pain catastrophizing.
When examining the relations among sleep quality, pain catastrophizing, and reports of pain, individuals’ ethnic background should be considered. A recent review of the literature [8] examined laboratory and clinical studies of ethnic differences in pain and revealed two general, yet key, themes: 1) findings from laboratory-based studies suggest greater sensitivity to experimental pain stimuli among African Americans compared with Caucasian Americans, and 2) higher levels of pain and disability have been reported among African Americans relative to Caucasian American patients that were treated for a variety of acute and persistent pain conditions. Furthermore, it has been recently demonstrated that individuals’ reports of catastrophizing during experimental noxious stimulation varied as a function of their self-selected ethnic background [9], yet findings are mixed with some studies reporting no relation between ethnicity and pain catastrophizing [10]. Lack of consistent results suggests that, in addition to exerting a direct effect, ethnicity may also act indirectly to affect catastrophizing. Lack of consideration for potential indirect effects is one possible explanation for why some studies have not demonstrated ethnic differences in catastrophizing. Although the direct relation between ethnicity and the use of passive coping strategies such as catastrophizing has previously been tested [11,12], whether ethnicity acts indirectly via a third variable (e.g., sleep quality) to influence pain catastrophizing remains untested. However, this seems reasonable given that ethnic differences in patterns of sleep and sleep quality have also previously been reported [13,14], with African Americans generally sleeping more poorly than their Caucasian counterparts. Despite documented ethnic differences in pain and sleep, the research investigating these topics has produced few firm generalizations, in part because ethnicity, sleep, and pain are multidimensional constructs that vary by individual and are shaped by culture. Explaining how and why ethnic differences in sleep and pain occurs remains a challenge and additional research seem warranted.
Ethnicity may be particularly relevant to the relation between sleep quality and pain catastrophizing since the biopsychosical model suggests that health behaviors (i.e., pain reporting) may be shaped by interactions among biological, psychological, and social variables [15], all of which are involved in an individual’s identification with one or more ethnic groups. A short-coming of the previous research investigating ethnic differences in sleep and pain has been the lack of inclusion of other ethnic groups beyond African and Caucasian Americans. For instance, much less is known about the sleep and pain experiences of Asian Americans. Therefore, the current study examined the associations between ethnicity, subjective sleep quality, standard and situation-specific pain catastrophizing, and pain reports to a cold pressor task in a multi-ethnic sample of self-identified Caucasian, African, and Asian Americans (Figure 1). Using laboratory pain testing procedures, we examined the following: 1) Is subjective sleep quality, and parameters of sleep quality such as sleep efficiency and total sleep time, related to catastrophizing processes and pain reports? 2) Are there significant ethnic differences in the report of pain catastrophizing and pain experiences? 3) Is there an interactive effect between ethnicity and sleep quality in relation to pain catastrophizing and pain reports?
Figure 1.
Putative study model
METHODS
Participants
For recruitment purposes, study flyers were hung throughout the campus of an urban mid-Atlantic university. Any person with faculty, staff, or student status was eligible for participation. Individuals interested in participation initially completed a web-based screening form, the address of which was posted on the study flyer. Eligible participants met the following criteria: (a) between the ages of 18 and 45 years; (b) no ongoing chronic pain problems; (c) not diagnosed with hypertension or taking medications for blood pressure; (d) no circulatory disorders; (e) no history of cardiac events; (f) not pregnant; (g) no history of metabolic disease or neuropathy; (h) not currently using prescription medications including analgesics, tranquilizers, antidepressants, or other centrally acting agents; and (i) no mental health disorders (e.g., depression). Following completion of the web-based screening, eligible participants were contacted via electronic mail to set a time when they were to present to our laboratory. Participants were instructed to refrain from using alcohol, nicotine products, and non-prescription medications on the day of participation. In addition, participants were asked to abstain from caffeinated beverages and vigorous exercise for at least two hours before study participation. A total of 155 healthy college students from four ethnic groups (86 Caucasian Americans, 35 Asian Americans, 28 African Americans, and 6 Hispanic American) participated in the current study. These college students encompassed a wide range of academic affiliations and major areas of study. Participants were compensated for their participation.
Procedures
Data were collected as part of a multifactorial study examining the ethnic and psychophysiological correlates of experimentally-induced acute pain sensations [16]. Participants completed a single 90-minute laboratory session. Several health behavior and pain-related questionnaires were administered before and after the pain testing. For quantitative sensory testing, participants were paired with experimenters of the same sex to minimize the effects of experimenter sex on participants’ report of pain [17]. All study procedures were approved by the University of Maryland, Baltimore County Institutional Review Board, and informed consent was obtained before the initiation of study procedures.
Cold Pressor Pain
Cold pressor pain was assessed by having participants immerse their hand up to the wrist in 4°C water. The water temperature was maintained (+/- 1°C) by a refrigeration unit (Neslab, Portsmouth, NH), and was constantly circulated to prevent local warming around the submerged hand. Participants were instructed to immerse their dominant hand into the water bath for as long as possible. Similar to Dixon et al. [18], participants were encouraged to keep their hands immersed for at least two minutes during the CPT immersion, but were told they could remove their hand at any time they chose. Unknown to participants, the maximum permitted immersion time was 300 seconds.
Pain intensity and unpleasantness
A description of the difference between pain intensity (“How strong the pain feels”) and pain unpleasantness (“How unpleasant or disturbing the pain is for you”) was read to all participants. Following this, pain intensity and pain unpleasantness were assessed during the CPT by asking participants for verbal self-reports on 0-100 scales, with 0 = “No pain” (or “Not at all unpleasant”) and 100 = “Pain as intense as I can imagine” (or “Pain as unpleasant as I can imagine). Pain intensity and unpleasantness ratings were obtained every 30 seconds throughout the 300 second painful procedure (maximum 10 ratings for each pain descriptor), and at the exact time the task was discontinued. Pain intensity and unpleasantness ratings were averaged over the course of the CPT trial and included in statistical analyses. Whether participants completed the entire CPT, or terminated the task prior to the allotted maximum time of exposure, the duration of exposure was recorded and classified as “pain tolerance”.
Questionnaires
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a self-rated questionnaire which assesses sleep quality and disturbances over a one month time interval [19]. Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. Each of the seven component scores is weighted equally on a scale from 0 to 3, 0 indicating no difficulty and 3 indicating severe difficulty. The sum of scores for these seven component scores yields one global score, ranging from 0 to 21. Higher scores indicate worse sleep quality, and a global PSQI score > 5 is consistent with poor sleep quality. The seven component scores of the PSQI have previously been shown to possess good internal consistency (α = .83), and the overall global score has demonstrated good test-retest reliability (r = .87) [16]. In the current study, internal consistency for the PSQI components was acceptable (α = .75)
Beck Depression Inventory (BDI)
The BDI is a 21-item self-report measure designed to assess DSM-IV depressive symptomatology in adolescents and adults [20]. Respondents are asked to rate each of the depressive symptoms, ranging from 0 (not present) to 3 (severe), in terms of how they have been feeling during the past two weeks. The BDI is designed to provide a single overall score that can range from 0 to 63. The BDI has well-established psychometric properties [21] and the internal consistency of the BDI in the current study was good (α = 0.81).
Standard Pain Catastrophizing Scale (PCS)
The standard PCS is a 13-item scale that assesses catastrophic thinking in response to pain [22]. The standard PCS assesses catastrophic pain-related cognitive-emotional processes by asking participants to recall their experiences during a past occurrence of pain. In the current study, as is typically done in experimental pain studies, the standard PCS was administered prior to the initiation of the laboratory pain task and was considered an assessment of individuals’ tendency to engage in pain-related catastrophizing. The PCS total score, calculated by summing the 13 item responses, provides a good index of the catastrophizing construct through the inclusion of the highly correlated subscales of helplessness, rumination, and magnification. Higher scores on the PCS are indicative of greater pain-related catastrophizing. The internal consistency of the standard PCS in the current study was very good (α = 0.86).
Situation-Specific Pain Catastrophizing Scale (PCS)
The situation-specific PCS used the same 13 items as the standard PCS, with modified instructions and item wording. The situation-specific PCS was administered immediately following the completion of the laboratory pain task and the instructions asked participants to refer to the pain experienced during the laboratory painful tasks when answering the questions pertaining to pain-related catastrophizing. The modified instructions used in the current study asked participants about “the thoughts and feelings you had during the painful procedures you just experienced.” The internal consistency of the situation-specific PCS in the current study was excellent (α = 0.91). Recent laboratory-derived reports suggest assessment of situational catastrophizing (i.e., the assessment of current catastrophic thinking during an experimental pain task) better predicts individuals’ propensity to report pain and distress during experimental acute pain induction [4,18].
DATA REDUCTION AND ANALYSIS
Using the PSQI global score as an index of sleep quality, each individual was categorized as either a “good sleeper” (global score < 5) or “poor sleeper” (global score >/= 5). In addition to the global score, we secondarily examined individuals’ scores on the habitual sleep efficiency and sleep duration components according to commonly accepted cut values. For habitual sleep efficiency, participants were categorized as “good sleepers” if sleep efficiency was >/= 85% or “poor sleepers” if sleep efficiency was < 85%. Similarly, participants were categorized as either “good sleepers” if sleep duration was > 6 hours or “poor sleepers” if sleep duration was </= 6 hours [23,24].
All statistical analyses were performed using SPSS version 17.0(SPSS Inc. Chicago, IL). Pearson correlations were used to assess zero-order relations among study variables. Descriptive statistics were computed, and the significance of ethnic group differences and sleep quality parameters on catastrophizing was determined by multifactorial analysis of variance (ANOVA). Due to unequal ethnic group and good versus poor sleep quality samples sizes, the parametric assumptions of data normality and homogeneity of variance were initially examined using Shapiro-Wilk and Levene’s tests, respectively. Significant interaction and main effects were further analyzed by Bonferroni adjusted post-hoc tests using Tukey’s HSD. Analyses include the partial η2 as a measure of effect size where appropriate. Following the conventions of Cohen [25], partial η2 = 0.01 is considered a small effect, partial η2 =0.06 a medium-sized effect and partial η2 = 0.14 large effect. In addition, chi-square analyses were completed to examine ethnic differences across categorical data. Analysis of covariance (ANCOVA) was used to examine relations between ethnicity, sleep quality (global score, efficiency, duration), and the various measures of catastrophizing. Significance was set at the 0.05 level unless multiple comparisons necessitated a control for type 1 error inflation.
RESULTS
Participant Characteristics
Within the full cohort of 155 persons, 78 (50%) participants were men and the mean age was 19.9 years, SD = 2.9 (range = 18 to 38 years). All were full-time college students; 60 (39%) were freshman, 33 (21%) were sophomores, 26 (17%) were juniors, 32 (20%) were seniors, and 4 (3%) were graduate students. At the time of assessment, 65 (42%) participants were working for pay in addition to being a full-time student; of those who were working, 48 (31%) worked less than 20 hours per week, 11 (7%) worked 21-30 hours per week, 4 (3%) worked 31-40 hours per week, and 2 (1%) worked more than 40 hours per week. For the sake of data analysis, occupational status was coded as either 0 = not currently working for pay or 1 = currently working for pay. Four participants (3%) reported being married, the rest were single. Eighty-six (55%) participants were Caucasian American, 35 (23%) were Asian American, 28 (18%) were African American, and 6 (4%) reported a Hispanic origin. Due to small sample size, the six Hispanic American participants were not included in any further analyses; these individuals did not significantly differ from any other ethnic group on subjective sleep quality or catastrophizing reports (all p values > .10). The final sample size was 149 individuals (Caucasian, Asian, and African Americans).
Zero-Order Correlations
The zero- order correlations for all study variables are shown in Table 1. Consistent with the study hypotheses, PSQI global sleep quality was significantly and positively related to standard and situation-specific pain catastrophizing. In addition, sleep quality was significantly correlated with depressive symptoms. Standard catastrophizing was positively correlated with depressive symptoms and occupational status, while CPT immersion time was inversely correlated with situation-specific catastrophizing.
Table 1.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1) Sex | — | |||||||
| 2) Occupational status | -.08 | — | ||||||
| 3) Depressive symptoms | -.01 | -.01 | — | |||||
| 4) Global sleep quality | -.04 | .09 | .35* | — | ||||
| 5) Standard pain catastrophizing | -.07 | -.26** | .28** | .17* | — | |||
| 6) Situation-specific pain catastrophizing | -.15 | .05 | .12 | .14 | .36** | — | ||
| 7) Pain tolerance | .20* | -.15 | .08 | .11 | -.14 | -.35** | — | |
| 8) Pain intensity | -.16 | .03 | .12 | .08 | .19* | .43** | -.43* | |
| 9) Pain unpleasantness | -.22** | .08 | .07 | .03 | .12 | .46** | -.47** | .87** |
= p < .05
= p < .01
Ethnic Differences
Descriptive statistics for depressive symptoms, CPT immersion time, global sleep quality, standard and situation-specific catastrophizing reports are presented separately for each ethnic cohort in Table 2. Univariate ANOVA (omnibus F test) demonstrated that Caucasian, Asian, and African Americans did not significantly differ on ratings of PSQI Global sleep quality (F(2, 146) = 2.07, p = .13) or standard reports of pain catastrophizing (F(2, 146) = 1.21, p = .30). Significant ethnic differences were detected for BDI scores (F(2, 146) = 4.86, p < .01); Asian Americans reported greater depressive symptoms than Caucasian but not African Americans. Ethnic group differences also emerged for CPT immersion time (F(2, 146) = 6.98, p < .01); Caucasian Americans kept their hands immersed longer than Asian and African Americans. Group differences were observed for situation-specific reports of pain catastrophizing (F(2, 146) = 14.84, p < .001); African Americans reported greater situational catastrophizing than Caucasian but not Asian Americans. Further, Chi-Square tests showed that ethnic group distribution significantly differed by sex (χ2(2)= 7.91, p = .02) and occupational status (χ2(2) = 4.03, p < 0.05). More African and Asian American women than men participated in the study; conversely, there were more Caucasian men than women. Additionally, more African Americans reported being employed (57%) relative to Asian Americans (31%) but not Caucasian Americans (42%).
Table 2.
| African Americans N = 28 | Caucasian Americans N = 86 | Asian Americans N = 35 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range |
| Depressive symptoms* | 6.0 | 5.1 | 0-18 | 5.3 | 4.4 | 0-20 | 8.3 | 5.4 | 1-22 |
| Global sleep quality | 4.2 | 2.8 | 1-8 | 4.4 | 2.0 | 1-10 | 4.4 | 2.7 | 1-12 |
| Standard catastrophizing | 15.4 | 11.5 | 0-41 | 13.2 | 8.6 | 0-37 | 15.9 | 9.9 | 1-35 |
| Situation-specific catastrophizing*** | 26.8 | 14.2 | 4-48 | 14.0 | 10.0 | 0-47 | 19.8 | 10.9 | 3-42 |
| Pain tolerance (seconds)** | 165.4 | 84.1 | 51-300 | 216.6 | 79.9 | 15-300 | 164.9 | 89.5 | 39-300 |
| Pain intensity | 74.3 | 19.3 | 10-99 | 63.6 | 20.6 | 15-98 | 72.6 | 18.9 | 29-100 |
| Pain unpleasantness | 78.9 | 19.2 | 22-100 | 64.4 | 21.6 | 5-100 | 74.1 | 19.9 | 26-100 |
| Occupational Status | 57% working | 42% working | 31% working | ||||||
| Sex | 71% women | 43% women | 60% women | ||||||
= p < .01, Asian > Caucasian = African
= p < .01, Caucasian > Asian = African
= p < .001, African > Asian > Caucasian
Covariates
The following variables were included as statistical controls in all subsequent analyses examining the interactive relation between sleep quality and ethnicity with catastrophizing: participants’ sex, depressive symptoms, CPT immersion time, and occupational status. The rationale for inclusion of these statistical controls is as follows: 1) Significant sex differences have previously been reported for pain catastrophizing [26]. 2). Given the overlap between measures of catastrophizing and negative affect, it is now customary (if not required) that any analysis of pain catastrophizing adjust for depressive symptoms or some general negative affective factor [1]. 3) Differences in CPT immersion time have the potential to significantly account for situation-specific catastrophizing reports (e.g., longer immersion time = less catastrophizing). 4) It stands to reason that maintenance of full-time college student status and active employment could negatively affect sleep quality more than full-time college student status alone.
Standard Catastrophizing
A 3 × 2 factorial analysis of covariance (ANCOVA) was conducted to evaluate the effects of subjective sleep quality and ethnicity on standard reports of pain catastrophizing. Despite unequal sample sizes, homogeneity of variance was not violated according to Levene’s test (F(5, 143) = 0.90, p = 0.48). The distributions of standard catastrophizing reports were approximately normal across ethnic groups, good quality sleepers and poor quality sleepers as indicated by Shapiro-Wilk statistics (p’s > 0.05). After adjustment for covariates, results indicated a significant main effect for global sleep quality (F(1, 139) = 5.27, p = 0.02, ηp2 = 0.037) and a non-significant main effect for ethnicity (F(2, 139) = 1.15, p = 0.32, ηp2 = 0.016). Additionally, there was a significant ethnicity X global sleep quality interaction for standard catastrophizing reports (F(2, 139)= 6.06, p = 0.003, ηp2 = 0.080). Because the interaction between ethnicity and global sleep quality was significant, ethnic differences in standard catastrophizing for “good sleepers” and “poor sleepers” were examined separately. To control for Type I error rates across the two simple main effects, alpha was set for each at 0.025. For good quality sleepers, there were no significant differences in standard catastrophizing across ethnic groups (F(2, 99) = 1.30, p = 0.28, ηp2 = 0.026); however, there were significant ethnic group differences among poor sleep quality sleepers (F(2, 36) = 4.75, p = 0.015, ηp2 = 0.209). Follow-up post-hoc tests with a Bonferroni adjustment (alpha set to 0.017) were conducted to evaluate the three pair-wise differences among the means for poor quality sleepers. It was demonstrated that African Americans produced significantly greater standard catastrophizing reports than both Caucasian (p = 0.013) and Asian Americans (p = 0.015). There was no significant difference between Caucasian and Asian Americans (p = 0.65). Figure 1 demonstrates these differences as a bar graph.
Situation-Specific Catastrophizing
Situation-specific catastrophizing was found to be approximately normally distributed with homogenous variances across ethnic groups and sleep quality parameters as indicated by Shapiro-Wilk statistics (p’s > 0.05) and Levene’s test (F(5, 143) = 1.05, p = 0.39), respectively. Although results did reveal significant mean differences across situation-specific catastrophizing for ethnicity (F(2, 139) = 8.19, p < 0.001, ηp2 = 0.105),there were no significant effects detected for global sleep quality (F(1, 139) = 1.47, p =0.23, ηp2 = 0.010) or the ethnicity X global sleep quality interaction (F(2, 139) = 0.20, p = 0.82, ηp2 = 0.003). Adjusted post-hoc contrasts demonstrated that African Americans reported significantly greater situation-specific catastrophizing than Caucasian (p < 0.001) and Asian Americans (p < 0.01) regardless of reported sleep quality (see Table 1 for exact means). There was no significant difference shown between Caucasian and Asian Americans (p = 0.27).
To further probe the relations among ethnicity, sleep quality, and situation-specific catastrophizing, additional exploratory analyses were completed as stated in the initial hypothesis. Specifically, the habitual sleep efficiency and total sleep duration components of the PSQI global composite were examined in relation to ethnicity to determine any interactive effect on situation-specific catastrophizing. Adjusted analysis revealed a significant ethnicity X habitual sleep efficiency interaction for situation-specific catastrophizing reports (F(2, 139) = 4.02, p = 0.02, ηp2 = 0.056). Considering ethnic differences in situation-specific catastrophizing for good (>/= 85% sleep efficiency) and poor (< 85% sleep efficiency) sleepers separately, no significant differences in situation-specific catastrophizing across ethnic groups was demonstrated for good sleep efficiency sleepers (F(2, 111) = 1.72, p = 0.18, ηp2 = 0.030). However,there were significant differences for poor sleep efficiency sleepers (F(2, 24) = 8.53, p =0.002, ηp2 = 0.415). Similar to the pattern demonstrated for standard catastrophizing reports, pair-wise differences demonstrated that African Americans produced significantly greater situation-specific catastrophizing reports relative to their Caucasian (p = 0.003) and Asian American (p = 0.004) counterparts. There was no significant difference between Caucasian and Asian Americans (p = 0.87). Figure 2 demonstrates these differences as a bar graph. Lastly, we did not detect a significant ethnicity X total sleep time (> 6 hours per night = good sleeper, </= 6 hours per night = poor sleeper)interaction for situation-specific catastrophizing reports (F(2, 139) = 1.63, p = 0.20, ηp2 =0.021).
Figure 2.
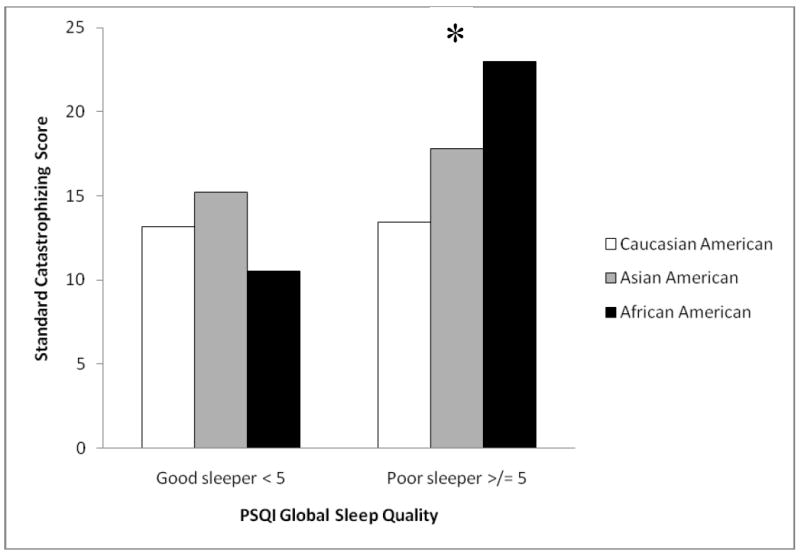
Ethnic differences in standard catastrophizing according to PSQI global sleep quality
Pain Reports
Caucasian Americans kept their hands immersed in the cold pressor (i.e., pain tolerance) significantly longer than Asian and African Americans (F(2, 146) = 6.98, p < .01). Furthermore, immersion time (i.e., pain tolerance) was found to correlate with pain intensity (r = -.39, p < .001) and pain unpleasantness reports (r = -.43, p < .001) regardless of ethnic affiliation. Because time in the water accounted for significant variance in pain reports, we controlled for this variable in analyses examining ethnicity, sleep quality, and pain intensity and unpleasantness. Results of ANCOVA did not demonstrate any significant main or interaction effects for ethnicity and sleep quality in relation to reports of pain intensity or unpleasantness (p’s > .30). However, correlational analyses did reveal significant associations between pain catastrophizing, pain reports, and pain tolerance. Specifically, standard catastrophizing was significantly correlated with reports of pain intensity (r = .19, p = .02) but not unpleasantness (r = .13, p = .11) or pain tolerance (r = -.14, p = .08). Situation-specific catastrophizing was significantly correlated with reports of pain intensity (r = .43, p < .001), unpleasantness (r = .47, p < .001), and pain tolerance (r = -.35, p < .001). These results provide preliminary and indirect evidence suggesting that ethnic differences significantly interact with sleep quality in relation to pain catastrophizing and, in turn, catastrophizing is significantly associated with pain ratings and CPT immersion time (pain tolerance).
DISCUSSION
Poor sleep quality has a deleterious impact on individuals’ physical and mental health, occupational functioning, and overall quality of life [27]. Of particular relevance here, research involving clinical samples and healthy individuals exposed to controlled laboratory stimuli has documented reliable relations between poor sleep and increased pain severity [28,29]. However, the association between sleep quality and psychosocial contributors (e.g., pain catastrophizing) to negative pain outcomes has not received much attention and remains unclear. Catastrophizing is a known psychosocial contributor to worsened pain outcomes, and it has been suggested that African Americans engage in greater pain catastrophizing compared to Caucasians [9,12]. As such, an examination of the relation between sleep quality and pain catastrophizing should consider ethnicity. This is because ethnicity has previously been shown to interact with sleep habits in relation to a host of clinical outcomes including blood pressure [30], catecholamine levels [31], and health-related quality of life [32]. Furthermore, in a study investigating sleep architecture, polysomnography revealed that African Americans experienced the greatest amount of non-restorative sleep, characterized by poor sleep efficiency, frequent awakenings, and little slow wave (delta) sleep, relative to other ethnic groups [33]. Taken together, it is plausible that ethnicity could interact with sleep quality in relation to pain catastrophizing and other pain-relevant outcomes. The purpose of this study was to address the interaction of ethnicity with sleep quality in relation to reports of standard and situation-specific pain catastrophizing in a multi-ethnic sample of young, healthy adults exposed to the cold pressor task.
Ethnic differences in the standard measure of pain catastrophizing were only significant for those who self-reported as “poor sleepers”. Among individuals with poor sleep quality, African Americans produced the greatest reports of standard catastrophizing when compared to Asian and Caucasian Americans, who did not differ from each other. Ethnic differences in standard catastrophizing were not significant for “good sleepers”. These findings provide one potential explanation for why some studies have found ethnic differences in catastrophizing [12], while others have not [10]. Ethnic differences in pain catastrophizing may be dampened when the sample is comprised of participants who sleep well, yet ethnic differences in catastrophizing may be potentiated when the sample is comprised of poorly sleeping participants. It should be mentioned that a particular strength of the current study is the inclusion of three separate ethnic groups. The majority of studies that have previously investigated ethnic differences in health-related outcomes only examined African Americans and Caucasian Americans. The finding that Asian Americans with poor sleep quality did not self-report greater standard or situation-specific pain catastrophizing compared to Caucasian Americans is novel and is deserving of further exploration. Future studies should more specifically examine whether African Americans in particular, rather than minority groups in general, may be susceptible to the ill effects of poor sleep quality on pain coping strategies.
Support was not provided for the interactive effect of ethnicity by PSQI Global sleep quality in relation to situation-specific measure of pain catastrophizing. While the reason(s) for the differences in relational patterns among ethnicity and sleep quality with standard and situation-specific catastrophizing is not abundantly clear, it may be that results vary as a function of catastrophizing assessment. Previous studies have shown that standard and situation-specific assessments of catastrophizing yield somewhat different information [34]. Therefore, the interaction between sleep quality and ethnicity may differently relate to catastrophizing as a function of situation-specific measurement (i.e., degree of catastrophizing in a specific painful situation) as opposed to standard measurement (i.e., a person’s general tendency to catastrophize when in pain). Despite the lack of an interactive effect between PSQI Global sleep quality and ethnicity in relation to situation-specific catastrophizing, additional analyses revealed that habitual sleep efficiency did significantly interact with ethnicity to affect situation-specific catastrophizing reports. Previous studies have confirmed the importance of sleep efficiency as an important sleep quality parameter in relation to pain [35,36]. Among those with poor sleep efficiency(i.e., </= 85%), African Americans reported elevated situational catastrophizing scores relative to Asian and Caucasian Americans; there was no significant difference between the latter two ethnic cohorts. Among those individuals with habitual sleep efficiency > 85%, no ethnic differences in situation-specific pain catastrophizing were demonstrated.
It is important to note that, in the present study, mean PSQI Global sleep quality scores were not significantly different across the three ethnic groups. This may be interpreted such that sleep quality did not directly differ as a function of individuals’ ethnic background. However, significantly elevated pain catastrophizing reports were found for poorly sleeping African Americans compared to their poorly sleeping Caucasian and Asian American counterparts. These findings suggest that individual differences in subjective sleep quality contribute to variability in the report of pain catastrophizing within the African American group, and that this association is less robust among Caucasian and Asian Americans (e.g., although individual differences exist among Caucasian and Asian Americans in their sleep quality, this variability is less influential on their reports of pain catastrophizing). Ethnicity and sleep quality also did not significantly interact in relation to CPT-induced pain reports or pain tolerance; however, both standard and situation-specific measures of catastrophizing were correlated with pain tolerance and ratings of pain intensity and unpleasantness. These results may indirectly support the ability of the sleep quality-ethnicity interaction to affect pain reports through pain catastrophizing processes; however, additional studies are necessary to confirm this hypothesis.
It should be noted that this cross-sectional study, with observational data, was not designed to demonstrate causal relations; however, it does preliminarily relate poor sleep quality with heightened pain catastrophizing and suggests that this relation varies as a function of self-reported ethnic background. The current study does not rule out the possibility that the associations among ethnicity, poor sleep quality, and pain catastrophizing may be bidirectional or co-occurring. That is, African Americans who express greater catastrophizing in relation to daily aches and pains may prime themselves for a poor night’s sleep, which in turn could elicit self-reports of poorer sleep quality. Another potential factor affecting the pattern of the sleep quality-pain catastrophizing relation might be whether the painful stimulation experienced is acute or chronic. For instance, in a sample of healthy, pain-free individuals, there may be a greater likelihood for poor sleep quality to influence catastrophizing about a single, acutely painful stimulus. Conversely, in a clinical sample of individuals afflicted with a painful condition, chronic rumination and feelings of helplessness characteristic of catastrophizing may be more likely to negatively impact sleep quality over time. This assertion is speculative and extends beyond well-accepted fact. Therefore, the explanation may be plausible but evidence supporting this explanation is currently lacking.
The current study’s findings call for consideration to be given to sleep and catastrophizing as potential treatment targets when treating painful conditions among African Americans. Addressing sleep quality may have a beneficial effect on African American’s pain experience or their perceptions regarding their ability to cope with their pain. Current evidence supports cognitive-behavioral therapies (CBT) for the effective treatment of chronic pain [37], and demonstrates improvements in pain, mood, coping and functional outcomes [38,39]. These approaches have more recently begun to address pain catastrophizing [40,41], but may not regularly incorporate assessment or treatment of sleep as a treatment module. Whether improvements in pain catastrophizing can be produced by improving African American’s sleep quality remains unknown and is a point for future investigation. Results of this study suggest that pain catastrophizing should continue to be a regular component of CBT for pain, but that sleep should also be included in treatment approaches, particularly among African Americans.
The present study possesses several limitations and qualifications that merit caution when interpreting the findings. First, study participants self-reported their ethnic background using relatively broad categories; further subdivision of individuals’ perceived ethnicities and cultural backgrounds may provide additional valuable information. Another limitation is that all study participants were college students, and college students have previously been shown to regularly maintain atypical sleep habits [42]. As a consequence, the generalizability of these findings is not perfectly clear and replication of these effects in other samples will be quite pertinent. Given that sleep deficits and catastrophizing are typically higher and more variable in clinical pain [1,5], the associations among ethnicity, sleep quality, and pain catastrophizing may well be stronger in clinical populations than was found in our non-clinical sample. Lastly, in the present study, sleep quality was subjectively reported using the Pittsburgh Sleep Quality Index. Although the PSQI is largely considered the gold standard for the self-report of sleep quality, future studies would benefit from a multimethod approach to sleep assessment that includes objectively derived sleep parameters (e.g., via polysomnography and actigraphy). Such assessment would allow for enhanced understanding of how specific domains of sleep quality (e.g., waking after sleep onset, latency, and sleep duration) may affect pain coping processes. In spite of these limitations, our findings identify self-reported sleep quality as a potentially important and previously unattended factor related to individual differences in the report of pain catastrophizing among African Americans. Continued investigation of ethnic differences in pain coping strategies, and the mechanisms underlying them, appears warranted. Researchers studying sleep in ethnic minority groups may wish to examine whether cognitive-behavioral treatments that improve sleep quality produce concomitant improvements in pain catastrophizing processes as a function of improved sleep.
Figure 3.
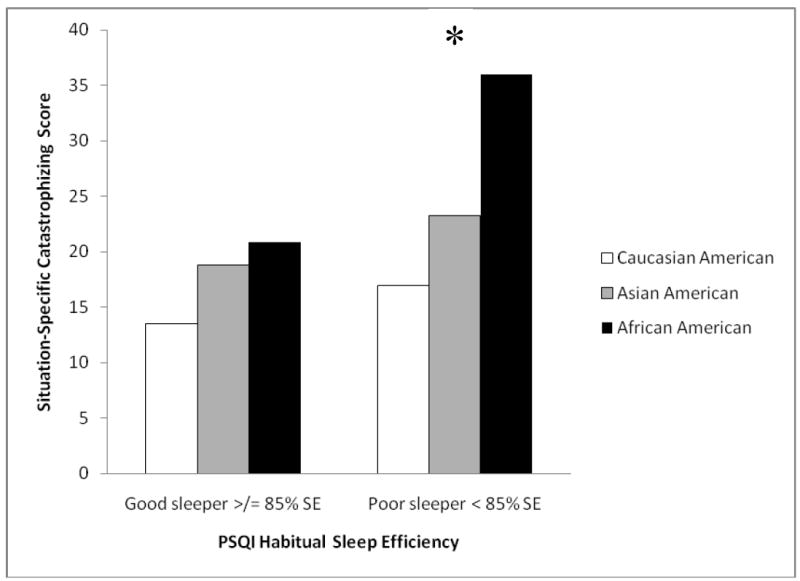
Ethnic differences in situation-specific catastrophizing according to habitual sleep efficiency
Acknowledgments
This work was supported by the Graduate Student Association of the University of Maryland, Baltimore County (B. Goodin) and by NIH Training Grant T32NS045551-06 provided to the University of Florida (B. Goodin).
Footnotes
Disclosures
None of the contributing authors have any conflicts of interest to declare.
References
- 1.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Turk DC, Okifuji A. Psychological factors in chronic pain. Evolution and revolution. J Consult Clin Psychol. 2002;70:678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 3.Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: Only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain. 2005;6:338–339. doi: 10.1016/j.jpain.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes L, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Edell-Gustaffson UM. Insufficient sleep, cognitive anxiety and health transition in men with coronary artery disease: a self-report and polysomnographic study. J Adv Nurs. 2002;37:414–422. doi: 10.1046/j.1365-2648.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression: The Johns Hopkins precursor study. Am J Epidemiol. 1997;146:105–114. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 8.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 9.Fabian LA, McGuire L, Goodin BR, Edwards RR. Ethnicity catastrophizing, and qualities of the pain experience. Pain Med. 2011;12:314–321. doi: 10.1111/j.1526-4637.2010.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and Caucasian patients. Pain Med. 2005;6:88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 11.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Hastie BA, Riley JL, III, Fillingim RB. Ethnic differences in pain coping: Factor structure of the coping strategies questionnaire and coping strategies questionnaire-revised. J Pain. 2004;5:304–316. doi: 10.1016/j.jpain.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hale L, Phoung Do D. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mezick EJ, Matthews KA, Hall M, Strollo PJ, Buysse DJ, Kamarck TW, et al. Influence of race and socioeconomic status on sleep: Pittsburgh sleepSCORE project. Psychosom Med. 2008;70:410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suls J, Rothman A. Evolution of the biopsychosocial model: Prospects and challenges for health psychology. Health Psychol. 2004;23:119–125. doi: 10.1037/0278-6133.23.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Goodin BR. Unpublished dissertation. 2010. A biopsychosocial explanation of experimental pain outcomes. [Google Scholar]
- 17.Levine FM, De Simone LL. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44:69–72. doi: 10.1016/0304-3959(91)90149-R. [DOI] [PubMed] [Google Scholar]
- 18.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 22.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assessment. 1995;7:524–532. [Google Scholar]
- 23.Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh sleep quality index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:564–571. [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts J, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; Hillsdale: 1998. [Google Scholar]
- 26.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen HK, Ducharme LJ, Roman PM. Job stress and poor sleep quality: data from an American sample of full-time workers. Soc Sci Med. 2007;64:1997–2007. doi: 10.1016/j.socscimed.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Raymond K, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized burn patients. Pain. 2001;92:381–388. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 30.Hughes JW, Kobayashi I, Deichart NT. Ethnic differences in sleep quality accompany ethnic differences in night-time blood pressure dipping. Am J Hypertens. 2007;20:1104–1110. doi: 10.1016/j.amjhyper.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32:417–423. doi: 10.1161/01.hyp.32.3.417. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin CM, Ervin AM, Mays MZ, Robbins J, Shafazand Z, Walsleben J, Weaver T. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;15:176–183. [PMC free article] [PubMed] [Google Scholar]
- 33.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;23:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 34.Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards RR, Grace E, Petersen S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 37.McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment processes. Spine. 2002;27:2564–2573. doi: 10.1097/00007632-200211150-00033. [DOI] [PubMed] [Google Scholar]
- 38.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 2006;121:181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Smeets RJEM, Vlaeyen JWS, Kester ADM, Knotterus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Spinhoven P, ter Kuile M, Kole-Snijders AMJ, Mansfeld MH, den Ouden DJ, Vlaeyen JWS. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur J Pain. 2004;8:211–219. doi: 10.1016/j.ejpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being, and sleepiness in college students. J Psychosom Res. 1997;42:583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]


