Abstract
Chronic obstructive pulmonary disease (COPD) is a common disease in the general population and it places a considerable burden on patients, with the disease negatively affecting quality of life. In practice, patients with COPD generally seek medical attention because of symptoms, particularly breathlessness, and the resulting physical limitations, which affect the health-related quality of life (HR-QOL) in patients. The defining feature of COPD is airflow limitation that causes air trapping and increased hyperinflation as the ventilation rate increases during physical effort. Hyperinflation causes or worsens breathlessness as breathing becomes inefficient, with the end result being an avoidance of physical exertion and a cycle of increasing dyspnea caused by inactivity and deconditioning, with deleterious effects on HR-QOL. Current published guidelines for COPD state that the goals of pharmacologic therapy should be to control symptoms, improve health status and exercise tolerance, and reduce the frequency of COPD exacerbations. Effective and sustained bronchodilation has emerged as a key strategy for improving dyspnea and ability to exercise. As there is no cure for COPD, a major goal of treatment and of research into new therapies is to improve HR-QOL in COPD patients.
Conclusion
More recently, indacaterol, an inhaled ultra-long-acting β2-agonist (24-hour action), has been approved in many countries at different doses (between 75 and 300 μg once daily) for treatment of patients with stable but symptomatic COPD. The aim of this review was to explore once-daily indacaterol clinical data as related to improvement in HR-QOL in COPD. Indacaterol studies have shown significant improvements in lung function of COPD patients, and these improvements have also translated into clinically meaningful improvements in patient symptoms and HR-QOL.
Keywords: airflow limitation, bronchodilation, long-acting β2-agonist, COPD
Introduction
Subjective indicators (based on self-rating) of quality of life (QOL) and health-related quality of life (HR-QOL) are increasingly incorporated in chronic obstructive pulmonary disease (COPD) because of the recognition of the importance of patient satisfaction and how patients with chronic disease feel, rather than how statistics imply these patients ought to feel. An American Lung Association survey states that half (51%) of all COPD patients say their condition limits their ability to work. The condition also limits COPD patients in normal physical exertion (70%), household chores (56%), social activities (53%), sleeping (50%), and family activities (46%).1
Although forced expiratory volume in 1 second (FEV1) is a measurement of lung function, because COPD is a progressively worsening disease, improvement in lung function can be transitory and not always indicative of symptoms improvement.2 It appears that improvements in patient-centered outcomes such as dyspnea and health status may better reflect the effectiveness of the particular pharmacotherapy.3,4 The concept of HR-QOL and its determinants in clinical trials have evolved to encompass those aspects of QOL that can be clearly shown to affect health, either physical or mental.5 In relation to health, health status is increasingly referred to as QOL, and, in order to narrow down the term’s operationalization in research studies, QOL is increasingly referred to as HR-QOL. Similar to subjective health status, HR-QOL is patient based; however, HR-QOL focuses more on the impact of a perceived health state on the patient’s ability to live a fulfilling life.6
The traditional approach to the diagnosis, staging, and treatment of COPD was based primarily on the percentage of predicted FEV1 and the rate of decline in FEV1. Previous recommendations for management of COPD were based solely on spirometric category. FEV1, while an important marker, is far from being the only measure available to characterize patients with COPD. In light of considerable cumulative evidence that the level of FEV1 is a poor descriptor of the disease status, Global Initiative for Chronic Obstructive Lung Disease guidelines7 have incorporated a strategy that considers both disease impact (determined mainly by symptoms burden and activity limitations) and future risk of disease progression (especially of exacerbations).7 Since the health status of patients with COPD is reported to be only weakly associated with spirometric values such as FEV1, measures of HR-QOL are increasingly used for evaluating the efficacy of bronchodilator medications in patients with COPD. Dyspnea, a key symptom in COPD, has been found to be strongly negatively associated with health status and to have the highest correlations with health status questionnaires.8–10 However, dyspnea is a subjective measure that poorly correlates with objective assessments of lung function, exercise capacity, and other outcomes. The other significant factors that determine QOL/health status in COPD patients are mood disorders (anxiety and depression) and exercise tolerance are all directly related to the disease – such as dyspnea and other symptoms – this will automatically result in these strong associations.11
Comprehensive assessment of the effects of COPD requires a battery of instruments that tap not only the disease-specific effects but also the overall burden of the disease on everyday functioning and emotional well-being. Health status as a concept of high complexity is assessed indirectly and requires the application of specially designed questionnaires. The most widely used instruments in studies addressing effects of pharmacologic treatment of COPD on HR-QOL are the Baseline Dyspnea Index (BDI) and the Transitional Dyspnea Index (TDI) for measuring breathlessness and the St George Respiratory Questionnaire (SGRQ) to assess the effects of bronchodilators on health status in COPD.12 The SGRQ is universally administered in COPD trials to measure improvements in HR-QOL. The use of an as-needed short-acting rescue inhaler (eg, salbutamol) has also become a quasi-standard measurement of symptom control in COPD trials. Additional outcomes of interest in clinical practice for management of COPD patients include mortality, hospitalizations, pneumonia, medication tolerability, and symptoms (especially exacerbations).
Indacaterol
The current standard of care for COPD patients is usually stepwise and is generally guided by disease severity. Current guidelines recommend treatment with one or more long-acting bronchodilators for patients with moderate or more severe disease.7 In clinical practice, bronchodilators are used for symptomatic relief in COPD patients with all stages of severity and on the basis of long-term improvements that can be achieved in clinical outcomes such as dyspnea, health status, and exacerbations. Indacaterol is the first ultra-long-acting β2-agonist (LABA) approved that has a 24-hour bronchodilatory effect, allowing for once-daily administration.
Indacaterol was first introduced throughout the European Union in 2009 at doses of 150 and 300 μg doses. In July 2011, indacaterol was also approved for use in the United States, but at a lower once-daily dose of 75 μg. The US Food and Drug Administration (FDA) approval of indacaterol was based on six phase III, randomized, double-blind trials: INLIGHT 1, INLIGHT 2, INVOLVE, INHANCE, INTENSITY, INSIST, and INDORSE (an extension of INHANCE).13–19 The designs of the six studies, plus the extension, are summarized in Table 1. Inclusion criteria were similar through each trial; patients were over 40 years old, with moderate to severe COPD and with at least a 10-pack-year smoking history. Short-acting β2-agonist (SABA) rescue medication and inhaled corticosteroids were permitted throughout all trials. A new drug application for indacaterol was submitted to the FDA in 2009; however, the drug was not approved at that time because a meaningful difference in efficacy between the proposed doses of 150 and 300 μg and a lower dose of 75 μg could not be discerned and because of concerns regarding safety with higher doses. To better delineate dose response at a lower dosage, the FDA requested the study in patients with asthma, a condition that is more responsive to bronchodilators than COPD. In addition to dose-ranging studies in asthma, the sponsor provided a second dose-ranging trial in patients with COPD and separate 12-week confirmatory trials using the 75 and 150 μg doses. In both the asthma and the COPD dose-ranging studies, there was no clear separation between 75 and 150 μg doses in the FEV1 time profile. As for the noncomparative confirmatory trials, both 75 and 150 μg doses had significantly higher trough FEV1 values than placebo. Because these studies did not directly compare the two doses, it is difficult to conclude whether the 150 μg dose is more effective than the 75 μg dose.20–22 As a result, the FDA supported approval of the 75 μg dose, based on the FDA’s own overall risk-benefit assessment. The primary efficacy outcome reported in all six studies was the difference in least squares mean trough FEV1 at 24 hours after 12 weeks of treatment. FEV1 is an important measurement in diagnosing and staging COPD. It is also commonly used in COPD clinical trials because it is an objective, reproducible measurement of lung function.
Table 1.
Pivotal trials of 12 weeks in duration or longer
| Reference | Study | Severity of COPD | Study duration (weeks) | Treatment arms |
|---|---|---|---|---|
| Feldman et al13 | B2346, INLIGHT1 | Moderate to severe | 12 | IND 150 μg (n = 211) Placebo (n = 205) |
| Kornmann et al14 | B2336, INLIGHT2 | Moderate to severe | 26 | IND 150 μg (n = 330) SAL 50 μg BID (n = 334) Placebo (n = 335) |
| Dahl et al15 | B2334, INVOLVE | Moderate to severe | 52 | IND 300 (n = 437) or 600 μg (n = 428) FOR 12 μg BID (n = 435) Placebo (n = 432) |
| Donohue et al16 | B2335S, INHANCE | Moderate to severe | 26 | IND 150 (n = 416) or 300 μg (n = 416) Placebo (n = 418) Open-label TIO 18 μg (n = 415) |
| Buhl et al17 | B2350, INTENSITY | Moderate to severe | 12 | IND 150 μg (n = 794) TIO 18 μg (n = 799) |
| Korn et al18 | B2349, INSIST | Moderate to severe | 12 | IND 150 μg (n = 559) SAL 50 μg BID (n = 562) |
| Chapman et al19 | Study 2335SE, INDORSE (extension of INHANCE) |
Moderate to severe | Additional 26 (total 52) |
IND 150 or 300 μg Placebo (n = 415) |
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; FOR, formoterol; IND, indacaterol; SAL, salmeterol; TIO, tiotropium.
The secondary outcome of interest to the FDA was the change from baseline in the SGRQ score. The FDA based approval of indacaterol on these two outcomes only. Because the FDA and the sponsor could not reach an agreement on the definition of “exacerbation,” this important outcome was not included in the FDA approval process.22 Because dyspnea is the key symptom of COPD and the need for rescue medication use is driven by dyspnea episodes, the data from the clinical development of indacaterol (eg, comparisons with active controls or scores on the BDI and the TDI) may have been weighed differently by the European Medicines Agency.21 Doses equal to or greater than 150 μg daily make up the majority of the available clinical data. Only 449 patients were exposed to the FDA-approved dose of 75 μg daily for up to 3 months.
Indacaterol data for health status/HR-QOL
The author has reviewed clinical evidence from the six indacaterol COPD studies mentioned earlier.13–19 The studies compared indacaterol with placebo, and inhaled tiotropium, salmeterol, or formoterol monotherapy. Indacaterol, along with all other bronchodilators, showed a statistically significant reduction in dyspnea compared with placebo. Importantly, improvements in lung function have translated into correspondingly beneficial effects on patient-reported outcomes, such as dyspnea, number of days with poor control, and clinically meaningful improvements in HR-QOL, particularly when comparisons are made with placebo.23
Breathlessness
The TDI was used to assess relief of dyspnea. The BDI and the TDI are validated clinical ratings that describe symptoms at a single point in time (eg, baseline) and measure changes in breathlessness from this baseline state over time, using a seven-point scale from −3 (major deterioration) to +3 (major improvement). TDI ratings are obtained in the course of an interview conducted by an experienced observer, with the observer asking open-ended questions about the patient’s experience of breathlessness during everyday activities that are then translated into numerical values. A change of one point is considered to be the minimum clinically important difference (MCID).24,25
Interviewer blinding to patients’ clinical status is necessary to prevent assessment bias. Mahler et al26 demonstrated for the first time that dyspnea ratings (including the TDI) were significantly related to the stage of disease severity based on the percentage of predicted FEV1. Indacaterol significantly improved dyspnea (measured by the TDI) compared with placebo.14–16 In general, indacaterol appeared to have greater effects on most COPD symptoms than open-label tiotropium, formoterol, or salmeterol, although differences between indacaterol and active comparators were not consistently statistically significant. Indacaterol subjects showed a 43% greater likelihood than tiotropium subjects of experiencing an MCID in the TDI of one point or higher (62% of indacaterol patients versus 52% of tiotropium patients).27 Tiotropium was used as an open-label comparator, thus introducing bias and limiting results interpretation. Nevertheless, when indacaterol was compared with tiotropium in blinded fashion in a study published by Buhl et al,17 indacaterol yielded greater improvements in TDI and SGRQ total scores than tiotropium.
Percentage of days with no rescue medication
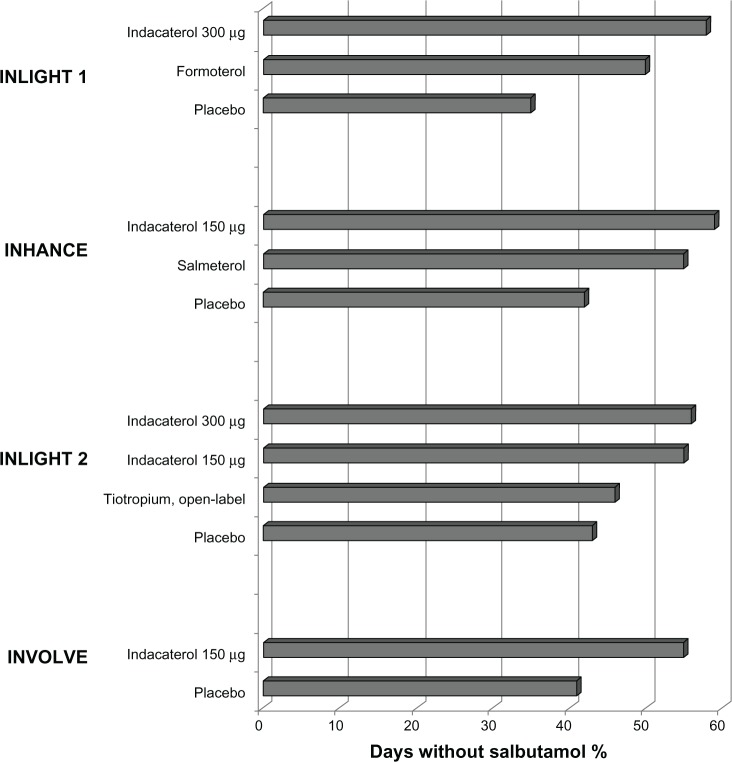
The use of salbutamol because of severity and/or frequency of COPD symptoms experienced by the patients serves as an indirect measure of how effective the study treatments are in controlling these symptoms. The percentage of days with no rescue medication was significantly higher in the indacaterol groups than in the active comparator groups in four studies (Figure 1).13–16 Indacaterol (both 150 and 300 μg doses) and formoterol recipients had significantly more days with no use of rescue medication than open-label tiotropium recipients in pooled analyses of 3-month28 and 6-month data.29 In addition to the four large (n > 400) placebo-controlled long-term indacaterol studies (Figure 1), significantly more patients receiving indacaterol had less need for a rescue inhaler (salbutamol) than salmeterol recipients over 12 weeks in the INSIST (Indacaterol: investigating superiority vs salmeterol) study.18
Figure 1.
Effect of treatment on use of as-needed salbutamol, averaged over the period of the studies, as percentage of days without use of salbutamol.
Use of an as-needed rescue inhaler (salbutamol)
Use of an as-needed short-acting rescue inhaler (salbutamol) has become a standard measure of symptom control in COPD trials. Rodrigo et al have conducted post hoc analysis of the five pivotal indacaterol studies.13–16,18 When compared with placebo, indacaterol significantly reduced the number of puffs per day of the rescue inhaler. Patients in the indacaterol group used less as-needed salbutamol, when compared with twice-daily LABA treatment, with a decrease of approximately one and a half puffs per day from baseline, although probably without clinical significance.23 Although use of a SABA in COPD during treatment with a LABA is recommended as needed, its effectiveness is unclear. To the best of the author’s knowledge, no clinical data have been reported to date about the effects of acute SABA administration on top of regular use of indacaterol in COPD treatment. The design of all phase III indacaterol COPD studies has included only stable patients. La Piana et al’s30 observational study, using a SABA on top of formoterol in patients with stable COPD, did not demonstrate improvement in FEV1 or peak expiratory flow after either acute or chronic treatment with formoterol. However, the authors concluded that the results of the study suggest a SABA was still effective in possibly reducing air trapping, as was demonstrated by the increase in forced vital capacity in the study.30
HR-QOL
Health status as a concept of high complexity is assessed indirectly and requires the application of specially designed questionnaires. The SGRQ has been widely used in clinical trials as a secondary end point to assess the effects of bronchodilators on health status in COPD and it has been demonstrated to be valid, reliable, and responsive among patients with COPD.31 The SGRQ is completed by the patient and has 50 questions covering three areas – symptoms, activity, and disease effects – summed to provide a total score of between zero (best) and 100 (worst). The instrument is time-consuming to implement and is therefore of limited applicability in day-to-day clinical practice. The SGRQ covers domains of symptoms (frequency and severity of respiratory symptoms), activity (effects on and adjustment of everyday activities), and psychosocial impact, from which a total score with a possible maximum of 100 points is calculated. A zero score indicates no impairment of QOL. The threshold for a MCID is a four-unit decrease in total SGRQ score. For example, a change of this size would occur in a patient who reports that he or she no longer takes so long to wash or dress, can walk up stairs without stopping, and is able to leave the house for shopping or entertainment.32,33 The SGRQ was used to assess HR-QOL in three studies.14–16 Significant improvements in HR-QOL were seen with indacaterol as compared with placebo, evidenced by statistically significantly lower (ie, better) mean SGRQ total scores at end-of-study assessments. Clinically important improvements (four points or greater) versus placebo were observed for indacaterol recipients.
Exercise endurance and lung hyperinflation
Limitation in exercise capacity represents an important feature of COPD and is one of the main factors that negatively affect the QOL in patients.34 Reduced exercise capacity is considered to be a consequence of airflow obstruction primarily because of dynamic hyperinflation occurring during exercise.35 Limited physical activity of patients with COPD, although a result of the disease, at the same time promotes worsening and progression of the disease and further decline in the patient’s HR-QOL. Consequently, exercise testing is increasingly being used in the functional and HR-QOL assessment of COPD patients and their response to bronchodilators. The possible methods of assessment of exercise capacity are field tests (6-minute and shuttle walking tests) and cardiopulmonary exercise testing (CPET).
CPET is considered the gold standard for studying a patient’s level of exercise limitation and its causes. O’Donnell et al36 undertook a study designed to assess the effect of indacaterol 300 μg once daily (dose not approved in the United States) on exercise endurance in patients with moderate to severe COPD using CPET.36 Indacaterol provided clinically meaningful improvements in exercise endurance with sustained reductions in lung hyperinflation both at rest and at the end of exercise. The authors postulated that the overall improvement in exercise capacity achieved with indacaterol at 300 μg once daily in the study may be an explanation for the improved health status (assessed using the SGRQ) seen with indacaterol in long-term studies.15,16
Mroz et al’s37 study of 34 patients with COPD used the 6-minute walking distance test to assess the impact of indacaterol add-on therapy on lung function, exercise tolerance, and QOL. This 3-month study showed that the patients’ QOL changed favorably in the indacaterol treatment arm, even though the FEV1 did not change significantly in the indacaterol group.37
Exacerbations of COPD
Exacerbations of COPD indicate clinical instability and progression of the disease and are associated with reduced health status, physical and physiologic deterioration, and an increased risk of morbidity and mortality.38,39 The prevention or reduction of exacerbations thus constitutes a major treatment goal. Although exacerbations are an important problem in COPD and a target of intervention, there is no universally standardized tool for assessing the frequency, severity, and duration of exacerbations. Health care databases and patient-based verification by interview, or prospectively from diary cards completed by patients, are the tools available for recording exacerbation rates during trials. Seasonal variations in exacerbation frequency usually require long-term studies of at least 1 year in duration.40 Analysis of time to first COPD exacerbation at 6 months has shown a reduced risk relative to placebo for indacaterol 150 μg. Similarly, the rate of exacerbation relative to placebo has been shown to be lower for indacaterol 150 μg but not for indacaterol 300 μg or tiotropium.16 Subjects with moderate to severe COPD who completed the 26-week INHANCE study were eligible for enrollment in an extension of the trial, during which double-blind treatment with indacaterol, 150 or 300 μg once daily, or placebo was continued for a further 26 weeks.19 Indacaterol treatment over this 12-month extension was accompanied by significant reductions in COPD exacerbations. However, indacaterol trials were not designed to measure exacerbations as the primary outcome, and all studies have only included stable patients without history of frequent exacerbations.
Discussion
The understanding of the merits and limitations of current methods for assessing physiological and clinical outcomes of COPD is crucial for the interpretation and design of clinical trials. Unfortunately, in contrast to spirometric category, there is no gold standard for measuring health status/HR-QOL, physical capacity for activities of daily living, and exacerbations, as none of the available methods is optimal in all regards.39 Nevertheless, there is considerable evidence that HR-QOL is a valid and important measure of the disease’s effect and activity.11 Therefore, it is fundamental that the study question be answerable by the study design. Since the relationship between spirometry, symptoms, and health status appears to be poor, measures of lung physiology alone may not adequately describe both the social impact of COPD and the effectiveness of therapeutic interventions.7
Indacaterol is a novel inhaled ultra-LABA providing 24-hour bronchodilation on once-daily dosing and is now approved in many countries for the management of patients with COPD. Indacaterol studies have shown significant improvements in lung function that, importantly, have translated into correspondingly beneficial effects on patient-reported outcomes such as dyspnea, number of days with poor control, and clinically meaningful improvements in HR-QOL as measured by the SGRQ, particularly when comparisons are made with placebo. Dyspnea is the primary reason for patients seeking medical care, and its measure may provide an insight into the practical effects of treatment on everyday life, reflecting whether or not patients perceive an improvement in this primary symptom of COPD. Results showed the sustained bronchodilator effect of indacaterol was accompanied by significant improvements in dyspnea and health status/HR-QOL when compared with placebo.23 Indacaterol was statistically superior to twice-daily LABAs (salmeterol and formoterol) in terms of exceeding the MCID in dyspnea BDI and TDI measurements. Indacaterol was also shown to be at least as effective as tiotropium in improving dyspnea and health status scores in indacaterol studies that included patients with mostly moderate to severe COPD.17,23
An important finding in phase III studies is that treatment with indacaterol showed in disease control, since sustained bronchodilation provided by indacaterol was associated with a 40% increase in rescue-free days compared with placebo. This meaningful reduction in salbutamol use for symptom relief characterized all indacaterol studies, suggesting that patients were experiencing fewer symptoms.41 The beneficial effects of indacaterol on breathlessness and health status suggest that the overall results of the indacaterol trials provide a useful guide to the level of efficacy that may be expected in milder patients who may be seen predominantly in a primary care patient population.27
Additionally, a new pooled analysis from three randomized studies (INVOLVE, INHANCE, and INLIGHT 2) in the indacaterol clinical trial program, INERGIZE, presented at the annual 2012 European Respiratory Society Congress in Vienna, Austria, showed that indacaterol 300 μg was superior to tiotropium in improving breathlessness in COPD patients who had more severe breathlessness symptoms on entry to the studies.42 Unfortunately, COPD is a progressive disease and often more therapies, with different mechanisms of action, are added in order to control symptoms and improve QOL. Recently, two studies investigating the approach of dual bronchodilation using indacaterol (150 μg) and tiotropium, compared with tiotropium alone, produced a significantly greater improvement in lung function than the tiotropium alone in patients with COPD.43 Sponsor Novartis introduced indacaterol in 2009. Novartis also added an investigational long-acting muscarinic antagonist, glycopyrronium bromide (NVA237), developed as once-daily inhaled maintenance therapy for the treatment of COPD. The results of phase III IGNITE data presented at the 2012 European Respiratory Society Congress demonstrated the efficacy of dual bronchodilation with indacaterol and glycopyrronium bromide (QVA149) and showed a superior effect on lung function and patient-reported outcomes versus all comparators used as monotherapy (placebo, indacaterol alone, glycopyrronium bromide alone, and tiotropium). QVA149 improved breathlessness as measured by the TDI, increased HR-QOL as measured by the SGRQ, and reduced rescue medication use.44 These results provide further support to current guidelines, which recommend use of one or more bronchodilators of different classes for treating moderate to severe disease.7 Additional studies assessing the impact of indacaterol and/or combination therapies such as a long-acting muscarinic antagonist and a LABA on COPD exacerbations, using a standardized definition of an exacerbation, are clearly needed.
Conclusion
Indacaterol is the first ultra-long-acting β2-agonist (LABA) approved that has a 24-hour bronchodilatory effect. Indacaterol studies have shown significant improvements in lung function that, importantly, have translated into correspondingly beneficial effects on patient-reported outcomes such as dyspnea, number of days with poor control, and clinically meaningful improvements in HR-QOL. Recent studies also suggest that dual bronchodilation with indacaterol in combination with long-acting muscarinic antagonists provide an additive impact on improving HR-QOL in patients with moderate to severe COPD with important implication for COPD management.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Chronic obstructive pulmonary disease (COPD) fact sheet [webpage on the Internet] Washington DC: American Lung Association; 2011Available from: http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.htmlAccessed September 14, 2012 [Google Scholar]
- 2.National Clinical Guideline Center Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care London: National Clinical Guideline Center; 2010Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/EnglishAccessed November 14, 2012 [PubMed] [Google Scholar]
- 3.Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(4):822–832. doi: 10.1183/09031936.06.00145104. [DOI] [PubMed] [Google Scholar]
- 4.Cazzola M, MacNee W, Martinez FJ, et al. American Thoracic Society; European Respiratory Society Task Force on outcomes of COPD Outcomes for COPD pharmacological trials: from lung function to biomakers. Eur Respir J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Measuring Healthy Days: Population Assessment of Health-Related Quality of Life Atlanta, GA: Centers for Disease Control and Prevention; 2000Available from: http://www.cdc.gov/hrqol/pdfs/mhd.pdfAccessed November 14, 2012 [Google Scholar]
- 6.Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res. 1993;2(6):451–459. doi: 10.1007/BF00422219. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary DiseaseGOLD; revised2011Available from: http://www.Goldcopd.comAccessed September 14, 2012
- 8.Mahler DA. How should health-related quality of life be assessed in patients with COPD? Chest. 2000;117(Suppl 2):S54–S57. doi: 10.1378/chest.117.2_suppl.54s. [DOI] [PubMed] [Google Scholar]
- 9.Reardon JZ, Lareau SC, ZuWallack R. Functional status and quality of life in chronic obstructive pulmonary disease. Am J Med. 2006;119(10 Suppl 1):32–37. doi: 10.1016/j.amjmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Martín A, Rodríguez-González Moro JM, Izquierdo JL, Gobartt E, de Lucas P; VICE Study Group. Health-related quality of life in outpatients with COPD in daily practice: the VICE Spanish study. Int J Chron Obstruct Pulmon Dis. 2008;3(4):683–692. doi: 10.2147/copd.s4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that infuence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20(3):257–268. doi: 10.4104/pcrj.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 13.Feldman G, Siler T, Prasad N, et al. INLIGHT-1study group Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med. 2010;10:11. doi: 10.1186/1471-2466-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kornmann O, Dahl R, Centanni S, et al. INLIGHT-2 study investigators Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 15.Dahl R, Chung KF, Buhl R, et al. INVOLVE study investigators Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 16.Donohue JF, Fogarty C, Lötvall J, et al. HANCE study investigators Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 17.Buhl R, Dunn LJ, Disdier C, et al. INTENSITY study investigators Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38(4):797–803. doi: 10.1183/09031936.00191810. [DOI] [PubMed] [Google Scholar]
- 18.Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C, INSIST study group Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med. 2011;105(5):719–726. doi: 10.1016/j.rmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, INDORSE study investigators Long-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140(1):68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- 20.VA Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives Indacaterol (Arcapta™ Neohaler™): National Drug Monograph Washington DC: US Department of Veterans Affairs; 2012Available from: http://www.pbm.va.gov/Clinical%20Guidance/drug%20Monographs/Indacaterol.pdfAccessed November 14, 2012 [Google Scholar]
- 21.Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol: the FDA’s review. N Engl J Med. 2011;365(24):2247–2249. doi: 10.1056/NEJMp1109621. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration Indacaterol FDA Summary Review Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022383Orig1s000SumR.pdfAccessedFebuary 2 2013
- 23.Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting beta-agonists for stable COPD: a systematic review. CHEST. 2012;142(5):1104–1110. doi: 10.1378/chest.11-2252. [DOI] [PubMed] [Google Scholar]
- 24.Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 25.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21(2):267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- 26.Mahler DA, Ward J, Waterman LA, McCusker C, Zuwallack R, Baird JC. Patient-reported dyspnea in COPD reliability and association with stage of disease. Chest. 2009;136(6):1473–1479. doi: 10.1378/chest.09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PW, Barnes N, Vogelmeier C, Lawrence D, Kramer B. Efficacy of indacaterol in the treatment of patients with COPD. Prim Care Respir J. 2011;20(4):380–388. doi: 10.4104/pcrj.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siler TM, Williams J, Yegen U, Owen R, Lassen C, Kramer B. The effect of once-daily indacaterol on health-related quality of life, rescue medication use, and exacerbation rates in patients with moderate-to-severe COPD: a pooled analysis of three months of treatment. Am J Respir Crit Care Med. 2010;181:A4430. [Google Scholar]
- 29.Kleerup E, Williams J, Yegen U, Owen R, Lassen C, Kramer B. The effect of indacaterol once-daily on health-related quality of life, symptoms and rescue medication use in moderate-to-severe chronic obstructive pulmonary disease: pooled analysis of six month. Am J Respir Crit Care Med. 2010;181:A4429. [Google Scholar]
- 30.La Piana GE, Corda L, Bertella E, Taranto Montemurro L, Pini L, Tantucci C. Dose-responsive curve to salbutamol during acute and chronic treatment with formoterol in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:399–405. doi: 10.2147/COPD.S22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 32.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–887. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 34.Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300. doi: 10.1183/09031936.00021409. [DOI] [PubMed] [Google Scholar]
- 35.Palange P, Ward SA, Carlsen KH, et al. ERS Task Force Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell DE, Casaburi R, Vincken W, et al. INABLE 1study group Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105(7):1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adv Exp Med Biol. 2013;756:23–28. doi: 10.1007/978-94-007-4549-0_4. [DOI] [PubMed] [Google Scholar]
- 38.Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res. 2010;11:79. doi: 10.1186/1465-9921-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anzueto A, Sethi S, Martinez FJ. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):554–564. doi: 10.1513/pats.200701-003FM. [DOI] [PubMed] [Google Scholar]
- 40.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:S46–S53. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 41.Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J. 2010;36(1):96–104. doi: 10.1183/09031936.00123709. [DOI] [PubMed] [Google Scholar]
- 42.Mahler DA, Buhl R, Lawrence D, McBryan D. Effectiveness of indacaterol and tiotropium in patients with severe dyspnea; European Respiratory Society Congress; September 1–5, 2012; Vienna, Austria. ERS abstract 850630, session 245. [Google Scholar]
- 43.Mahler DA, D’Urzo A, Peckitt C, Lassen C, Kramer B, Filcek S. Concurrent Use of Indacaterol Plus Tiotropium in Patients with COPD Provides Superior Bronchodilation Compared with Tiotropium Alone. Thorax. 2012;67(9):781–788. doi: 10.1136/thoraxjnl-2011-201140. [DOI] [PubMed] [Google Scholar]
- 44.Bateman E, Ferguson GT, Barnes N, et al. Benefits of dual bronchodilation with QVA149 once daily versus placebo, indacaterol, NVA237 and tiotropium in patients with COPD: the SHINE study; European Respiratory Society Congress; September 1–5, 2012; Vienna, Austria. ERS abstract 700179, session 306. [Google Scholar]