Abstract
Background: objectively measured population physical activity (PA) data from older persons is lacking. The aim of this study was to describe free-living PA patterns and sedentary behaviours in Icelandic older men and women using accelerometer.
Methods: from April 2009 to June 2010, 579 AGESII-study participants aged 73–98 years wore an accelerometer (Actigraph GT3X) at the right hip for one complete week in the free-living settings.
Results: in all subjects, sedentary time was the largest component of the total wear time, 75%, followed by low-light PA, 21%. Moderate-vigorous PA (MVPA) was <1%. Men had slightly higher average total PA (counts × day−1) than women. The women spent more time in low-light PA but less time in sedentary PA and MVPA compared with men (P < 0.001). In persons <75 years of age, 60% of men and 34% of women had at least one bout ≥10 min of MVPA, which decreased with age, with only 25% of men and 9% of women 85 years and older reaching this.
Conclusion: sedentary time is high in this Icelandic cohort, which has high life-expectancy and is living north of 60° northern latitude.
Keywords: physical activity, accelerometry, sedentary behaviour, older adults, BMI, AGES-Reykjavik, older people
Introduction
Physical activity (PA) is an important indicator of health [1] and overall PA level decreases with age [2]. In old age, low PA has been linked with reduced physical functioning, such as mobility limitation [3], which is one of the most important factors in maintaining an individual's independence [4]. Sustained PA over the lifespan has been shown to have protective effects on mobility, even in those who start participating in PA at a later stage in life [5].
In epidemiological studies, PA has often been assessed by self-report measurements. Self-reports can be helpful but tend to overestimate true PA and underestimate sedentary time [6, 7]. Light PA is the most difficult intensity category to recall or remember accurately [8, 9]. However, light PA is the most common intensity category for the activities in which older adults engage [7, 10]. Although other measures exist for assessing overall energy expenditure (doubly-labelled water) and for the assessment of activity type and context (surveys and diaries), accelerometers are useful tools to explore patterns of PA objectively in terms of the elemental characteristics such as intensity, duration and frequency [11, 12]. Accurate assessments of PA levels and patterns, using objective portable activity monitors (pedometers and accelerometers), have been shown to be sensitive and feasible for measuring general activity patterns in older adults [13]. Although the accelerometer has been used extensively to assess PA in other age groups [2], it has been sparsely utilised in older populations.
The AGES-Reykjavik study (Age, Gene/Environment Susceptibility Reykjavik Study) has investigated the contributions of environmental factors, genetic susceptibility and gene–environment interactions to ageing of the neurocognitive, cardiovascular, musculoskeletal, body composition and metabolic systems in population with high life-expectancy [14]. The AGES-Reykjavik cohort was recruited from survivors of the Reykjavik Study. Data collection on the original Reykjavik Study cohort dates back to 1967 and there have been two waves of data collection for the AGES-Reykjavik studies, separated by 5 years (AGES-Reykjavik in 2002–06 and the AGESII-Reykjavik study in 2007–11). In this well-characterised population, the goals of this study are: (i) to assess free-living PA patterns in a subsample in the AGESII-Reykjavik study with accelerometers; (ii) to investigate the features of PA and sedentary patterns in the same study with respect to age, sex and body mass index (BMI). This study is, to our knowledge, the first one to objectively measure PA using accelerometry in a large, well characterised cohort of older people living north of 60° northern latitude.
Methods
Participants and protocol
This study was a part of the AGESII-Reykjavik study which is a follow-up of the AGES-Reykjavik study. Between April 2009 and June 2010, objective PA measurement by accelerometers was added to the AGESII-Reykjavik study protocol. Details on the study design and the baseline AGES-Reykjavik assessments have been described elsewhere [15].
During the PA measurement period, 1,194 subjects participated in the AGESII-Reykjavik study (73–98 year old). For the PA measurements, participants (n = 55) were excluded due to cognitive impairment (MMSE <20), as those participants were not expected to be able to reliably wear and use the accelerometer [16], 95 were excluded for other reasons (e.g. blindness and other physical obstructions), 84 refused and 294 did not participate because of scheduling conflict. The remaining 671 (56.2%) participants received an accelerometer to measure their daily activity. Five monitors were lost and 12 files were unusable because of device failures. The final number was 579 participants who had four or more valid days (≥10 h of wear time) of accelerometry data. The study was approved by the Icelandic National Bioethics Committee (VSN: 00–063), the Icelandic Data Protection Authority, and the institutional review board of the US National Institute on Aging, National Institutes of Health. Signed informed consent was given by all participants.
Measurements and data analysis
Participants were asked to wear the ActiGraph activity monitors (model GT3X ActiGraph, Inc., Pensacola FL, USA) monitor at the right hip for 7 days and to remove the monitor only before going to bed and during showers, bathing or other water activities. Also, participants completed a self-reported questionnaire on swim habits. To explore the general patterns of PA, we only report the data in the vertical axis. Activity intensity categories were defined as: sedentary, low-light intensity, high-light intensity and, moderate to vigorous intensity (see Table 1 for cut-points). Please see Supplementary data available Age and Ageing online, Appendix S1.
Table 1.
Descriptive statistics for subjects with four or more days with 10 or more hours of wear time
| Variable | Men |
Women |
P-value | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Age (years) | 221 | 79.7 (4.2) | 358 | 80.2 (5.1) | 0.23 |
| Weight (kg) | 220 | 83.2 (14.2) | 357 | 70.1 (13.6) | <0.001* |
| BMI (kg × m−2) | 220 | 26.7 (3.9) | 357 | 26.8 (4.8) | 0.69 |
| Valid wear time (min × day−1) | 221 | 832 (92) | 358 | 815 (83) | 0.024* |
| Age groups | |||||
| ≤74.9 | 53 | 859 (96) | 73 | 837 (78) | <0.001** |
| 75–79.5 | 76 | 831 (91) | 130 | 833 (90) | |
| 80–84.9 | 64 | 818 (77) | 90 | 802 (77) | |
| ≥85 | 28 | 816 (109) | 65 | 773 (65) | |
| BMI groups | |||||
| Normal weighte | 77 | 835 (102) | 132 | 816 (75) | 0.17 |
| Overweighte | 97 | 823 (82) | 141 | 814 (88) | |
| Obesee | 46 | 843 (95) | 84 | 816 (89) | |
| Total PA (1,000 counts × day−1) | 221 | 117 (68) | 358 | 105 (58) | 0.048* |
| Age groups | |||||
| ≤74.9 | 53 | 146 (67) | 73 | 130 (71) | <0.001** |
| 75–79.5 | 76 | 125 (67) | 130 | 116 (55) | |
| 80–84.9 | 64 | 95 (61) | 90 | 94 (45) | |
| ≥85 | 28 | 89 (60) | 65 | 72 (41) | |
| BMI groups | |||||
| Normal weight | 77 | 128 (78) | 132 | 113 (61) | <0.001*** |
| Overweight | 97 | 114 (64) | 141 | 103 (56) | |
| Obese | 46 | 106 (56) | 84 | 97 (53) | |
| Wear time PA (count × min−1) | 221 | 139 (78) | 358 | 128 (65) | 0.092 |
| Age groups | |||||
| ≤74.9 | 53 | 171 (77) | 73 | 154 (80) | <0.001** |
| 75–79.5 | 76 | 150 (78) | 130 | 139 (62) | |
| 80–84.9 | 64 | 115 (69) | 90 | 116 (51) | |
| ≥85 | 28 | 106 (67) | 65 | 92 (52) | |
| BMI groups | |||||
| Normal weight | 77 | 152 (89) | 132 | 137 (69) | <0.001*** |
| Overweight | 97 | 137 (74) | 141 | 125 (63) | |
| Obese | 46 | 125 (63) | 84 | 119 (62) | |
| Sedentary time (hours × day−1)a | 221 | 10.5 (1.5) | 358 | 10.0 (1.3) | <0.001* |
| Age groups | |||||
| ≤74.9 | 53 | 10.5 (1.8) | 73 | 9.9 (1.4) | 0.15 |
| 75–79.5 | 76 | 10.3 (1.5) | 130 | 10.0 (1.5) | |
| 80–84.9 | 64 | 10.7 (1.3) | 90 | 10.0 (1.3) | |
| ≥85 | 28 | 10.7 (1.7) | 65 | 10.2 (1.1) | |
| BMI groups | |||||
| Normal weight | 77 | 10.5 (1.6) | 132 | 9.9 (1.2) | 0.13 |
| Overweight | 97 | 10.3 (1.4) | 141 | 10.0 (1.4) | |
| Obese | 46 | 10.7 (1.5) | 84 | 10.3 (1.5) | |
| Sedentary time (percent × weartime−1)a | 221 | 75.9% (8.3%) | 358 | 73.9% (8.6%) | 0.001* |
| Age groups | |||||
| ≤74.9 | 53 | 73.5% (8.8%) | 73 | 71.5% (9.2%) | <0.001** |
| 75–79.5 | 76 | 74.3% (7.8%) | 130 | 72.1% (7.7%) | |
| 80–84.9 | 64 | 78.2% (7.4%) | 90 | 75.0% (8.2%) | |
| ≥85 | 28 | 79.0% (8.2%) | 65 | 78.9% (7.9%) | |
| BMI groups | |||||
| Normal weight | 77 | 75.8% (8.2%) | 132 | 72.9% (8.7%) | 0.002*** |
| Overweight | 97 | 75.5% (8.6%) | 141 | 73.8% (8.8%) | |
| Obese | 46 | 76.7% (7.7%) | 84 | 75.5% (8.0%) | |
| Low-light PA (min*day−1)b | 221 | 163 (55) | 358 | 182 (60) | <0.001* |
| Age groups | |||||
| ≤74.9 | 53 | 178 (63) | 73 | 197 (64) | <0.001** |
| 75–79.5 | 76 | 169 (50) | 130 | 198 (53) | |
| 80–84.9 | 64 | 150 (51) | 90 | 175 (59) | |
| ≥85 | 28 | 148 (56) | 65 | 145 (55) | |
| BMI groups | |||||
| Normal weight | 77 | 161 (56) | 132 | 189 (62) | 0.012*** |
| Overweight | 97 | 165 (56) | 141 | 184 (61) | |
| Obese | 46 | 163 (54) | 84 | 171 (54) | |
| High-light PA (min × day−1)c | 221 | 29 (22) | 358 | 27 (23) | 0.23 |
| Age groups | |||||
| ≤74.9 | 53 | 35 (22) | 73 | 36 (28) | <0.001** |
| 75–79.5 | 76 | 34 (25) | 130 | 30 (23) | |
| 80–84.9 | 64 | 21 (18) | 90 | 24 (16) | |
| ≥85 | 28 | 19 (16) | 65 | 17 (18) | |
| BMI groups | |||||
| Normal weight | 77 | 28 (19) | 132 | 28 (23) | 0.028*** |
| Overweight | 97 | 30 (25) | 141 | 27 (24) | |
| Obese | 46 | 29 (22) | 84 | 25 (22) | |
| MVPA (min*day−1)d,f | 221 | 9.9 (13) | 358 | 5.0 (7.2) | <0.001* |
| Age groups | |||||
| ≤74.9 | 53 | 14.5 (15.0) | 73 | 7.6 (9.6) | <0.001** |
| 75–79.5 | 76 | 10.0 (11.7) | 130 | 5.6 (7.5) | |
| 80–84.9 | 64 | 7.4 (12.6) | 90 | 3.7 (5.1) | |
| ≥85 | 28 | 6.8 (10.3) | 65 | 2.3 (4.0) | |
| BMI groups | |||||
| Normal weight | 77 | 13.9 (17.2) | 132 | 6.3 (8.1) | <0.001*** |
| Overweight | 97 | 8.4 (9.5) | 141 | 4.1 (5.6) | |
| Obese | 46 | 6.6 (8.6) | 84 | 4.3 (7.7) | |
Genders compared by t-test; for PA and sedentary variables t-tests were conducted on square root transformed data. PA measured by sex, age group and BMI. For cut-points see Troiano et al. [2] and Matthews et al. [17].
a0–99 counts/min.
b100–759 counts/min.
c760–2,019 counts/min.
d≥2,020 counts/min.
eNormal weight BMI < 25kg × m−2, overweight BMI = 25–29.9 kg × m−2, obese BMI ≥ 30 kg × m−2.
fOf the 579 participants, 25 (4.3%) had zero minutes of MVPA, 10 (4.5%) men and 15 (4.2%) women.
*Significant difference between genders (P < 0.05).
**Significant correlation with age, adjusted for BMI and gender (multiple linear regression).
***Significant correlation with BMI, adjusted for age and gender (multiple linear regression).
Results
Subject participation
Detailed demographic and anthropometric characteristics of subjects are presented in Table 1. There were no significant differences between men and women in age and BMI (P = 0.23 and 0.69, respectively) and no difference in total PA (counts × day−1) between different educational levels (P = 0.21), data not shown. Participants who wore the accelerometers had similar subject characteristics compared with those participants who did not receive an accelerometer: average values (SD) for men who did not receive an accelerometer; age = 81.8 year (5.1), weight = 83.0 (13.6), BMI = 26.7 (4.0) and for women who did not receive an accelerometer; age = 81.2 year (5.4), weight = 70.8 (12.3) and BMI = 27.3 (4.6).
Sex differences
The detailed summary of the PA is shown in Table 1. Men had ∼18 min longer average daily wear time than women (P = 0.024). While the average PA level during the valid days among participants varied widely (9,300–400,000 total PA (counts × day−1); 15–491 wear time PA (counts × min−1)), men had slightly higher average PA than women (total PA: P = 0.048; wear time PA: P = 0.092). Sedentary time was the largest component of the total wear time (∼74.5%), followed by low-light PA (21.3%) and MVPA (1%) in the participants as a group. Women spent more time in low to-light PA and less in MVPA compared with men, but had less sedentary time compared with men (all P < 0.001). The average time spent in MVPA per week was 9.9 min/day for men and 5.0 min/day for women.
Age differences
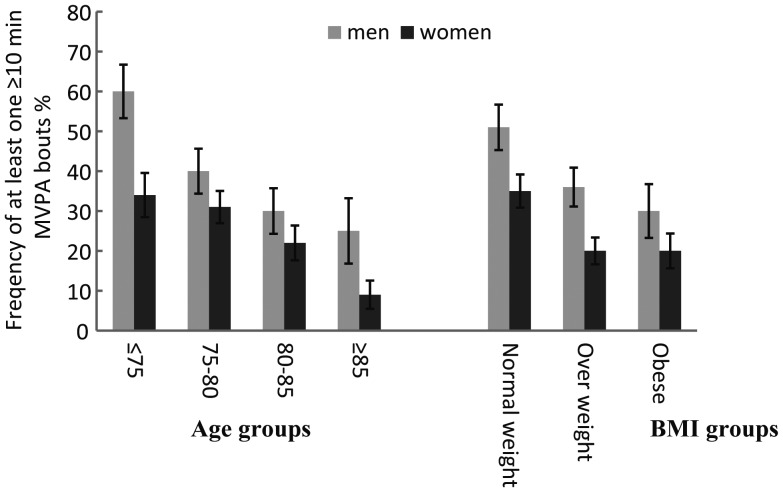
In both men and women, except for sedentary time, the PA variables total PA, wear time PA, low-light PA, high-light PA and MVPA decreased progressively with advancing age (Table 1). In the <75 years age group, 60% of men and 34% of women had at least one bout ≥10 min of MVPA (MVPA10+) during PA measurements (Figure 1). This proportion declined with age, and only 9% of the women and 25% of men in the >85-year age group had at least one bout of MVPA10+.
Figure 1.
Proportion (SEp) of subjects with more than one MVPA10+ bouts by age groups and gender (n = 579; men = 221, women = 358) and by BMI categories and gender (n = 577; men = 220, women = 357).
BMI differences
Except for sedentary time, BMI was negatively related to all PA variables. Furthermore, multiple linear regression analysis, using BMI and age as continuous variables, showed that the association of BMI and the PA parameters was independent of age and gender (Table 1). The proportion of subjects having at least one bout of MVPA10+ during PA measurements also declined with an increasing BMI (Figure 1).
Average daily PA patterns
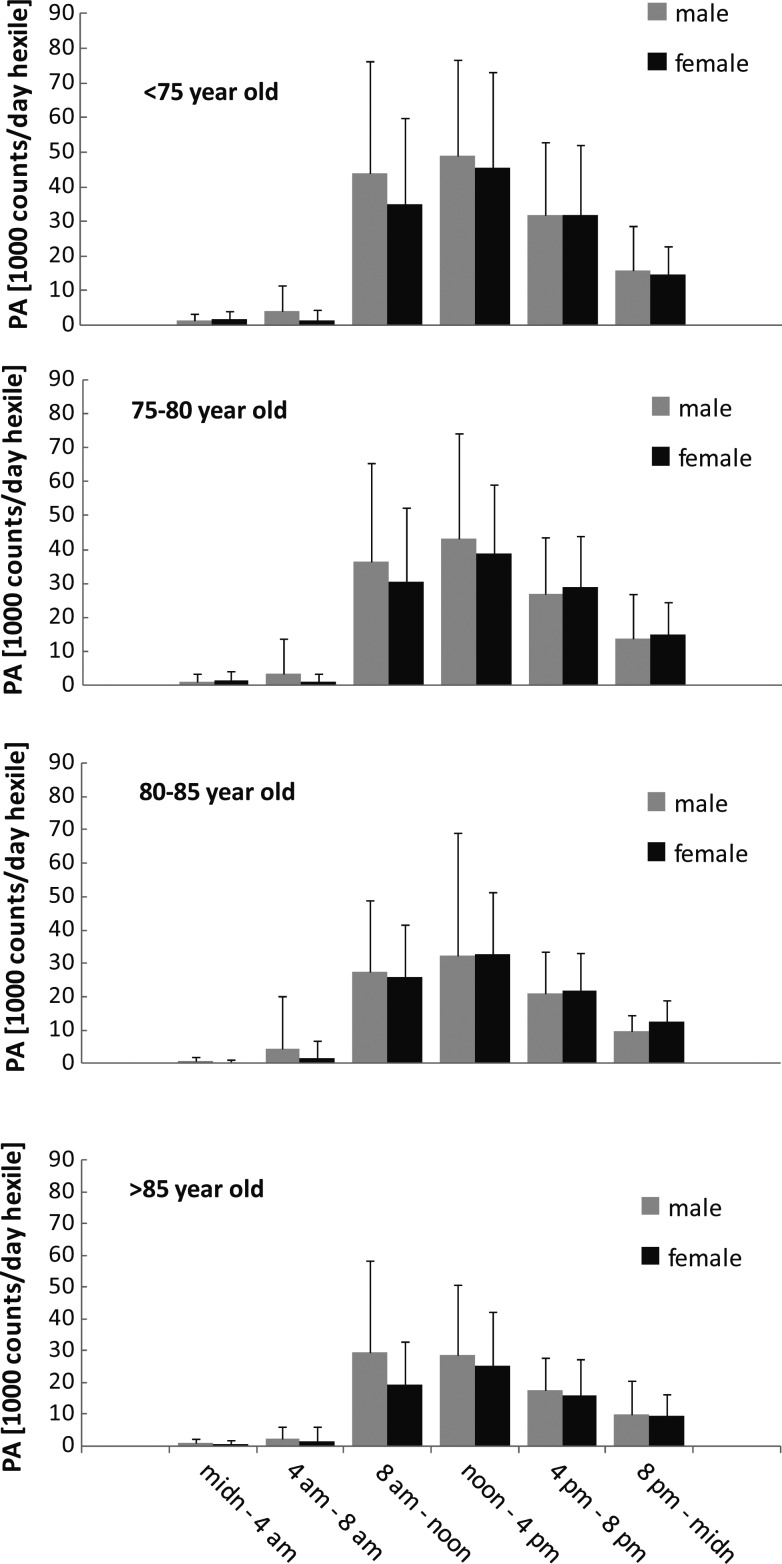
Within an average day, the majority of the PA occurred during the hours between 8 a.m. and midnight (Figure 2). In all age groups, there was a significant (P < 0.001) sex difference in within-day PA variation. Post hoc analysis (Bonferroni) indicated that men were more physically active than women between both 4 a.m.–8 a.m. and 8 a.m.–noon, Also, there was a significant difference in within-day PA variation and age groups (P < 0.001), which is explained by more decline in PA with increasing age during the day than during the night. Further examination showed the relative decline in PA with age to be very similar for all day hexiles, except for the midnight–4 a.m. hexile that show relatively more decline in PA with age than the other day hexiles.
Figure 2.
Distribution of PA between day hexiles (4 h periods) for different age groups and sexes. Beside a significant difference in PA between different day hexiles (P < 0.001), ANOVA for repeated measures (on square root transformed data) showed significant interactions of the within-day distribution of PA with both gender (P < 0.001) and age groups (P < 0.001); three-way interactions were non-significant (P = 0.65).
Day of week effects
There was a significant difference in activity by day of the week when adjusted for difference between subjects (P < 0.001). Participants were significantly less active on Sundays (total PA 98 × 103 counts × day−1) compared with other days of the week (114 × 103 counts × day−1; TukeyHSD: P < 0.001). Saturdays also had less activity than Wednesdays and Thursdays. Age and gender distribution of valid accelerometer data was the same for all days of the week.
Self-reported swim habits
About quarter of all participants reported swimming as an exercise, both during summer and winter, but of those who swim, only 25% swim for >30 min each time. Those who reported swimming as an exercise, also had more total PA and wear time PA (total PA: 121 × 103 counts × day−1 for swimmers vs. 91 × 103 counts × day−1 for non-swimmers; P < 0.001). Men used swimming more as an exercise compared with women, both during summer and winter (Chi-Square: P < 0.001). There was no age group difference in using swimming as an exercise during the winter (P = 0.069), but an age group difference was present during the summer (P = 0.013).
Discussion
The main finding of this study is that older adults spend on average 74.5% of their non-sleeping time as sedentary and 21.3% as low-light activity, indicating that this age group has very low activity. Furthermore, the PA is reduced as age and BMI increase. Women spend more time in low-light activity than men, where men had more MVPA than women.
Sedentary time, as proportion of wear time, was somewhat higher than has been reported for older populations [17, 18]. Harris et al [19] reported more than twice the total PA than we found (mean age 74 years). Also, average wear time PA has been reported around twice as high than in the present study [2, 20, 21]. Some of these differences may be explained by the older mean age of the participants in our study compared with the others. However, Davis and Fox [22] studied a group of older adults with a similar mean age and reported an average wear time PA intensity that was twice as high as that found here. In a recent study, PA was considerably higher in all age groups, except for the oldest group [23]. MVPA accumulated in our cohort, is only about half what has been previously shown for this age group [18, 22]. Furthermore, relatively fewer participants in our study reach at least one MVPA10+ bout per day as has been reported before [22].
Our results indicate that most older adults fail to meet general recommendations for PA, i.e. 30 min of MVPA each day [9, 24]. Older individuals find it difficult to take part and maintain MVPA [25, 26]. Therefore, more frequent periods of rest between bouts might be required for them to accumulate enough PA to meet recommendations. As Buman et al. [18] pointed out, it may be more realistic to focus the recommendations for older individuals on replacing sedentary behaviour by high-light PA rather than on accumulating MVPA. In our study, men accumulate 29 min of high-light PA each day on average and women 27 min.
The low PA level in our cohort can possibly be explained by several factors. It may be partly counterbalanced by swimming activity as a quarter of our cohort reported swimming as an exercise. Also, we have not taken into account seasonal effects. The summer is shorter in Iceland compared with the summer season of locations of comparable studies. Data in our study were accumulated from April to June with a summer-break in July, and then from August to June of the next year. Part of the free-living summer activity was missed. The reason that older adults accumulate low amount of MVPA in our and other studies [18, 22, 23] may also be due to the fact that cut-points were not adjusted according to individual aerobic fitness as should be done according to the recommendations [9, 24]. Factors such as illness and worse physical health may explain some of this reduced activity in older adults. The lack of social support and encouragement from family is an important factor, but fear of getting injured and moving outdoors is common [27, 28]. Icelandic winters can be cold, windy- and icy, so easy access to facilities is important and should be provided. PA pattern earlier in life can also be an indicator of later life activity [29].
The difference between sex and BMI groups in PA we report here is comparable with other previous population data [17–19, 22, 23]. Also, PA and valid wear time declines with age in both men and women which was similar to existing literature [18, 23]. Sedentary time remains the same for all age groups, which is similar to Davis et al. [23], except he showed the oldest age group to be most sedentary. Buman et al. [18] showed a decrease with age in low-light PA, high-light PA and MVPA, despite minor differences in how activity categories were defined compared with our definition. Sedentary behaviour in the USA, based on NHANES 2003–04, shows a large change from the age 60–69 to 70–85 age groups, where sedentary time is increased by 87 min/day on average [17].
Most of the PA in older Icelandic populations occurred from noon till 4 p.m. for both genders and all age groups, except for men in the oldest age group. Others have reported that older persons are active earlier in the day [22, 23]. The difference in the timing of PA could possibly be explained by the fact that Iceland is constantly on daylight saving time. Because of this, the solar noon is ∼1:30 p.m. in Reykjavik. As the PA in our and the other studies seems to peak around solar noon, the late solar noon in Reykjavik explains at least to some extent different daily pattern of activity in older Icelanders in comparison with the other studies.
The strength of this study is that the findings are based on a well-characterised large-population-based cohort of older Icelandic adults. However, there are some limitations that need to be accounted for when interpreting the results. It is known that accelerometers miss some movement patterns, like upper body movements during activities like weight lifting and heavy carrying. They also have limitations on detecting non-ambulatory activities like cycling [11]. However, in Iceland this kind of activity is not common in this age group. There might also be a problem measuring older individuals with accelerometers, where the quality of the data depends on participant's compliance [30]. This can be challenging for older persons suffering from some kind of memory loss, but those who were most cognitively impaired were excluded from our study. However, compliance in our study was very high as 86% of the participants had four or more valid days. Overall, the results from this study generally exhibited a low PA level in the population of older Icelanders who live at high latitude with high life-expectancy. This contradicts studies that show PA to reduce mortality and extend life expectancy [31]. The reason for high life expectancy in the Icelandic population may be due to a good health-care system, low infant mortality and high fish consumption [14, 32, 33]. Future studies are needed to further investigate how PA is related to health outcomes and other risk factors of health in this unique population.
Key points.
Very low level of PA in older population who live at high latitude with high life-expectancy.
PA declines with increasing age and BMI.
Women spent more time in low-light activity than men, where men had more moderate activity than women.
Conflicts of interest
None declared.
Funding
This study has been funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). This study was also supported by National Institutes of Health Intramural Research Program, grant number: Z01 DK071013 and Z01 DK071014 to R.J.B. and K.Y.C. The researchers are indebted to the participants for their willingness to participate in the study.
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
References
- 1.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Spor. 2006;16:3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 2.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 3.Leveille SG, Guralnik JM, Ferrucci L, Langlois JA. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999;149:654–64. doi: 10.1093/oxfordjournals.aje.a009866. [DOI] [PubMed] [Google Scholar]
- 4.Penninx BWJH, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the health, aging and body composition study. J Gerontol A-Biol. 2009;64:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Arch Int Med. 2009;169:1476–83. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- 6.Chinapaw MJ, Slootmaker SM, Schuit AJ, van Zuidam M, van Mechelen W. Reliability and validity of the activity questionnaire for adults and adolescents (AQuAA) BMC Med Res Methodol. 2009;9:58. doi: 10.1186/1471-2288-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Baranowski T. Validity and reliability of self report measures of physical-activity—an information-processing perspective. Res Q Exercise Sport. 1988;59:314–27. [Google Scholar]
- 9.US Department of Health and Human Services. 2008. Physical activity guidelines for Americans. 2012. April 10th http://health.gov/paguidelines/guidelines/chapter5.aspx . [Google Scholar]
- 10.Westerterp MR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93:1039–43. doi: 10.1016/j.physbeh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37(11 Suppl):S490–500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 12.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Brit J Sport Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OECD. Health at a Glance. 2011. April 9th 2012 http://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance_19991312;jsessionid=31e5looe0fck7.epsilon .
- 15.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 17.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–65. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris TJ, Owen CG, Victor CR, et al. A Comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sport Exer. 2009;41:1392–402. doi: 10.1249/MSS.0b013e31819b3533. [DOI] [PubMed] [Google Scholar]
- 20.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Activ. 2009;17:17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Hagstromer M, Troiano RP, Sjostrom M, Berrigan D. Levels and patterns of objectively assessed physical activity—a comparison between Sweden and the United States. Am J Epidemiol. 2010;171:1055–64. doi: 10.1093/aje/kwq069. [DOI] [PubMed] [Google Scholar]
- 22.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007;100:581–9. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]
- 23.Davis MG, Fox KR, Hillsdon M, et al. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exerc. 2011;43:647–54. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- 24.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 25.Burchfiel CM, Sharp DS, Curb JD, et al. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol. 1995;141:360–8. doi: 10.1093/aje/141.4.360. [DOI] [PubMed] [Google Scholar]
- 26.Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3 Suppl 2):172–83. doi: 10.1016/s0749-3797(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 27.Rantakokko M, Manty M, Iwarsson S, et al. Fear of moving outdoors and development of outdoor walking difficulty in older people. J Am Geriatr Soc. 2009;57:634–40. doi: 10.1111/j.1532-5415.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 28.Lord S, Chastin SF, McInnes L, et al. Exploring patterns of daily physical and sedentary behaviour in community-dwelling older adults. Age Ageing. 2011;40:205–10. doi: 10.1093/ageing/afq166. [DOI] [PubMed] [Google Scholar]
- 29.Loland NW. Exercise, health, and aging. J Aging Phys Activ. 2004;12:170–84. doi: 10.1123/japa.12.2.170. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox S, Tudor-Locke CE, Ainsworth BE. Physical activity, patterns, assessment and motivation in older adults. In: Shephard RJ, editor. Gender, Physical Activity, and Aging. Boca Raton, FL: CRC Press, 2002; 13–39 (chapter 2) [Google Scholar]
- 31.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 32.Michaud PC, Goldman D, Lakdawalla D, Gailey A, Zheng Y. Differences in health between Americans and Western Europeans: effects on longevity and public finance. Soc Sci Med. 2011;73:254–63. doi: 10.1016/j.socscimed.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramel A, Jonsdottir MT, Thorsdottir I. Consumption of cod and weight loss in young overweight and obese adults on an energy reduced diet for 8-weeks. Nutr Metab Cardiovasc Dis. 2009;19:690–6. doi: 10.1016/j.numecd.2008.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.