Abstract
While performance-based tests of everyday functioning offer promise in facilitating diagnosis and classification of HIV-associated neurocognitive disorders (HAND), there remains a dearth of well-validated instruments. In the present study, clinical correlates of performance on one such measure (i.e., Medication Management Test—Revised; MMT-R) were examined in 448 HIV+ adults who were prescribed antiretroviral therapy. Significant bivariate relationships were found between MMT-R scores and demographics (e.g., education), hepatitis C co-infection, estimated premorbid IQ, neuropsychological functioning, and practical work abilities. MMT-R scores were not related to HIV disease severity, psychiatric factors, or self-reported adherence among participants with a broad range of current health status. However, lower MMT-R scores were strongly and uniquely associated with poorer adherence among participants with CD4 T-cell counts <200. In multivariate analyses, MMT-R scores were predicted by practical work abilities, estimated premorbid functioning, attention/working memory, learning, and education. Findings provide overall mixed support for the construct validity of the MMT-R and are discussed in the context of their clinical and research implications for evaluation of HAND.
Keywords: HIV, medication management, neuropsychological functioning, adherence, construct validity, instrumental activities of daily living
INTRODUCTION
While it is well established that HIV damages brain structure and function, combination antiretroviral therapy (cART) has significantly changed the prevalence of HIV-associated neurocognitive disorders (HAND) [1, 2, 3]. Specifically, severe dementia related to HIV during the era of cART has decreased dramatically [4,3], but less severe forms of HAND, which adversely impact everyday functioning [5], persist and may be more prevalent in the cART era [2]. HIV effects on both neuropsychological (NP) and everyday functioning have supported the use of laboratory-based functional measures to complement NP assessment for studies in order to understand the impact of HIV on everyday life functioning and to facilitate diagnosis of HAND [5, 6, 7].
For example, there is evidence that impaired neurocognition in HIV is associated with diminished medication management capacity [8, 9, 10, 5, 11, 12] and poorer medication adherence [13, 14]. Specific cognitive domains such as retrospective learning [10, 5] and memory [8], prospective memory [12], executive functions [8, 10, 5], and psychomotor speed [8] are most reliably associated with medication management and adherence [13, 14]. Moreover, the impact of various NP abilities on adherence appears to be independent of other factors known to influence adherence, such as depression and substance use [13, 14]. Strict adherence to cART remains essential to the successful management of HIV disease [15]. Reciprocal interactions between medication adherence and NP functioning in HIV have raised the possibility that neurocognitive dysfunction leads to diminished medication adherence that permits HIV replication in the CNS and leads to further neurocognitive decline [16, 17].
Few published studies have investigated laboratory-based measures of medication management as predictors of actual adherence, and fewer still have specifically investigated the predictive validity of measures of medication management in persons with HIV. Albert and colleagues [8] were the first to address this limitation, adapting the medication component of the well-validated Observed Tasks of Daily Living paradigm [19] into an objective performance test tailored specifically to antiretroviral medications (the Medication Management Test, or MMT). This simulation evaluates both “pill dispensing” (i.e., compliance with an HIV dosage/regimen indicated by correct placement in a one-week pillbox) and “medication inference” (i.e., correct interpretation of medication insert and dosing instructions). Other studies have begun to investigate the NP, demographic, and disease factors that influence medication management, and the relationships between laboratory tests of medication management and actual patient adherence and its biological outcomes [8].
The three studies used to develop the MMT [8] indicated that lower NP functioning was associated with poorer performance on both dimensions of the MMT. These effects were greatest in those with NP scaled scores greater than 2 standard deviations below the mean, and varied by domains. Executive functions and psychomotor speed domain performance were significantly associated with pill dispensing scores, while memory domain performance was significantly associated with medication inference scores. When both cognitive status and MMT performance were used to group participants, those with poor memory and poor MMT performance were more likely to make medication recall errors [8]. A follow-up study [9] replicated the relationships between pill dispensing and executive functions and psychomotor domain performance, and further suggested that individuals with NP impairment may adapt by adopting a fixed medication schedule to manage medications (see also [13]). Demographic and disease correlates of MMT performance have also been identified. For example, fewer years of educational attainment and lower medication numeracy in women and African-Americans have been associated with lower scores on the MMT [11, 20]. Time since HIV diagnosis is the only index of HIV disease of which we are aware that has thus far been associated with MMT performance [21, 20].
Performance on the MMT-Revised (a 10-minute subset of the most reliable and valid MMT items based on an HIV sample pilot test) is associated with both NP and everyday functioning. NP impaired participants were more likely to fail the MMT-R than unimpaired individuals and the two NP domains that predicted medication management failures were executive dysfunction and learning deficits [22]. Older HIV-positive adults with NP impairment may be at particular risk of poorer MMT-R performance [23]. MMT-R scores also significantly differed by IADL functional status, such that dependent individuals performed significantly more poorly than independent individuals [22]. MMT-R scores are not predicted by self-reported medication adherence, suggesting that functional impairment is better detected by performance-based measures than patient self-report [23].
A reliable and valid measure of medication management capacity to complement NP testing in patients with HIV would be valuable to research and potentially clinical care. Therefore, we examined the relationship of medication management capacity, as measured by the MMT-R, to NP functioning and adherence in a large sample of participants with HIV on ART. These relationships were evaluated in the context of other factors that are known or suspected to be involved in adherence, such as demographics, health and disease characteristics, psychiatric status (e.g., depression, alcohol substance use), and other abilities of everyday functioning. We tested the following hypotheses: 1) that poorer medication management capacity (i.e., lower MMT-R scores) would be associated with lower adherence, 2) that lower NP functioning would be associated with lower MMT-R scores, with the domains of executive functions and episodic memory best predicting poorer performance on the MMT-R, and 3) that MMT-R scores would uniquely contribute to the prediction of adherence beyond that which can be explained by psychiatric functioning, NP functioning, and other factors that are thought to affect adherence.
METHODS
This study was conducted on retrospective data from the multi-site, NIH-funded CNS Antiretroviral Therapy Effects Research (CHARTER; www.charterresource.ucsd.edu). Procedures for CHARTER were approved by the Human Subjects Protection Committees of each participating institution. Written informed consent was obtained from all study participants. All participants underwent an extensive neuromedical and NP evaluation that included medical history, structured neurologic and medical examination, collection of blood and urine samples, and administration of measures of neurocognition, psychosocial functioning, substance use, and everyday functioning. Medical procedures were performed by physicians, nurse practitioners, or trained nurses and research associates according to established protocols. NP measures were administered by trained psychometrists.
Participants
Data were from 448 adult CHARTER participants who currently were prescribed ART. Utilizing published guidelines [6] for classifying the likely influence of conditions other than HIV on the brain and neurocognitive functioning, CHARTER participants are classified with respect to whether the most commonly encountered comorbid conditions should be considered incidental, contributing, or confounding (see [24] for details). Only participants that were classified as having incidental (n = 300) or contributing (n = 148) conditions were included in the current study. Participants ranged in age from 19 to 69 years (M = 44.7, SD = 8.5), were mostly male (81%), and on average were high school educated (M = 12.9, SD = 2.4). Forty-two percent were Black or African American, 45% were Caucasian, and 11% were Hispanic. Estimated duration of HIV infection ranged from 6 months to just over 27 years (M = 11.4 years; SD = 6.3) and 74% were categorized as having a history of AIDS according to CDC guidelines. For estimated premorbid functioning, scores generally fell in the average range. Participant demographic and health characteristics are presented in Table 1.
Table 1.
Participant demographic and health characteristics (n = 448).
| Mean (SD) or % | |
|---|---|
|
| |
| Age (years) | 44.7 (8.5) |
|
| |
| Education (years) | 12.9 (2.4) |
|
| |
| Women | 19%F |
|
| |
| Non-Hispanic white | 44.9% |
|
| |
| African American | 41.7% |
|
| |
| Hispanic | 11.4% |
|
| |
| Other ethnicity | 2.0% |
|
| |
| Estimated Duration of HIV Infection (years) | 11.4 (6.3) |
|
| |
| History of AIDS Diagnosis | 74% |
|
| |
| Current CD4 (absolute) | 476.6 (255.2) |
|
| |
| Current CD4 Category | |
| CD4 < 200 (i.e., immunosuppression) | 12.3% |
| CD4 = 200-499 | 45.5% |
| CD4 = 500+ | 40.8% |
|
| |
| CDC Clinical Classification | |
| Category A | 30.1% |
| Category B | 28.8% |
| Category C | 41.0% |
|
| |
| Nadir CD4 | 148.8 (138.9) |
|
| |
| Viral Load (HIV log10, Plasma) | 2.2 (0.9) |
|
| |
| Hepatitis C (HCV) | 25.2% |
| Influence of Comorbid Condition(s) on Neurocognitiona | |
| Incidental | 67% |
| Contributing | 33% |
|
| |
| Estimated Premorbid Functioning (WRAT-3 Reading) | 94.5 (14.6) |
Likely influence of non-HIV comorbid conditions on neurocognitive functioning as classified according to Antinori et al. (2007) criteria (see Heaton et al., 2010 for details).
Measures
Medication Management Test, Revised (MMT-R; [5])
The MMT-R is a measure of one’s ability to accurately dispense medications according to a fictitious prescription regimen and answer questions about mock medications. The MMT-R takes about 10 minutes to complete and includes elements from a “pill dispensing” component and a “medication inference” component. In the former component, participants are observed and scored with respect to their ability to dispense one day’s dosage and follow a fictitious prescription regimen, which includes 5 different mock medications consistent with many of the current therapies that are used in the treatment of HIV. Pill bottles are provided with realistic instructions on standardized labels. As part of this task, participants must transfer the correct number of pills from the pill bottles to a medication organizer designed to hold a 1-week’s supply of medication. Participants are scored on the total percentage of prescriptions that they correctly place in the organizer. The “medication inference” component includes 7 items presented in ascending order of difficulty, and requires participants to answer questions regarding the mock medications as well as one over-the-counter medication insert. Total scores on the MMT-R range from 0 to 10. Consistent with prior research, impairment on the MMT-R was defined as scores of five or below (Heaton et al., 2004).
ACTG Adherence Questionnaire [25]
The ACTG Adherence Questionnaire asks respondents to provide information about each of their HIV medications, such as name of drug, prescribed doses per day, prescribed number of pills per dose, and any specific instructions, (e.g., “with food,” “on an empty stomach,” etc.). Respondents are also asked about HIV medications that they took for each of the last four days, including the name of each medication and how many pills they had skipped taking that day. They are then asked about more distal adherence, such as skipping medications over the past weekend (Saturday/Sunday) and identifying the last time they skipped any medications (e.g. within the past four days, within the past two weeks, two-four weeks ago, one-three months ago, more than three months ago, and never skip medications). Respondents who report ever skipping their medications are presented a list of reasons commonly given for missing doses of medications (e.g. away from home, busy with other things, simply forgot) and are asked to rate on a four-point scale (never, rarely, sometimes, often) how often each reason applies to them. Finally, respondents are given a list of 12 symptoms commonly experienced by people with HIV (e.g. nausea, weight loss) and are asked to rate how frequently they experienced each symptom during the prior two weeks (never, rarely, sometimes, often). Participants self-reporting adherence levels ≥ 90% were classified as “adherent,” with < 90% classified as “non-adherent.”
Neuropsychological Measures
Participants completed a comprehensive NP test battery emphasizing tests that are known or expected to be sensitive to the deficits associated with HIV infection. More specifically, the NP battery consisted of a premorbid estimate of intellectual functioning (Wide Range Achievement Test 3rd Edition –Reading subtest [26]), and tests to assess the following domains: 1) motor (Grooved Pegboard Test, dominant and non-dominant hands [27]), 2) attention/working memory (WAIS-III Letter-Number Sequencing [28] and PASAT—1st channel only [27]), 3) Verbal fluency (Controlled Oral Word Association Test [27] and Category Fluency [27]), 4) executive functions (Wisconsin Card Sorting Test, 64-item version [29] and Trail Making Test, Part B [27, 30]), 5) speed of information processing (WAIS-III Digit Symbol and Symbol Search subtests [28, 31] and Trail Making Test Part A [27, 30]), and 6) learning and memory, two separate domains (Story Memory Test and Figure Memory Test [27]). After conversion to demographically-corrected T-scores using published normative data, clinical ratings of global functioning and functioning in each NP domain were made by senior neuropsychologists on a 9-point scale (1 = above average to 9 = severe impairment), consistent with published procedures [32]. For the current study, impairment was defined as a rating of 5 (i.e., definite mild impairment) or greater.
Everyday Functioning
Measures of everyday functioning included the COMPASS Computerized Assessment [33] of job knowledge, skills, interests, and abilities, as well as a modified measure of ability to perform instrumental activities of daily living (IADLs) required for living independently [5].
The COMPASS is a computerized assessment involving three practical subtests that assess work-related abilities (i.e., finger dexterity, general motor coordination, and ability to follow instructions) and takes approximately an hour to complete. Each subtest includes a practice session to familiarize the examinee with the instructions and controls (e.g., knob, button press). Questions are presented from the lower to the higher levels of difficulty, with successful completion of easier items required for advancement to more difficult items. The 15 subtest scores are converted into a 14-domain Dictionary of Occupational Titles [34] profile based on previous factor analyses [33]. COMPASS validity is supported by research linking the measure to performance to employment, neuropsychological functioning, and performance of instrumental activities of daily living [5], as well as by the construct validity studies of its precursor, the MESA [35, 36]. Test-retest reliability coefficients for the COMPASS range from .78 to .81 in HIV-infected individuals. Consistent with prior research, impairment on the COMPASS was defined as scores of 24 or below [22].
The IADL is a 13-item scale on which individuals are rated in terms of their ability to independently function in the areas of Financial Management, Home Repair, Medication Management, Laundry, Transportation, Grocery Shopping, Comprehension of Reading/TV Materials, Shopping, Housekeeping, Cooking, Bathing, Dressing, and Telephone Use. For each activity the participant separately rates his/her current level of independence and highest previous level of independence. The total score is the total number of activities for which the individual currently requires more assistance compared to highest previous level of ability (ranging from minimal to complete assistance). Scores range from zero (no change) to 13 (increased dependence in all activities). A cut-off score of 2 or higher was established as a criterion for overall IADL dependence in previous analyses [5] and was used to define impairment in the current study.
Mood and Alcohol & Substance Use
Psychiatric diagnoses were assessed using the computer-assisted Composite International Diagnostic Interview (CIDI [37]), a comprehensive, structured interview used for the assessment of mental disorders according to the definitions and criteria of ICD-10 and DSM-IV. The CIDI is widely used in research and classifies current and lifetime diagnoses of mood disorders and substance use disorders, as well as other mental disorders. Presence and severity of current depressive symptoms was assessed with the Beck Depression Inventory II [38].
Laboratory Assessment
HIV infection was diagnosed by ELISA with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cells (flow cytometry) were performed at each site’s Clinical Laboratory Improvement Amendments (CLIA)–certified, or CLIA equivalent, medical center laboratory. HIV RNA levels were measured centrally in plasma and CSF by reverse transcriptase PCR (Roche Amplicor, v. 1.5, lower limit of quantitation 50 copies/mL).
Data Analyses
Statistical analyses were performed using PASW Statistics version 18.0 software (SPSS, Inc., Chicago, IL, USA). MMT-R scores were non-normally distributed the according to Shapiro-Wilk test (p < 0.05) and they were resistant to transformation. Therefore, non-parametric tests including the Mann-Whitney U and Kruskal-Wallis were used to test bivariate relationships between the MMT-R and nominal variables, with alpha set at .05. Relationships between the MMT-R and continuous variables were performed using Spearman’s ρ. In order to determine variables that were predictive of MMT-R scores, hierarchical multivariate regression analyses were used including the variables that demonstrated significant (p < 0.05) bivariate relationships with MMT-R scores. Regression diagnostics for outliers, error residuals, and multicollinearity were assessed.
RESULTS
Description of the Study Population
Participant psychiatric, NP, everyday functioning, and adherence characteristics are presented in Tables 2 and 3. About 35% of participants reported at least mild current depressive symptoms on the BDI-II, with 8.5% currently having Major Depressive Disorder (MDD) and 26.3% having had MDD at some point during their lifetime. Few participants endorsed current alcohol (AUD) and substance use disorders (SUD) (1.1% and 1.6%, respectively), but lifetime occurrence of AUD (54.7%) and SUD (20.3%) was substantial.
Table 2.
Participant psychiatric characteristics.
| Mean (SD) or % | |
|---|---|
|
| |
| BDI-II Total Score | 12.2 (11.9) |
|
| |
| BDI-II Classification | |
| Minimal (< 13) | 64.9% |
| Mild (14-19) | 14.5% |
| Moderate (20-28) | 12.1% |
| Severe (>28) | 8.5% |
|
| |
| Major Depressive Disorder (MDD) | |
| Current | 8.5% |
| Lifetime | 26.3% |
|
| |
| Alcohol Use Disorder (AUD) | |
| Current | 1.1% |
| Lifetime | 54.7% |
|
| |
| Substance Use Disorder (SUD) | |
| Current | 1.6% |
| Lifetime | 20.3% |
Table 3.
Participant NP, everyday functioning, and adherence characteristics.
| Mean (SD) | % Impaired | |
|---|---|---|
|
| ||
| Neuropsychological Clinical Ratings | ||
| Global | 4.4 (1.6) | 40.2% |
| Motor | 2.7 (1.9) | 19.9% |
| Attention/Working Memory | 3.0 (1.9) | 23.7% |
| Verbal Fluency | 2.4 (1.6) | 13.8% |
| Executive | 3.8 (2.0) | 35.0% |
| Speed of Information Processing | 2.9 (1.8) | 17.6% |
| Learning | 3.0 (1.7) | 21.9% |
| Memory | 3.1 (1.9) | 25.7% |
|
| ||
| Number of Impaired NP Domains | 1.6 (1.7) | -- |
|
| ||
| Everyday Functioning Measures | ||
| COMPASS | 28.8 (7.5) | 30.3% |
| IADL | -- | 14.5% |
|
| ||
| Medication Management Test-Revised Score | 7.5 (2.2) | 19.6% |
|
| ||
| ACTG Adherence | ||
| Percent adherence | 96.7 (11.1) | 10.3% were < 90% adherent |
| Total Pills Prescribed | 23.8 (18.2) | -- |
| Total Pills Skipped | 0.78 (3.1) | -- |
A substantial proportion of participants (40%) were rated as globally NP impaired, with impairment in a specific NP domain ranging from as low as 13.8% (verbal fluency) to as high as 35% (executive functioning). Participants were impaired in 1.6 NP domains on average and mean clinical ratings were in the low average range (i.e., a rating of 3). On measures of everyday functioning, impairment was found in 30.3% for practical work abilities (i.e., the COMPASS), 14.5% in IADLs, and 19.6% on the MMT-R. Self-reported adherence rates generally were quite high (mean = 96.7, SD = 11.1), with only 10.3% reporting adherence levels below 90%.
Bivariate Analyses
Results of bivariate analyses between the MMT-R and other variables are presented in Table 4 (relationship to demographic and disease characteristics) and Table 5 (relationship to psychiatric, NP, everyday functioning, and adherence characteristics). In a bivariate analysis of demographic characteristics, MMT-R performance was significantly related to age (Spearman’s ρ = −.10, p = 0.031), education (Spearman’s ρ = .32, p < 0.001), gender (Mann-Whitney U test; z = −2.98, p = 0.003), and race/ethnicity (Kruskal-Wallis test, χ2 = 28.02, < .001). Overall, younger, more-educated, non-Hispanic whites, and men performed better on the MMT-R than their counterparts.
Table 4.
Bivariate relationships of MMT-R scores to demographic and disease characteristics.
| Variable | Test Statistic | p-value |
|---|---|---|
| Age | ρ = −0.10a | .031* |
| Education | ρ = 0.32a | < .001*** |
| Gender | z = −2.98b | .003**† |
| Ethnicity | X2 = 28.02c | < .001***† |
|
Estimated Duration of HIV Infection (years) |
ρ = −0.06a | .179 |
| History of AIDS Diagnosis | z = −1.24b | .216 |
| Current CD4 (absolute) | ρ = 0.21a | .659 |
|
Current CD4 Category (i.e., < 200, 200 -499,500+) |
X2 = 0.51c | .775 |
| CDC Clinical Classification | X2 = 0.34c | .842 |
| Nadir CD4 | ρ = 0.07a | .178 |
| Viral Load (HIV log10, Plasma) | ρ = −0.02a | .637 |
| Hepatitis C (HCV) | z = −2.86b | .0040** |
Spearman’s ρ
Mann-Whitney test
Kruskal-Wallis test
p < 0.05
p < 0.01
p < 0.001
Note. Males > females and non-Hispanic white > African American for MMT-R. However, findings appear to reflect differences in education and/or other factors. See text and multiple regression analyses for further details.
Table 5.
Bivariate relationships of MMT-R scores to psychiatric,NP, everyday functioning, and adherence characteristics.
| Variable | Test Statistic | p-value |
|---|---|---|
|
| ||
| BDI-II Total Score | ρ = 0.05a | .260 |
|
| ||
| BDI-II Classification | X2 = 0.06c | .997 |
|
| ||
| Major Depressive Disorder (MDD) | ||
| Current | z = −0.10b | .920 |
| Lifetime | z = −1.51b | .131 |
|
| ||
| Alcohol Use Disorder (AUD) | ||
| Current | z = −0.53b | .600 |
| Lifetime | z = −0.81b | .415 |
|
| ||
| Substance Use Disorder (SUD) | ||
| Current | z = −0.57b | .571 |
| Lifetime | z = −1.43b | .152 |
|
| ||
| Estimated Premorbid Functioning (WRAT-3 Reading) | ρ = 0.44a | < .001*** |
|
| ||
| Neuropsychological Clinical Ratings | ||
| Global | ρ = −0.24a | < .001*** |
| Motor | ρ = −0.07a | .126 |
| Attention/Working Memory | ρ = −0.35a | < .001*** |
| Verbal Fluency | ρ = −0.13a | .007** |
| Executive | ρ = −0.26a | < .001*** |
| Speed of Information Processing | ρ = −0.20a | < .001*** |
| Learning | ρ = −0.20a | < .001*** |
| Memory | ρ = −0.15a | .002 ** |
|
| ||
| Number of Impaired NP Domains | ρ = −0.20a | < .001*** |
|
| ||
| Everyday Functioning Measures | ||
| COMPASS | ρ = 0.47a | < .001*** |
| IADL | z = −0.83b | .409 |
|
| ||
| ACTG Adherence | ||
| Percent adherence | ρ = 0.03a | .497 |
| Adherent vs. Non-Adherent (90% cutoff) | z = −1.04b | .299 |
| Total Pills Prescribed | ρ = 0.02a | .669 |
| Total Pills Skipped | ρ = −0.03a | .520 |
Spearman’s ρ
Mann-Whitney test
Kruskal-Wallis test
p < 0.05
p < 0.01
p < 0.001
Similar bivariate analysis of MMT-R scores and disease characteristics revealed significantly lower MMT-R scores in those with hepatitis C infection (Mann-Whitney U test; z = −2.86, p = 0.004). MMT-R was not significantly associated (p > .10) with other factors such as psychiatric variables (BDI-II total scores, BDI-II clinical classification, or current or prior history of MDD, AUD, or SUD) or HIV-related variables (duration of HIV, history of AIDS, current CD4 count, CDC clinical classification, nadir CD4, or viral load).
With the exception of fine-motor skills (Spearman’s ρ = −.07, p = 0.13), MMT-R scores significantly correlated with all other clinical ratings of NP functioning, including global (Spearman’s ρ = −.24, p < 0.001), attention/working memory (Spearman’s ρ = −.35, p < 0.001), verbal ability (Spearman’s ρ = −.13, p = 0.007), executive functions (Spearman’s ρ = −.26, p < 0.001), speed of information processing (Spearman’s ρ = −.20, p < 0.001), learning (Spearman’s ρ = −.20, p < 0.001), and memory (Spearman’s ρ = −.15, p = 0.002). Additionally, MMT-R scores were significantly related to a premorbid estimate of intellectual functioning (Spearman’s ρ = .46, p < 0.001)
Significant correlations were also found between MMT-R scores and COMPASS scores (Spearman’s ρ = −.47, p < 0.001), but not with IADLs (Mann-Whitney U test; z = −0.83, p = 0.41) or indices of adherence (e.g., MMT-R Percent Adherence: Spearman’s ρ = .03, p = 0.50).
Post-Hoc Bivariate Analyses
Given that our findings were dissimilar to those of Albert et al. [8], we performed exploratory post hoc analyses to assess the association between MMT-R scores and other variables in a subsample of our participants more closely matching the CD4 counts of their study population. Specifically, bivariate correlations were computed for participants (n = 56) with CD4 counts of 200 or less at the time of evaluation. In this subset, MMT-R scores were positively related to self-reported adherence (Spearman’s ρ = .36, p < 0.007) as they were in the Albert et al. (1999) study.
Regression Analyses
Multivariate regression analyses were utilized to evaluate the prediction of MMT-R scores by the demographic, health, NP, and everyday functioning variables found to be significant at the bivariate level, as described above. Variables were entered in blocks, with demographic variables (i.e., age, education, gender and race/ethnicity) entered in Step 1, health variables (i.e., Hepatitis C status) in Step 2, and NP and everyday functioning variables (i.e., premorbid estimate, global, attention/working memory, verbal fluency, executive functioning, speed of information processing, learning, memory, number of impaired domains, and COMPASS scores) entered in Step 3 (Table 6). The initial model was significant (R2 = .15, Adj. R2 = .14, F(4, 402) = 18.1, p < 0.001), with education, race/ethnicity, and age each contributing to the prediction of MMT-R scores. Addition of hepatitis C in Step 2 did not significantly improve the model (i.e., p > .10). With the addition of NP and everyday functioning variables in the third step, the model was significantly improved (R2 = .34, Adj. R2 = .32, F(15, 390) = 13.61, p < 0.001), and MMT-R scores were predicted in order of their contribution by COMPASS scores (β =.20; p = 0.002), premorbid estimate (β =.19; p = 0.001), attention/working memory ratings (β = −.19; p = 0.001), learning ratings (β = −.16; p < 0.02), and education (β = .14; p = 0.01).
Table 6.
Prediction of MMT-R Scores: Results of hierarchical multiple regression analyses.
| b | SE b | β | R2 | Δ R2 | |
|---|---|---|---|---|---|
|
| |||||
| Step 1 | 0.15*** | ||||
| Constant | 5.07 | 0.91 | |||
| Age | −0.04 | 0.01 | −0.15** | ||
| Education | 0.25 | 0.05 | 0 27*** | ||
| Race/Ethnicity | 0.46 | 0.11 | 0 20*** | ||
| Gender | 0.05 | 0.28 | 0.01 | ||
|
| |||||
| Step 2 | 0.15*** | 0.002 | |||
| Constant | 5.18 | 0.92 | |||
| Age | −0.04 | 0.01 | −0.14** | ||
| Education | 0.24 | 0.05 | 0 26*** | ||
| Race/Ethnicity | 0.44 | 0.11 | 0 20*** | ||
| Gender | 0.06 | 0.28 | 0.01 | ||
| Hepatitis C Status | −0.22 | 0.25 | −0.04 | ||
|
| |||||
| Step 3 | 0.34*** | 0.19*** | |||
| Constant | 2.64 | 1.18 | |||
| Age | −0.001 | 0.01 | −0.003 | ||
| Education | 0.13 | 0.05 | 0.138* | ||
| Race/Ethnicity | 0.06 | 0.12 | 0.025 | ||
| Gender | 0.19 | 0.25 | 0.034 | ||
| Hepatitis C Status | −0.22 | 0.23 | −0.043 | ||
| Est. Premorbid Functioning (WRAT-3 Reading) | 0.03 | 0.01 | 0.192** | ||
| Global | −0.10 | 0.13 | −0.073 | ||
| Attention/Working Memory | −0.22 | 0.06 | −0.19** | ||
| Verbal Fluency | 0.07 | 0.07 | 0.050 | ||
| Executive | −0.06 | 0.07 | −0.058 | ||
| Speed of Information Processing | −0.05 | 0.07 | −0.039 | ||
| Learning | −0.20 | 0.09 | −0.158* | ||
| Memory | 0.06 | 0.09 | 0.050 | ||
| Number of Impaired NP Domains | 0.16 | 0.13 | 0.124 | ||
| COMPASS | 0.0 | 0.02 | 0.202** | ||
p < 0.05
p < 0.01
p < 0.001
Post-Hoc Regression Analyses
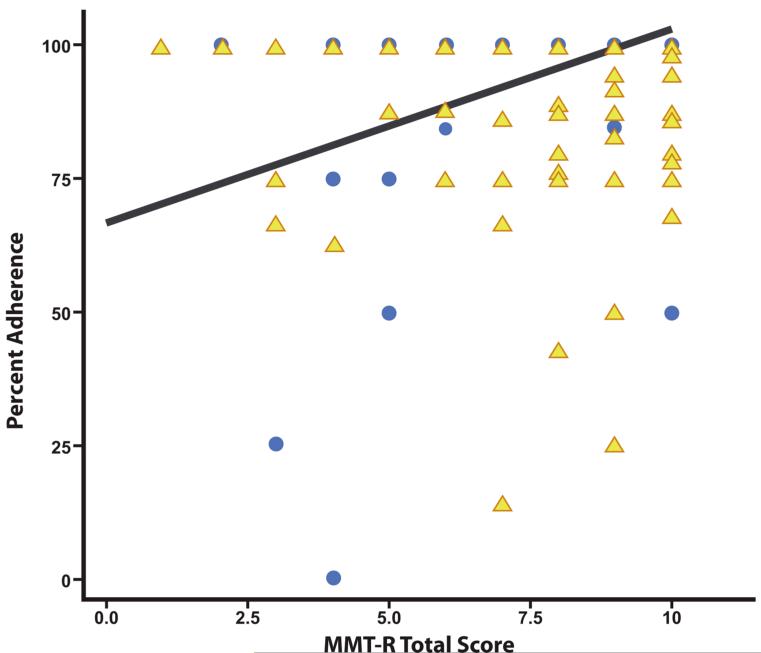
As discussed above, discrepancies between our findings and those of Albert et al. [8] prompted exploratory post hoc analyses. A significant bivariate correlation was found between MMT-R scores and adherence in participants with current immunosuppression (n = 56). When we tested CD4 count and MMT-R scores for interactions in determining adherence we found such an interaction (F(8,423) = 10.61, p < .001)., Higher MMT-R scores were associated with higher levels of adherence for participants with CD4 ≤ 200 (n = 56, F(8,423) = 12.07, p < .001), but not for those with CD4 > 200 (F(8,423) = 0.77, p = .64). This interaction is depicted in Figure 1. A multivariate regression model predicting adherence by MMT-R among participants with CD4 ≤ 200 and adjusted for depression (i.e., BDI-II Total Score) and global NP ratings was significant (R2 = .19, Adj. R2 = .15, F(3, 52) = 4.18, p = 0.01). Only MMT-R scores significantly predicted adherence (β =.46; p = 0.001) whereas depression (β = −0.04; p = 0.74) and global NP functioning (β =.21; p < 0.13) did not.
Figure 1.
Relationship of MMT-R Scores to Adherence among participants with CD4 ≤ 200 (represented by circles, n = 56) compared to those with CD4 > 200 (represented by triangles n = 386).
DISCUSSION
While performance-based tests of everyday functioning (e.g., medication management) offer promise in facilitating diagnosis of HAND, no instruments have yet been validated for the clinic or research. Our findings support the construct validity of the MMT-R in a well-characterized, geographically diverse cohort of HIV-infected patients attending six academic clinics.
The most robust support for the construct validity of the MMT-R was its associations with clinical measures of NP functioning. At the bivariate level, MMT-R scores were moderately correlated with most NP domains measured, including tests of executive functions, memory, information processing speed, verbal fluency, and attention/working memory. These domains are consistent with the requirements of both components of the MMT-R.
Contrary to our expectations, multivariate analyses revealed that attention/working memory and learning were the strongest predictors of MMT-R scores, domains that have been historically been more highly related to the medication inference items [8]. The burdens on learning and working memory for this section are high, as participants are required to encode, consolidate, retrieve, and manipulate information in order to answer questions about the sample regimen as well as an over-the-counter medication insert. Because an unfamiliar sample regimen was used in the task, participants’ adherence to their own well-practiced regimen may be less reliant on learning and/or working memory. Surprisingly, fine-motor skill deficits were not associated with performance on the MMT-R despite the fact that participants were required to have sufficient dexterity to place each pill in its requisite pillbox compartment. However, without a time limit or penalty for slow completion of test items on the MMT-R, it may be less sensitive to motor slowing.
Although MMT-R performance was associated with several sociodemographic factors (e.g., age), the most robust predictors were education, vocational functioning (i.e., COMPASS scores), and a reading-based estimate of premorbid IQ. One interpretation of these data is that these three sociodemographic predictors of MMT-R reflect the common influence of cognitive reserve, which may play an important role in the expression of HAND [39]. Specifically, cognitive reserve refers to the relative strength and utilization of neural networks and adaptive compensatory strategies that may moderate the clinical manifestation of HIV-associated neuropathology [40]. HIV-infected individuals with lower cognitive reserve (e.g., lower levels of education and occupational attainment) are at greater risk for HAND as compared to persons with high reserve [41]. Thus, in the current study, those persons with higher levels of occupational functioning, premorbid IQ, and educational attainment may have been protected against deficits in MMT-R performance as a function of higher cognitive reserve. This interpretation is commensurate with recent data showing that low literacy (i.e., lower cognitive reserve) is a risk factor for medication non-adherence in persons living with HIV [42].
In the current study, MMT-R scores were not related to adherence in the full study sample. Given that impaired neurocognition in HIV is associated with diminished medication management capacity [8, 9, 10, 5, 11] and poorer medication adherence [13, 14], we expected that medication management capacity would be related to adherence among those with HIV. However, this relationship has yet to be specifically demonstrated. While the Albert study (1999) found increased likelihood of medication recall errors (60% vs. 31%) in those with impaired memory and low medication inference scores, its small overall sample size precluded statistical comparisons of error likelihood across NP status groups [8]. More recently, Thames et al. [23] found no association between self-reported and performance-based assessments of both medication management and financial management, concluding that deficits were more readily identified through performance-based measures. It remains to be seen whether an objective measure of adherence (e.g., electronic monitoring) may correspond more closely to MMT-R performance. Alternatively, associations between diminished performance-based medication management performance and adherence may be obscured by the standardization involved in developing a simulated task regimen used for all participants. By testing with a sample regimen, the task may specifically evaluate a given participant’s aptitude for initial adherence to a novel regimen. This ability may become gradually diminished even as participants continue to adapt to cognitive impairments using compensatory strategies (e. g., fixed medication schedules [9]) to remain adherent to their own familiar and well-practiced regimen.
Several similarities and distinctions in our study compared to that of Albert et al. [8] warrant discussion to better understand our findings. Most notably, participants in our study had better immune functioning at the time of evaluation (mean CD4 = 477 vs. 199 [8]), despite having longer estimated duration of HIV infection (i.e., 11 years vs. 7 years [8]). These disparities in health status likely reflect historical differences in treatment options and effectiveness, since participants in the Albert et al. [8] study were evaluated at a time during which cART had just become available. More to the point, could these differences account for differences in findings between the two studies? In order to examine this possibility, we performed exploratory post-hoc analyses, which suggested that current immune health significantly influenced the relationship between MMT-R scores and adherence. Specifically, MMT-R scores strongly predicted adherence among participants who were immunosuppressed (i.e., CD4 ≤ 200), but not in those with CD4 > 200. Among immunosuppressed participants, MMT-R scores significantly predicted adherence, but other factors that have historically been predictors (i.e., depression and global NP functioning) did not.
While these exploratory post-hoc findings require replication, we hypothesize that immunosuppression unmasks the relationship between medication management capacity and adherence. That is, in the setting of severe immunosupression, an individual’s medication management capacity may become a more salient factor in the manifestation of adherence. Immunosuppression increases risk for neuropsychological compromise that may compromise adherence. A lack of association between NP and adherence in our post-hoc analyses would appear to argue against this explanation, but our NP assessment may not have targeted the critical abilities that are lost and limit adherence in advanced patients.
Limitations of the current study include the exclusive use of a self-report measure of adherence and cross-sectional design. Several methods to measure adherence, including objective (e.g., electronic monitoring, pharmacy refill verification) and subjective (e.g., self- and other-report) procedures might have increased the accuracy of its assessment. Additionally, a longitudinal study design could have enabled us to evaluate the ability of MMT-R to predict adherence and test whether changes in MMT-R scores predict decline in adherence. Finally, the MMT’s use of “sample” medications that are in common use may also introduce a heightened possibility of intrusion errors if a patient’s current or past medication is used with different dosing instructions as part of the MMT-R. Albert et al. [8] reports that approximately 40% of physicians report not following guidelines for protease inhibitors, so it may be helpful for future investigations to assess whether the test includes medications that a patient uses or has used in the past.
The relationship between medication management capacity and adherence is likely complex with multiple factors, such as motivation, social support, stigma, and many others contributing to adherence level. While a level of medication management capacity seems necessary, other factors may be more relevant and overshadow it in some patients. Thus, future studies might utilize more complex statistical modeling (e.g., structural equation modeling) to better understand the nature of these relationships. Finally, cART has been dramatically simplified since the MMT and its later versions were developed. These changes suggest that measures of medication management capacity should more closely reflect current cART regimens.
CONCLUSIONS
In summary, our findings provide mixed support for the construct validity of the MMT-R. Although the MMT-R was moderately associated with current NP functioning and sociodemographic factors and predicts adherence in those with advanced immunosuppression, it failed to predict self-reported medication adherence in more immunocompetent patients. We recommend that interpretation of MMT-R data be supplemented with collateral information, including neuropsychological testing, psychiatric assessments, and neuromedical examinations, particularly when used for clinical purposes. Because MMT-R scores were strongly predictive of adherence among a subset of individuals with more advanced disease (i.e., CD4 ≤ 200), it may be most useful screening tool in this population.
Acknowledgements
This research was supported by NIH contract N01 MH2205 (CHARTER; PI: I. Grant). Participating sites include: Johns Hopkins University (J. McArthur); Mt. Sinai School of Medicine (S. Morgello & D. Simpson); University of California, San Diego (J.A. McCutchan); University of Texas Medical Branch, Galveston (B. Gelman); University of Washington, Seattle (A. C. Collier & C. Marra); Washington University, St. Louis (D. Clifford).
REFERENCES
- 1.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18(Suppl 1):S75–78. [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 4.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 6.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackstone K, Moore DJ, Franklin DR, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: Self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. doi: 10.1017/S135561771100141X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert SM, Weber CM, Todak G, et al. An observed performance test of medication management ability in HIV: Relation to neuropsychological status and medication outcomes. AIDS Behavior. 1999;3:121–128. [Google Scholar]
- 9.Albert SM, Flater SR, Clouse R, et al. Medication management skill in HIV: I. Evidence for adaptation of medication management strategies in people with cognitive impairment. II. Evidence for a pervasive lay model of medication efficacy. AIDS Behavior. 2003;7(3):329–338. doi: 10.1023/a:1025404105378. [DOI] [PubMed] [Google Scholar]
- 10.Benedict RH, Mezhir JJ, Walsh K, Hewitt RG. Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch Clin Neuropsychol. 2000;15(6):535–544. [PubMed] [Google Scholar]
- 11.Waldrop-Valverde D, Jones DL, Jayaweera D, Gonzalez P, Romero J, Ownby RL. Gender differences in medication management capacity in HIV infection: The role of health literacy and numeracy. AIDS Behavior. 2009;13(1):46–52. doi: 10.1007/s10461-008-9425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods SP, Moran LM, Carey CL, et al. Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Arch Clin Neuropsychol. 2008;23:257–270. doi: 10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SP, Dawson MS, Weber E, et al. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Intl Neuropsycholl Soc. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Priority Interventions HIV/AIDS prevention, treatment and care in the health sector. WHO; Geneva: 2010. [Google Scholar]
- 16.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behavior. 2010;14(6):1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74(15):1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis MP, Colbert A, Erlen J, Meyers M. A qualitative study of persons who are 100% adherent to antiretroviral therapy. AIDS Care. 2006;18(2):140–148. doi: 10.1080/09540120500161835. [DOI] [PubMed] [Google Scholar]
- 19.Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: observational assessment and cognitive correlates. Psychol Aging. 1995;10(3):478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- 20.Waldrop-Valverde D, Osborn CY, Rodriguez A, Rothman RL, Kumar M, Jones D. Numeracy skills explain racial differences in HIV medication management. AIDS Behavior. 2010;14(4):799–806. doi: 10.1007/s10461-009-9604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldrop-Valverde D, Jones DL, Gould F, Kumar M, Ownby RL. Neurocognition, health-related reading literacy, and numeracy in medication management for HIV infection. AIDS Patient Care STDS. 2010;24(8):477–484. doi: 10.1089/apc.2009.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead–Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- 23.Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: The impact of age and cognition. J Clin Exp Neuropsychol. 2011;33(2):200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton RK, Clifford DB, Woods SP, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson GS. Wide Range Achievement Test. 3rd ed. Wide Range; Wilmington, DE: 1993. [Google Scholar]
- 27.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Inc; Lutz, FL: 2004. 2004. [Google Scholar]
- 28.Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky D, et al., editors. Clinical Interpretation of the WAIS-III and WMS-III. Academic Press; San Diego, CA: 2002. pp. 183–210. [Google Scholar]
- 29.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version: Professional Manual. Psychological Assessment Resources Inc.; Lutz, FL: 2000. [Google Scholar]
- 30.Reitan RM. Manual for administration of neuropsychological test batteries for adults and children. Reitan Neuropsychology Laboratories, Inc; Tucson, AZ: 1979. [Google Scholar]
- 31.Psychological Corporation . WAIS-III and WMS-III technical manual. Author; San Antonio, TX: 1997. [Google Scholar]
- 32.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exper Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 33.Valpar International Corporation . Computerized Assessment (COMPASS) Author; Tucson, AZ: 1992. [Google Scholar]
- 34.U.S. Department of Labor . Dictionary of occupational titles. 4th ed. U.S. Government Printing Office; Washington, DC: 1991. [Google Scholar]
- 35.Janikowski TP, Bordieri JE, Musgrave JR. Construct validation of the academic achievement and general educational development subtests of the Microcomputer Evaluation Screening and Assessment (MESA) Voc Eval Work Adjust Bull. 1990;23:11–16. [Google Scholar]
- 36.Stoelting C. A study of the construct validity of the MESA. Voc Eval Work Adjust Bull. 1990;23:85–91. [Google Scholar]
- 37.World Health Organization . Composite International Diagnostic Interview, version 2.1. WHO; Geneva: 1997. [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 39.Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12-months. J Clin Exper Neuropsychol. 2000;22:208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- 40.Satz P, Morgenstern H, Miller EN, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the Multicenter AIDS Cohort Study (MACS) J Acquir Immune Defic Syndr. 1993;66(5):503–511. [PubMed] [Google Scholar]
- 41.Pereda M, Ayuso-Mateos JL, Gomez Del Barrio A, et al. Factors associated with neuropsychological performance in HIV-seropositive subjects without AIDS. Psychol Med. 2000;30:205–217. doi: 10.1017/s0033291799001348. [DOI] [PubMed] [Google Scholar]
- 42.Waldrop-Valverde D, Jones DL, Weiss S, Kumar M, Metsch L. The effects of low literacy and cognitive impairment on medication adherence in HIV-positive injecting drug users. AIDS Care. 2008;20(10):1202–1210. doi: 10.1080/09540120801927017. [DOI] [PubMed] [Google Scholar]