Abstract
Background
Essential tremor (ET) patients seem to have impaired gait and balance, yet surprisingly few studies have utilized quantitative analysis to study these impairments. With one exception, these prior studies examined gait on a treadmill, which does not approximate functional environmental conditions (level ground). Moreover, these studies tested middle-aged subjects so it remains unclear whether ET patients maintain a pattern of deficits that is in excess of that seen in controls, even into advanced ages. Methods: 104 ET subjects (86.0 ± 4.6, range = 75–97 years) and 40 similarly aged controls (84.1 ± 4.2, range = 74–94 years) underwent gait testing using the GAITRite® mat under standard walk and tandem walk conditions on level ground.
Results
In standard walk, ET patients demonstrated deficits related to gait speed (lower velocity and cadence, p = 0.0001), dynamic imbalance (increased double support percent, p = 0.01), and gait asymmetry (increased step time difference, p = 0.003). During tandem walk, ET patients had lower velocity (p = 0.002) and cadence (p = 0.003), and more mis-steps (p < 0.008) compared with controls. For all variables, ET patients performed more poorly than controls even into advanced ages, as demonstrated in linear regression models.
Conclusions
ET patients demonstrated decrement in gait speed, dynamic balance and gait symmetry during standard walk and clear balance impairment during tandem walk. This constellation of impairments is consistent with a cerebellar deficit. ET patients maintained this pattern of deficits, in excess of that seen in controls, into advanced age, reinforcing the importance of gait and balance impairment in this disorder.
Keywords: Essential tremor, Gait, Tandem walk, Aging, Motor control
1. Introduction
Essential tremor (ET), one of the most common neurological disorders, is characterized by both motor and non-motor impairments [1]. The most prominent motor impairment is progressive kinetic tremor, particularly in the arms, although tremor may be present in the head and other body regions [1–3]. Other motor impairments include postural instability [4,5] and gait and balance impairments [6–8].
Gait and balance impairments are functionally important because they increase risk for falls and associated morbidity. Despite their importance, only a few studies have examined gait and balance in patients with ET. Three studies used simple bedside clinical measures to assess tandem gait in ET patients [6,7,9]. Two of the three studies reported that ET patients had more mis-steps compared with age-matched controls during tandem walking [7,9]. Recently, we reported among ET patients that tandem mis-steps were positively correlated with age and cranial tremor (i.e., tremor involving the jaw, voice and/or head) [6].
Three other studies examined gait and balance in ET using a more sophisticated approach – quantitative movement analysis [4,8,10]. Two of these studies examined gait on a treadmill and reported greater number of mis-steps and increased step width during tandem walking in ET patients [8,10]. In contrast, examination of standard walking on a treadmill revealed minimal impairments (increased step width) in ET patients compared with controls [8]. The only quantitative paper that examined gait on level ground tested 13 ET patients and reported lower velocity, cadence and higher double support time [4].
Thus, findings have been mixed and the exact nature of gait and balance impairments in ET is unclear. One likely contributor is the varied methodology used: walking on level ground [4] vs. walking on a treadmill [8,10]. Treadmill walking imposes unnatural spatial (narrow path) and temporal constraints (constant speed) on walking, which is very different compared with functional ambulation on level ground. The other likely reason for the mixed results is the numbers of patients sampled in each study have been small (N = 13 [4] and N = 25 patients [8,10]).
Another limitation of previous studies is that ET patients were mainly middle-aged (mean age = 50.3 ± 21.1 years [8], 46.3 ± 22.6 [10]), or middle-aged to elderly (61.6 ± 16.5 years) [4]. Since gait impairments progress with age in otherwise healthy elderly [11–13], and since the gait impairment in ET is subtle to begin with, it is unclear whether (1) things even out during advanced age such that ET patients and controls are both similarly impaired with respect to gait or, alternatively, (2) ET patients maintain a pattern of deficits that is in excess of that seen in controls, even into advanced age. To address this question, one would need to enroll cases in advanced age groups (70s, 80s and 90s). An important clinical issue is whether excessively disordered gait represents a continued deficit that follows ET patients through their disease course.
Our primary aim was to use a computerized instrumented walkway on level ground to further clarify the exact nature of the gait and balance impairments in ET. We used a large sample of 104 ET patients in order to do so and compared their performance to that of age-matched controls. A second aim of this study was to examine whether differences in gait performance between ET patients and controls persist with advanced age. Thus, our subjects ranged in age from 74 to 97 years.
2. Methods
2.1. Subjects
ET patients and controls were enrolled in an ongoing clinical-pathological study at Columbia University Medical Center (CUMC), New York. Thus, ET patients were enrolled as future brain donors to the Essential Tremor Centralized Brain Repository (ECBR) at Columbia University, a national repository for the collection of ET brains. They were ascertained by (1) advertisements in the International Essential Tremor Foundation website and newsletters, (2) advertisements on the Tremor Action Network website, and (3) an ETCBR study website (www.essentialtremor.us). ET patients were recruited throughout the United States and were not restricted to the local New York area. Using data from a clinical questionnaire and videotaped neurological examination administered during their in-person assessment (see below), the diagnosis of ET was re-confirmed in each ET case using published diagnostic criteria (moderate or greater amplitude kinetic tremor during three or more activities or a head tremor, in the absence of Parkinson’s disease) [14,15].
Spousal controls were recruited when the ET subject had a living spouse who did not have a diagnosis of ET or another movement disorder (such as Parkinson’s disease or dystonia).
2.2. Testing
Subjects were recruited across the United States and were tested at home in a single day by a trained tester. Upon enrollment, each subject signed a written informed consent form, approved by the CUMC institutional ethics committee. Prior to testing, we ensured that subjects had access to a hallway long enough accommodate testing with the gait mat. Testing consisted of two parts, a clinical assessment and quantitative gait assessment. Subjects were provided with rest, as needed, during testing.
2.3. Clinical assessment
All ET patients and controls underwent an in-person clinical assessment that included collection of demographic and clinical (e.g., height, weight) data. As part of a more detailed clinical assessment, ET patients also underwent a standardized videotaped neurological examination [16] and a Folstein Mini Mental State Examination (MMSE, range = 0–30) [17].
2.4. Quantitative gait examination
The GAITRite®, a 4.6 m long computerized mat (CIR Systems, Havertown, PA) was used for collecting quantitative gait data. The GAITRite contains pressure sensors embedded in the mat, which register the location and timing of each footfall, and computes spatio-temporal measures from customized algorithms. Gait data were stored on a laptop computer for off-line processing. Subjects began walking at a starting position 3 m from the beginning of the mat and continued walking ~3 m beyond the end of the mat. This enabled us to record steady-state gait without the effects of gait initiation and termination. ET patients and controls were tested under two conditions: (a) standard walk, in which subjects were asked to walk at their preferred speed; and (b) tandem walk, in which they were asked to walk in a straight line with the heel of one foot touching the toe of the other foot [6]. We requested subjects not to use assistive devices (such as canes or walkers) for this task. Five subjects (three ET patients and two controls) were unable to perform the task without assistive devices. Their data were not included in the analysis.
Data were analyzed by AKR, who was blinded to clinical diagnosis and age. We computed (1) measures of gait speed (velocity, cadence and step length), (2) dynamic balance (double support percent of gait cycle, support base and tandem mis-steps [the latter was measured only during tandem walk]), (3) gait symmetry (step time difference), and (4) gait variability (coefficient of variation in stride length and swing time), for further analysis.
The variables were defined as follows:
1a. Velocity was meters traveled per second.
1b. Cadence (or step frequency) was the number of steps per minute.
1c. Step length was the distance, in meters, between the heels of successive footfalls.
2a. Double support, expressed as a percentage of the gait cycle, was the percent time that both feet were simultaneously on the ground.
2b. Support base was the distance, in meters, between the heels of the left and right feet in a direction perpendicular to the line of progression.
2c. Tandem mis-steps were defined as the number of steps in a direction away from the line of progression.
3. Step time difference was the difference, in seconds, between the step time of the right and left feet. This was computed from the step time, which was the time each foot spent on the ground.
4a. Variability of stride length was computed as the coefficient of variation (CoV) in stride length.
4b. Variability of swing time was computed as the CoV in swing time, which was defined as the time in seconds that each foot was off the ground.
2.5. Statistical analyses
Demographic and clinical variables such as age, gender, height, and weight were compared in ET patients vs. controls using a chi-square test or an independent Student’s t-test (p = 0.05). During each condition (standard walk and tandem walk), the pre-specified quantitative gait measures (velocity, cadence, step length, double support percent, support base, step time difference, variability of stride length, variability of swing time) were compared in ET patients vs. controls using independent Student’s t-tests. For tandem walk, we also examined the number of mis-steps in ET patients vs. controls. Level of significance was adjusted to 0.0125 reduce the risk of committing a Type I error during multiple comparisons.
In order to evaluate the influence of age on gait, we performed a series of linear regression analyses (with age and diagnostic group [ET vs. control] as independent predictors in the same model) and selected as the dependent variable, those gait variables that differed across diagnostic groups (ET vs. controls) in initial bivariate analyses. All statistical analyses were carried out by AKR in SPSS® (version 16.0).
3. Results
3.1. Subject characteristics and correlates of gait performance
Given the advanced age of ET patients (Table 1), the number of available spouses was limited. Thus, the ratio of ET patients to controls was 2.5:1 (i.e., 104 ET patients and 40 controls, Table 1). ET patients and controls were similar in terms of gender, age, weight, and height (Table 1). Age of tremor onset for ET patients was 43.3 (SD = 23.6 years, (range = 11–90 years). None of the gait variables tested in our study was associated with age of tremor onset. The mean MMSE score for ET patients was 26.7 ± 2.9, which is consistent with the reported median score for population-based healthy individuals >80 years, [18] indicating that ET patients did not have significant cognitive impairments. Among ET patients, none of the gait variables were correlated with the total score on the MMSE, indicating that gait performance was not influenced by cognitive ability.
Table 1.
Demographic and clinical characteristics of ET patients and controls
| ET patients | Controls | |
|---|---|---|
| Number of subjects | 104 | 40 |
| Female subjects (percent) | 62 (59.6) | 25 (62.5) |
| Age (years) ± SD | 86.0 ± 4.6 | 84.1 ± 4.2 |
| Range years | 75-97 | 74-94 |
| Weight ± SD (kilogram) | 66.81 ± 13.3 | 63.59 ± 11.1 |
| Height ± SD (m) | 1.69 ± 0.5 | 1.70 ± 0.4 |
| Folstein mini mental state Examination score ± SD |
26.7 ± 2.9 | Not tested |
| Age of tremor onset ± SD (years) |
43.3 ± 23.6 | Not applicable |
3.2. Gait (standard walk)
ET patients walked with slower speed compared with controls: they demonstrated lower velocity (p = 0.0001) and lower cadence (p = 0.0001). Gait velocity for our control subjects (0.94 ± 0.25 m/sec) was comparable to published norms for a comparable age group. [12] No differences were seen in step length across groups (p > 0.05). ET patients also demonstrated impaired dynamic balance, as seen by increased percent time in double support (Table 2). No differences were seen in support base (p > 0.05). Finally, ET patients demonstrated gait asymmetry as indicated by increased step time difference compared with controls (p = 0.003). No differences were seen in any of the variables related to gait variability, such as CoV in stride length or CoV in swing time (p > 0.05 in both cases). Means and standard deviations for a number of gait variables are shown in Table 2.
Table 2.
Means ± SD for gait measures across diagnostic groups for standard walk and tandem walk conditions
| Standard walk |
Tandem walk |
|||
|---|---|---|---|---|
| Control | ET patients | Control | ET patients | |
| Measures related to gait speed | ||||
| Velocity (m/sec) | 0.94 ± 0.25 | 0.76 ± 0.26*** | 0.27 ± 0.13 | 0.20 ± 0.13** |
| Cadence (steps/min) | 102.90 ± 10.36 | 92.85 ± 14.8*** | 52.05 ± 17.28 | 41.17 ± 18.55** |
| Step Length (m) | 0.51 ± 0.09 | 0.46 ± 0.13 | 0.28 ± 0.067 | 0.28 ± 0.074 |
| Measures related to dynamic balance | ||||
| Double Support percent | 33.83 ± 6.79 | 39.01 ± 12.87* | 55.93 ± 15.83 | 59.49 ± 21.28 |
| Support base (m) | 0.11 ± 0.04 | 0.11 ± 0.03 | 0.035 ± 0.026 | 0.044 ± 0.031 |
| Tandem mis-steps | NA | NA | 2.23 ± 3.68 | 4.43 ± 4.67** |
| Measure related to gait symmetry | ||||
| Step time difference (sec) | 0.03 ± 0.03 | 0.05 ± 0.07** | 0.46 ± 0.68 | 0.62 ± 0.59 |
| Measures related to gait variability | ||||
| Stride length CoV | 5.2 ± 7.35 | 5.7 ± 5.5 | 43.62 ± 23.74 | 48.2 ± 25.85 |
| Swing time CoV | 7.31 ± 5.7 | 7.64 ± 6.14 | 76.03 ± 97.63 | 63.47 ± 34.99 |
NA= not applicable.
p < 0.01.
p < 0.005.
p < 0.001.
3.3. Gait (tandem walk)
ET and control subjects were able to complete the tandem walk test. This was confirmed by narrow support base for both controls (0.035 ± 0.026 m) and ET patients (0.04 ± 0.031 m); no differences were seen across groups (p > 0.05) for support base. In comparison with standard walk, both controls and ET patients demonstrated significant decrements in gait velocity and cadence. Despite the overall decrease in speed related variables for both groups, gait velocity (p = 0.002) and cadence (p = 0.003) were significantly lower for ET patients compared with controls. No differences were seen for step length across groups, indicating that regulation of speed occurred through modulation of cadence (step frequency). In addition, balance was impaired in ET patients, as evidenced by greater number of tandem mis-steps (ET patients (4.43 ± 4.67) vs. controls (2.23 ± 3.68), p = 0.008). No differences were seen in double support percent or support base. Gait variability, evaluated by the CoV in stride length and CoV in swing time, was substantially increased for both controls and ET patients compared with standard walk condition (Table 2). However, no differences were seen across diagnostic groups for any of the variability measures.
3.4. Gait (effect of age)
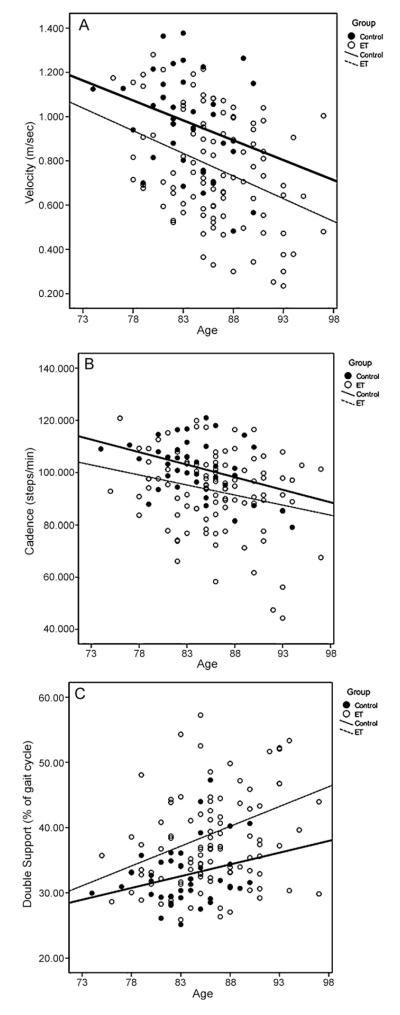
In order to evaluate the influence of age on gait, we performed a series of linear regression analyses (with age and diagnostic group [ET vs. control] as independent predictors in the same model) and, selected as dependent variables those gait measures that differed across diagnostic groups (ET vs. controls) in initial bivariate analyses (p < 0.05) (Table 3). Fig. 1 also graphically shows the results of the three regression analyses in which gait velocity (A), cadence (B) and double support percent (C) for standard walk were the dependent variables. Diagnostic group (p = 0.0001) and age (p = 0.001) were significant independent predictors of gait velocity (Table 3). Age and diagnostic group were significant independent predictors of cadence and double support percent as well (Table 3). The regression lines for velocity (Fig. 1A), and cadence (Fig. 1B) were parallel, indicating that despite age-related decrements in these two variables for both groups, ET patients were more impaired compared than controls at all ages in our sample. Regression lines for double support percent (Fig. 1C) indicate that performance of ET patients worsened with age compared with controls (seen by the divergent regression lines).
Table 3.
Regression coefficients and significance for selected gait variables with diagnostic group and age as predictors
| Predictor: diagnostic group |
Predictor: age |
|||
|---|---|---|---|---|
| Regression coefficient | Significance | Regression coefficient | Significance | |
| Standard walk | ||||
| Velocity | −0.34 | 0.0001a | −0.26 | 0.001a |
| Cadence | −0.26 | 0.002a | −0.24 | 0.004a |
| Double support (%) | 0.22 | 0.01a | 0.18 | 0.03a |
| Tandem walk | ||||
| Velocity | −0.29 | 0.001a | 0.13 | 0.12 |
| Cadence | −0.28 | 0.001a | 0.15 | 0.07 |
| Mis-steps | 0.28 | 0.004a | −0.13 | 0.16 |
a Significant regression coefficients.
Fig. 1.
Relationship between age and gait velocity (A), cadence (B) and double support percent (C) for controls (filled circles) and ET patients (empty circles) during standard walking. Linear regression fit is shown for both groups (controls: thick line, ET patients: thin hatched line).
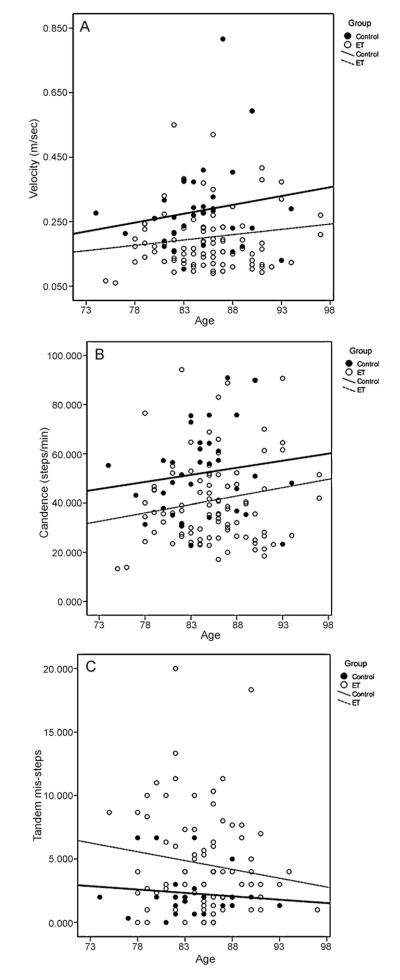
Results of regression analyses for tandem walk are presented in Table 3 and Fig. 2. Diagnostic group was a significant independent predictor of gait velocity (Table 3 and Fig. 2A), cadence (Fig. 2B) and tandem mis-steps (Fig. 2C). The parallel regression lines for diagnostic groups for velocity and cadence in Fig. 2 indicates that ET patients were more impaired (lower velocity, lower cadence and increased tandem mis-steps) compared with controls even into advanced ages. The regression lines for tandem mis-steps indicate that ET patients had greater number of mis-steps compared with controls, despite the slight decrease in mis-steps with age (Fig. 2C). Age was not a significant factor in any of these analyses (Table 3).
Fig. 2.
Relationship between age and gait velocity (A), cadence (B) and tandem mis-steps (C) for controls (filled circles) and ET patients (empty circles) during tandem walk. Linear regression fit is shown for both groups (controls: thick line, ET patients: thin hatched line).
4. Discussion
Quantitative gait analysis for patients with movement disorders has helped to characterize gait impairments to include one or more of the following: impairment in gait speed (decrease in velocity, cadence and/or step length), dynamic imbalance (increase in double support percent, base of support, mis-steps), gait asymmetry (step time difference), or gait variability (increase in variability in stride length or variability in swing time) [19–23]. Gait and balance impairments have recently been described in ET, but the precise nature of the impairments is unclear, as evidenced by observed differences across studies. Contributors to the differences are small sample sizes and, even among those that used quantitative gait analysis, the testing methods differed considerably (walking on a gait mat on level ground [4] vs. walking on a moving treadmill [8,10]). Of three studies that conducted quantitative gait analysis, only one tested walking on level ground with a small number of subjects (N = 13) [4]. The other two studies tested gait on a treadmill, which is not optimal because it imposes artificial spatial and temporal constraints on gait not seen in functional ambulation [8,10]. Here we examined gait in a large sample of ET patients (N = 104) and controls (N = 40) using quantitative gait analysis on level ground. Our results show that ET gait, under standard walking conditions, is characterized by slower gait speed, dynamic imbalance and temporal asymmetry. However, we did not observe increased stride-to-stride variability in ET patients compared with control subjects [23,24].
We observed a similar pattern of results during tandem walking. While both controls and ET patients demonstrated decrements during tandem walk, ET patients in particular demonstrated slower gait speed and dynamic imbalance (indicated by greater number mis-steps). ET patients did not demonstrate high stride-to-stride variability compared to controls, though variability was significantly higher (by – an order of magnitude) for both groups during tandem walk.
The motor phenotype of ET in our study includes decreased speed, dynamic imbalance, step asymmetry and tandem gait difficulty, and this pattern could reflect underlying cerebellar pathology [2,6,20]. However, results from the present study and previous studies [4,8,10] did not find increased stride-to-stride variability, considered an important hallmark of cerebellar pathology, suggesting that the changes in ET are not as advanced as those seen in the cerebellar ataxias. The pathology in the cerebellar ataxias is centered in the cerebellum as well as select brainstem nuclei. Although the underlying anatomical pathological of ET is still under active investigation, several recent studies have detailed the constellation of microscopic changes (e.g., increased numbers of torpedoes, Purkinje cell loss, increased Purkinje cell heterotopias, hypertrophic changes in Basket cell axonal processes) that are present in the cerebellum of ET cases on post-mortem examination [25,26].
Our results show that while both ET patients and controls demonstrated decline in gait performance with age, ET patients continued to perform more poorly than controls for most gait measures, even into and through advanced ages (70s, 80s and 90s). Thus, despite the fact that gait impairments progress with age in otherwise healthy elderly, age was not an equalizer for gait measures. The clinical implication of these findings is that excessively disordered gait represents a continued deficit that follows ET patients to the end of their disease course and which continues to distinguish them from their age-matched counter-parts without the disease. This emphasizes the continued presence of gait dysfunction in ET and should encourage its inclusion in the clinical evaluation of and clinical dialogues with patients living into later decades, something that, at the moment is not routine.
To summarize, our study has elucidated a clear pattern of impairments during standard walk and tandem walk in elderly patients with ET. We found that, when compared with similarly aged controls, ET patients demonstrated decrement in gait speed, impaired dynamic balance and gait asymmetry during standard walk and clear balance impairment during tandem walk. ET patients maintained a pattern of deficits during standard walking that was in excess of that seen in controls, even into advanced age, indicating that the gait decrement that typically occurs with age does not approximate that of ET patients, thereby serving to further reinforce the permanence and potential importance of gait and balance impairment in this disorder.
Acknowledgements
This work was supported by the National Institutes of Health (Bethesda, MD) under grant number 5R01NS042859-07. We thank all ET subjects, their families and control subjects for participating in the study. Statistical analyses were conducted by Ashwini K. Rao.
Funding: Ashwini K. Rao has received research support from the National Institute of Child Health and Human Development (1K01HD060912-01A1, PI), and National Institute of Neurological Disorders and Stroke (5R01NS042859-06A2, Co-I). Arthur Gillman has not received any support. Elan D. Louis has received research support from the National Institutes of Health: NINDS #R01 NS42859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R56 NS042859 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NIA #2P01 AG0027232-16 (principal investigator), and NINDS #R01 NS36630 (co-Investigator), as well as the Parkinson’s Disease Foundation (principal investigator), the Arlene Bronstein Essential Tremor Research Fund (Columbia University), and the Claire O’Neil Essential Tremor Research Fund (Columbia University).
Footnotes
Conflicts of interest: None of the authors report a conflict of interest with respect to financial or personal relationships with organizations that may have an influence on the work.
References
- [1].Louis ED. Essential tremor. Lancet Neurol. 2005;4(2):100–10. doi: 10.1016/S1474-4422(05)00991-9. [DOI] [PubMed] [Google Scholar]
- [2].Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2(12):666–78. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- [3].Benito-Leon J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31(3):191–2. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- [4].Earhart GM, Clark BR, Tabbal SD, Perlmutter JS. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009;24(3):386–91. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parisi SL, Heroux ME, Culham EG, Norman KE. Functional mobility and postural control in essential tremor. Arch Phys Med Rehabil. 2006;87(10):1357–64. doi: 10.1016/j.apmr.2006.07.255. [DOI] [PubMed] [Google Scholar]
- [6].Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. 2010 doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9(2):193–6. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- [8].Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. 2001;124(Pt 11):2278–86. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- [9].Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age. Mov Disord. 1997;12(6):969–72. doi: 10.1002/mds.870120620. [DOI] [PubMed] [Google Scholar]
- [10].Kronenbuerger M, Konczak J, Ziegler W, Buderath P, Frank B, Coenen VA, et al. Balance and motor speech impairment in essential tremor. Cerebellum (London England) 2009;8(3):389–98. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- [11].Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60(6):835–9. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- [12].Shumway-Cook A, Guralnik JM, Phillips CL, Coppin AK, Ciol MA, Bandinelli S, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55(1):58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wolfson L. Gait and balance dysfunction: a model of the interaction of age and disease. Neuroscientist. 2001;7(2):178–83. doi: 10.1177/107385840100700212. [DOI] [PubMed] [Google Scholar]
- [14].Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic criteria for essential tremor: a population perspective. Arch Neurol. 1998;55(6):823–8. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- [15].Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–33. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- [16].Louis ED, Ford B, Lee H, Andrews H. Does a screening questionnaire for essential tremor agree with the physician’s examination? Neurology. 1998;50(5):1351–7. doi: 10.1212/wnl.50.5.1351. [DOI] [PubMed] [Google Scholar]
- [17].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [18].Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–91. [PubMed] [Google Scholar]
- [19].Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10(3):247–59. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- [20].Morton SM, Bastian AJ. Mechanisms of cerebellar gait ataxia. Cerebellum (London England) 2007;6(1):79–86. doi: 10.1080/14734220601187741. [DOI] [PubMed] [Google Scholar]
- [21].Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13(3):428–37. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- [22].Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS. Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov Disord. 2008;23(8):1100–7. doi: 10.1002/mds.21987. [DOI] [PubMed] [Google Scholar]
- [23].Palliyath S, Hallett M, Thomas SL, Lebiedowska MK. Gait in patients with cerebellar ataxia. Mov Disord. 1998;13(6):958–64. doi: 10.1002/mds.870130616. [DOI] [PubMed] [Google Scholar]
- [24].Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130(Pt 3):786–98. doi: 10.1093/brain/awl376. [DOI] [PubMed] [Google Scholar]
- [25].Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9(6):613–22. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- [26].Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70(16 Pt 2):1452–5. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]