Abstract
Late adulthood is associated with increased hippocampal atrophy and dysfunction. Although there are multiple paths by which hippocampal deterioration occurs in late life, the authors discuss the evidence that a single nucleotide polymorphism in the brain-derived neurotrophic factor (BDNF) gene and age-related changes in BDNF protein or receptor expression contribute to hippocampal atrophy. The authors conclude that few studies have tested whether BDNF mediates age-related hippocampal atrophy and memory impairment. However, there is strong evidence that decreased BDNF is associated with age-related hippocampal dysfunction, memory impairment, and increased risk for depression, whereas increasing BDNF by aerobic exercise appears to ameliorate hippocampal atrophy, improve memory function, and reduce depression. Importantly, the most consistent associations between BDNF and hippocampal dysfunction have emerged from research on BDNF protein expression in rodents and serum and plasma concentrations of BDNF in humans. Current research suggests that the BDNF val66met polymorphism may be only weakly associated with hippocampal atrophy in late adulthood. These conclusions are interpreted in relation to age-related memory impairment and preventions for hippocampal atrophy.
Keywords: aging, BDNF, depression, exercise, hippocampus
There are many common negative stereotypes of older adults in Western culture: lonely, grumpy, slow, frail, sickly, and cognitively feeble. These negative stereotypes often have the unfortunate effect of perpetuating ageism and expectations that cognitive decline is normal and inevitable. Stereotypes of aging are also present in science. For example, in human neuroscience, the aged brain has historically been stereotyped as brittle and uncomforming with little room for augmentation or enhancement. Luckily, more recent investigations have begun to question these stereotypes and to suggest that the aged brain is considerably more plastic and responsive than previously thought. Importantly, these breakthroughs challenge the definition of normal aging and the amount of brain deterioration or cognitive decline that should be deemed acceptable and “normal.”
Despite the growing recognition that the aged brain retains some capacity for plasticity, there is significant evidence for brain atrophy with advancing age (Figs. 1 and 2). For example, longitudinal studies have reported between 1% and 2% annual hippocampal atrophy in adults older than 55 years without dementia (e.g., Jack and others 1998). Other regions also atrophy in late adulthood, including the prefrontal cortex, caudate nucleus, and cerebellum, at estimated rates between 0.5% and 2% per year, whereas the striate, primary motor, and sensorimotor regions remain relatively preserved (Fjell and others 2009).
Figure 1.
The mean hippocampal volume (for both left and right hemispheres) atrophies in late adulthood. These data were collected using a high-resolution magnetic resonance imaging scan and the hippocampus demarcated using an automated segmentation algorithm (data adapted from Erickson, Prakash, and others 2010).
Figure 2.
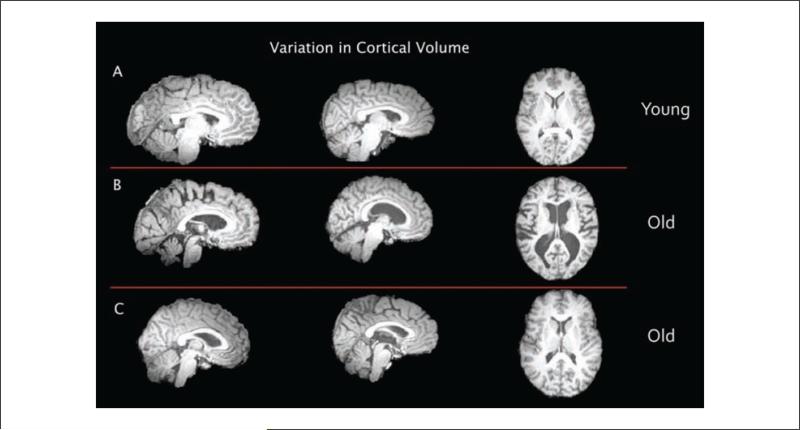
There is significant variation in brain atrophy with advancing age, such that some older adults show little atrophy whereas others show considerable atrophy. In this array of nine brains, we demonstrate that some of the variation can be easily detected by the naked eye. (A) Brain images from young subjects at an average age of 22 years. (B) Brain images from older adult subjects at an average age of 72 years. (C) Brain images from older adult subjects at an average age of 72 years. The average age of (B) and (C) are identical yet the morphology and amount of atrophy are quite disparate between these subjects. For example, the ventricles and sulci are enlarged in (B) compared with (C) or (A). These data showing differential trajectories of brain atrophy support the idea that brain decay might be slowed or prevented.
Alzheimer disease (AD) is characterized by significant tissue loss in many brain regions but most consistently with the hippocampal formation (Jack and others 1998). The loss of tissue in AD occurs at a considerably enhanced rate compared with adults without AD. For example, longitudinal studies find that the hippocampus deteriorates at 3% to 5% annually in adults with AD and that in adults with mild cognitive impairment, smaller hippocampal volumes are predictive of more rapid conversion to dementia (Jack and others 1998). This significant and rapid atrophy of hippocampal tissue is thought to cause the characteristic memory loss associated with AD.
Discovering the molecular mechanisms contributing to brain tissue loss in late adulthood has become an important objective in recent years. On a cellular level, there are manifold explanations for brain atrophy in humans, including increased apoptosis, decreased cell proliferation, axonal degeneration, reduced dendritic extent and spine density, shrinkage of cells, reduced vascularity, or any combination of these factors (von Bohlen und Halbach 2010). In fact, given the nonuniformity and nonlinearity in the rates of atrophy across the brain, it is unlikely that changes in any single molecule or cell structure will be able to fully explain volume loss. Indeed, rodent research suggests that increased rates of cell death are unlikely to explain atrophy associated with normal aging (Rasmussen and others 1996), suggesting that other factors (e.g., loss of dendritic spines) are contributing to increased atrophy in aged humans. Nonetheless, there are several molecules that have generated considerable interest for their putative roles in AD and cellular senescence. These molecules include β-amyloid and τ (Fjell and others 2010), inflammatory markers such as interleukin-6 (Marsland and others 2008), and stress hormones such as cortisol (Bruehl and others 2009). Brain-derived neurotrophic factor (BDNF) is another molecule found in high concentrations in the hippocampus and cortex and is critical in cellular analogs of memory formation, plasticity, and cell proliferation. For the remainder of this review, we outline the evidence that changes in the BDNF system are associated with age-related memory impairment, depression, and atrophy of the hippocampus in humans and the factors that might offset declining BDNF levels in an aged population.
The BDNF Polymorphism
A functional single nucleotide polymorphism on the BDNF gene produces an amino acid substitution of Val to Met at codon 66 in the prodomain of the BDNF peptide, with the Met allele impairing the regulated secretion and intracellular trafficking of BDNF (Egan and others 2003). Early work on the BDNF polymorphism demonstrated that the Met allele was associated with poorer episodic memory, smaller hippocampal volumes, and reduced levels of n-acetyl-aspartate in the hippocampus of young adults and schizophrenics (Egan and others 2003). Although the association with schizophrenia remains controversial, the BDNF Met variant has been associated with increased risk of depression (see below) and anxiety. Overall, these results argue that the BDNF polymorphism explains significant individual variability in hippocampal morphology and function, a result consistent with research in rodents demonstrating that BDNF is critical for memory formation and hippocampal plasticity. Furthermore, these results lead to the prediction that the BDNF Met variant might explain significant variation in age-related hippocampal atrophy and memory function.
Some of the earliest studies to examine the association of the BDNF gene on cognitive and brain function in older adults examined whether the BDNF Met variant confers an increased risk for AD. Despite several postmortem studies showing less BDNF protein in the hippocampus of AD patients (see Murer and others 2001 for a review), most genetic association studies find that the BDNF Met allele is unrelated to the risk for AD (e.g., Combarros and others 2004). However, there might be a sexually dimorphic effect of the BDNF gene on risk for AD such that the Met allele confers a greater risk only in women (Fukumoto and others 2010). In any case, these studies are important not only in that they reduce the likelihood that the BDNF polymorphism is a major risk factor for AD but also because they demonstrate the critical distinction between genetic variants and protein levels. That is, AD is associated with lower BDNF protein levels, but lower protein levels do not appear to be mediated by the BDNF gene that regulates protein secretion. There are several possible explanations for this discrepancy. First, lower BDNF levels may be a marker of neuronal death in AD, having nothing to do with the BDNF gene or masking any effect that the BDNF gene has on protein levels. An alternative hypothesis is that epistasis moderates the influence of the BDNF gene on mRNA expression and protein levels. Finally, a third explanation is that there are environmental factors that influence BDNF protein levels independently of the Val66Met BDNF variant. Evidence to support these latter two hypotheses will be discussed in greater detail below.
One of the first studies to examine the association between the BDNF Val66Met polymorphism and cognitive function in healthy older adults found that Met homozygotes outperformed Val carriers on a measure of fluid reasoning (Harris and others 2006). This finding was perplexing because prior studies in young adults, schizophrenics, and depressed patients have consistently shown that the Met allele was associated with poorer episodic memory function and reduced secretion of BDNF (Egan and others 2003). Adding to this confusion, a study with 722 elderly people without dementia found that the Met allele was associated with poorer cognition in several domains including fluid intelligence, processing speed, and memory (Miyajima and others 2008), results that were clearly inconsistent with the findings on reasoning (Harris and others 2006). One possible explanation for this discrepancy is that the Harris and others (2006) study tested individuals who were on average nearly 10 years older than the individuals in the Miyajima and others (2008) study. Within this 10-year span, the incidence of age-related diseases increases, which may result in decreasing the penetrance of or reversing gene-phenotype associations (Lindenberger and others 2008). Consistent with this explanation, a small longitudinal study over a 10-year period with 54 elderly individuals without dementia found that the genetic association of BDNF with cognition changes in late life (Erickson and others 2008). In this study, when participants were first tested at the average age of 65 years, the Val homozygotes outperformed Met carriers on an executive functioning task, a result consistent with Miyajima and others (2008) and research in younger populations. However, after a span of 10 years, the participants, now at an average age of 75 years, showed a reversal such that Met carriers performed better than their Val homozygote counterparts (Fig. 3). Although the sample size of this study was small, the findings suggest that the association between the BDNF gene and cognitive function might change in late life because of the increasing prevalence of other brain-related changes, age-related diseases that might moderate the expression of the BDNF gene, or environmental factors that influence BDNF expression or expression of its high affinity receptor tyrosine kinase (trkB). Consistent with this prediction, two studies have reported that vascular risk factors might moderate the effect of the BDNF gene on associative memory performance in older adults (Raz and others 2008, 2009).
Figure 3.
Evidence for a shift in the brain-derived neurotrophic factor genotype-phenotype association in executive function over a 10-year span in older adults. Participants in this study performed a switching task, a common measure of executive function twice over a 10-year period. There were two conditions: a repeat condition in which two tasks of the same type follow each other and switch conditions when the tasks switch from one trial to the next. There was a main effect of condition such that all participants were slower on the switch condition than repeat. Although Val/Val carriers performed faster at time 1, they were the only group to show a decline in performance over a 10-year span (data adapted from Erickson and others 2008).
These data might also be interpreted in terms of the capacity or allocation of cognitive resources (Lindenberger and others 2008). This hypothesis suggests that the magnitude of the genetic effect on cognitive function should increase with increasing age because of diminishing cognitive resources. In other words, the effect sizes for gene-cognition associations should be considerably smaller in younger cohorts compared with older cohorts (Li and others 2009; Lindenberger and others 2008; Nagel and others 2008). However, the gene-cognition association might also dissipate in very late age when incipient dementia, cognitive impairment, or other age-related diseases are independently influencing cognitive function (Lindenberger and others 2008). In one of these studies with participants at an average age of 65 years, Met carriers performed more poorly on an episodic memory test when the association demands were highest (Li and others 2009). However, in another study, the BDNF gene influenced cognitive performance only by modulating the effect of the catechol-o-methyltransferase (COMT) gene on executive function and working memory (Nagel and others 2008). Indeed, epistasis is likely in late adulthood and could explain a significant amount of the variation observed between studies. Nonetheless, these studies argue that 1) the penetrance of the BDNF gene on memory and cognition appears to change in late adulthood such that the Met variant might be protective against further age-related loss and 2) the association between the BDNF gene and cognition might be moderated by epistasis, linkage disequilibrium, or the comorbidity of vascular disease. Indeed, there are other factors including differential mortality that might also affect these associations.
Adding to this apparent complexity between the BDNF gene and cognition in late adulthood, several studies have measured hippocampal volume in older adults and have reported no differences between Met and Val carriers (Benjamin and others 2010; Miyajima and others 2008), a result that is inconsistent with studies of the BDNF gene on hippocampal volume in younger populations (Bueller and others 2006). However, several studies have reported that the BDNF Met allele might be associated with reduced volume in the prefrontal cortex (Nemoto and others 2006) and splenium (Kennedy and others 2009). In short, the research on the association between the BDNF gene, cognition, and hippocampal volume in late adulthood appears inconsistent with research on the BDNF polymorphism in other populations (e.g., younger adults, schizophrenics), suggesting an age-related change in the penetrance of the BDNF gene on cognitive and brain atrophy phenotypes (Table 1).
Table 1.
Studies Examining the Association between the Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism and Cognitive Function in Elderly Samples
| Author | Year | Sample Size | Mean Age | Finding |
|---|---|---|---|---|
| Harris and others | 2006 | Group 1: 471 Group 2: 433 |
Group 1: 64 Group 2: 79 |
Met homozyogotes outperformed Val carriers on reasoning measures |
| Miyajima and others | 2008 | 722 | 63 | Val homozygotes outperformed Met carriers on processing speed, memory, and fluid intelligence |
| Erickson and others | 2008 | 53 | Time 1: 66 Time 2: 76 |
Val homozygotes outperformed Met carriers at T1 on a task-switch paradigm but performed worse 10 years later at age 76 |
| Nagel and others | 2008 | Younger: 164 Older: 154 |
Young: 25 Old: 65 |
The BDNF gene was not associated with performance on the Wisconsin Card Sort or spatial memory but moderated the effect of the COMT gene |
| Raz and others | 2008 | 103 | 53 (range, 19–77) | The BDNF Met polymorphism moderated the association between blood glucose and memory |
| Raz and others | 2009 | 189 | 54 (range, 18–82) | BDNF Met carriers performed more poorly on memory and processing speed tasks |
| Kennedy and others | 2009 | 82 | 64 (range, 43–81) | Met carriers had smaller splenium volumes |
| Li and others | 2010 | Younger: 382 Older: 566 |
Young: 25 Old: 65 |
Met carriers performed more poorly on primacy and middle portions of backward recall; no differences between Met and Val young adults |
| Karnik and others | 2010 | 129 | 48 (range, 18–100) | No differences between Met and Val groups in fluency or memory; no differences in hippocampal volume |
| Benjamin and others | 2010 | 264 | 70 | No effect of BDNF genotype on digit span, logical memory, word recall; no differences in hippocampal volume |
One study (Harris and others 2006) found that Met carriers outperformed Val homozygotes, three studies found that Val carriers outperformed Met carriers (Li and others 2010; Miyajima and others 2008; Raz and others 2009), and three studies (Benjamin and others 2010; Karnik and others 2010; Nagel and others 2008) found no differences in cognition between the genotypes. One study (Erickson and others 2008) reported an age-related decline in executive function, but only in the Val homozygotes. In short, there is mixed evidence that the BDNF gene contributes to neuropsychological performance or brain volume. However, the BDNF polymorphism might be moderated by illness, other genes, or environmental factors.
Based on this reviewed literature, it is difficult to attribute with any certainty an important role for the BDNF Val66Met polymorphism on age-related memory impairment and hippocampal atrophy. However, it is also possible that the BDNF polymorphism moderates associations with other genes or interacts with environmental factors in such a way that main effects of the BDNF gene are uninterpretable. In this case, functional effects of the BDNF polymorphism on hippocampal volume or memory would have to be assessed in relation to other factors. We now turn our attention to the BDNF protein to examine the evidence that variation in BDNF protein levels is associated with cognitive function and, more specifically, hippocampal atrophy.
The BDNF Protein
The BDNF protein is found in high concentrations in the hippocampus, hypothalamus, cortex, and cerebellum, where it can be visualized in dendrites, soma, and terminals (see Murer and others 2001 for a review). Although there is high overlap between mRNA and protein levels in most brain regions, BDNF can be transported (anterograde or retrograde) to other regions and either stored in vesicles or immediately released. Transcription of the BDNF gene results in the production of proBDNF, a precursor molecule that is converted by plasmin activators to its mature form (mBDNF). ProBDNF and mBDNF influence neuronal function by binding to separate receptors and activating different signaling pathways. mBDNF binds with a high affinity to trkB, which is coexpressed with BDNF. Activation of trkB induces genesis of dendritic spines, whereas selective inhibition of trkB prevents dendritic spine outgrowth. Furthermore, activation of trkB promotes cell growth and survival of serotonergic neurons, which decline in number in AD and are critically involved in the pathophysiology of depression. On the other hand, proBDNF binds to a p75NGFR receptor that induces an apoptotic cascade and induces long-term depression (LTD) and dendritic retraction. In short, proBDNF and mBDNF have opposing actions on both neuronal morphology and physiology, with mBDNF promoting dendritic growth, cell survival, and long-term potentiation (LTP) and proBDNF inducing dendritic retraction, LTD, and apoptosis (see Lu and others 1995 for a review). This difference could be critical in interpreting the effect of BDNF on age-related atrophy.
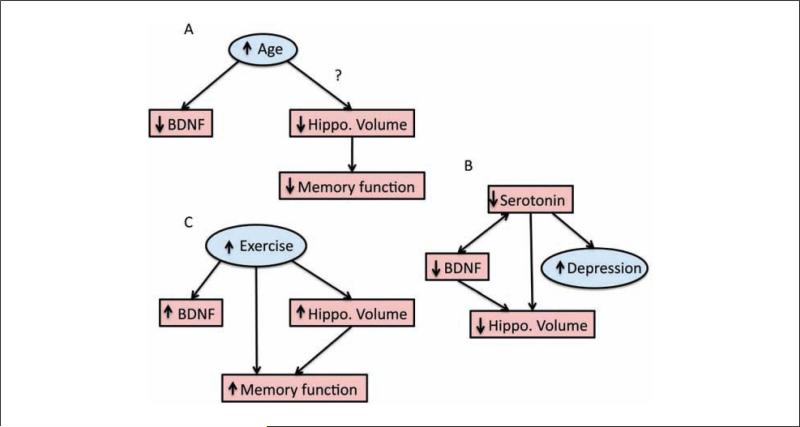
Given the neural signaling cascades of BDNF and its putative role in cellular analogs of memory formation, decreases in BDNF signaling have been hypothesized to contribute to memory impairment in aging. However, it is important to consider the ways in which changes in BDNF signaling could influence age-related impairments. First, age-related memory impairments could be mediated by reduced production of BDNF, suggesting that methods that increase the production and/or secretion of BDNF could improve memory and elevate hippocampal function. Second, if production and secretion of BDNF do not change throughout the life span, age-related memory impairments might still be mediated by a reduction in cleavage molecules that convert proBDNF to mBDNF or in the concentration of trkB receptors (see Silhol and others 2007 for a discussion on full and truncated forms of the trkB receptor on age-related impairments). That is, if the concentration of trkB receptors declines with advancing age, as has been shown in rodent models (Silhol and others 2005), mBDNF will be unable to elicit neuronal growth and LTP cascades. Alternatively, if conversion of proBDNF to mBDNF is disrupted with aging, then greater proBDNF levels might elicit greater cell death and dendritic retraction in a neuronal system already weakened by other cellular changes associated with aging. According to these scenarios, methods that increase the production or secretion of BDNF might have very little benefit to memory function and may even have the consequence of elevating memory impairment. Finally, it is also possible that other factors that are either downstream from BDNF or work in concert with BDNF (e.g., estrogen) could be altering the efficacy of the BDNF signaling pathway, thereby mediating memory and cognitive deficits with age. Given that each of these three possible scenarios could result in memory impairment, it remains a challenge for researchers to directly link, in a causal fashion, one of these scenarios with age-related memory impairment while excluding the possibility of the others (Fig. 4).
Figure 4.
Mediation models for how (A) reductions in hippocampal volume mediate age-related decline in memory function and (B) reductions in brain-derived neurotrophic factor (BDNF) signaling result in reduced hippocampal volume (e.g., dendritic branching and cell proliferation), which in turn results in poorer memory function. Note that in both of these models, there are additional direct paths between age and memory function because hippocampal volume most likely only makes up one node in a network of brain structures that are leading to memory impairment. Furthermore, in (B), a direct path is modeled between age and hippocampal volume because changes in many different molecules besides BDNF are probably contributing to decreased hippocampal volume and function.
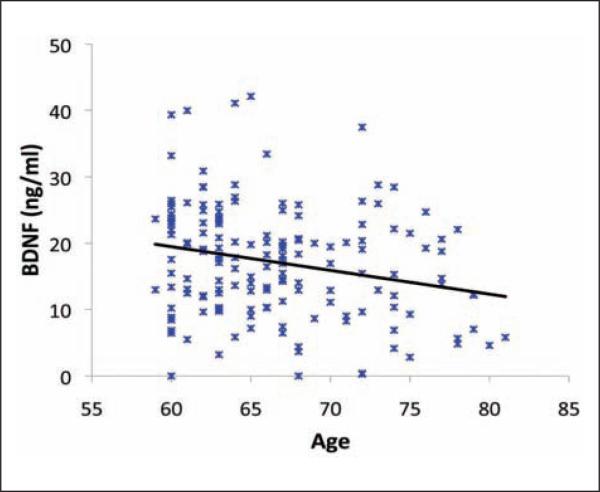
Despite these challenges, there has been growing evidence that alterations to the BDNF system influence hippocampal function and memory in aged adults and rodents (Tapia-Arancibia and others 2008). For example, both rodent and monkey studies have found that BDNF levels in the hippocampus decline in older ages (Silhol and others 2005). The trkB protein is also reduced in aged rodents (Silhol and others 2005) but can be rescued by extensive cognitive training (Silhol and others 2007). BDNF-induced LTP is reduced in aged rodents, but exogenous administration of BDNF rescues LTP and improves spatial memory performance (Rex and others 2006). Peripheral administration of BDNF can also reduce depression-like symptoms and induce neurogenesis in rodents (Schmidt and Duman 2010). In humans, older adults have lower serum (Erickson, Prakash, and others 2010; Ziegenhorn and others 2007) and plasma (Lommatzsch and others 2005) BDNF as determined by enzyme-linked immunosorbent assays (ELISAs; Fig. 5). ELISA techniques do not currently differentiate between mBDNF and proBDNF, so any age-related changes in BDNF detected by this method cannot be attributed to any one form of the molecule. Furthermore, because BDNF is produced and secreted by peripheral tissues, changes in serum or plasma BDNF might not reflect central changes in BDNF.
Figure 5.
Serum brain-derived neurotrophic factor levels decline with advancing age (P < 0.05; data adapted from Erickson, Prakash, and others 2010).
There is also evidence that BDNF plays an important role in AD-related pathophysiology. For example, in humans, lower levels of both BDNF and trkB have been found in postmortem brains of individuals with AD (Murer and others 2001). Furthermore, cells containing neurofibrillary tangles, one of the putative causes of AD, do not contain measureable amounts of BDNF, whereas cells with high levels of BDNF are free from neurofibrillary tangles (Murer and others 1999). Also in humans, lower serum levels of BDNF are found in more severe stages of AD (Laske, Stellos, and others 2006; however, see Ziegenhorn and others 2007). Furthermore, acetylcholinesterase treatments for AD increase serum levels of BDNF to a level equivalent to that of healthy controls, and higher serum levels of BDNF in AD are predictive of slower rates of decline (Laske, Stellos, and others 2010). Overall, the evidence from humans with AD suggests that there is a link between BDNF and AD and that at the very least, BDNF could be considered a biomarker for memory impairment. However, studies in humans are inherently limited in determining whether BDNF mediates age-related memory impairments or AD pathology.
Animal models have the ability to measure and manipulate the molecular pathways and connections between the putative causes of AD, memory impairment, and BDNF and can therefore make stronger claims about the causal mechanisms of BDNF. These studies find that β-amyloid disrupts BDNF signaling, including the expression of trkB receptors and NMDA and glutamatergic processes (Tapia-Arancibia and others 2008). Furthermore, BDNF protects against β-amyloid toxicity via trkB signaling in a dose-dependent manner (Tapia-Arancibia and others 2008). Interestingly, entorhinal administration of BDNF reverses synaptic loss, regulates gene expression, improves neuronal signaling, and enhances learning and memory in transgenic mouse models of AD, aged rats, and aged monkeys (Nagahara and others 2009). Overall, these data argue for a critical role of BDNF in regulating β-amyloid toxicity and suggest that disruptions in the BDNF pathway, including protein and receptor expression, contribute to age-related hippocampal dysfunction, loss of dendrites, and AD-related memory impairment.
The link between BDNF and hippocampal impairment in humans is more tenuous, but two recent studies have shed some light on the associations. In one study, BDNF was measured in cerebrospinal fluid (CSF) and cognitive function assessed by a comprehensive neuropsychological battery (Li and others 2009). CSF BDNF levels were reduced in older adults and in adults with AD, and lower BDNF levels were predictive of poorer memory performance at baseline and after a longitudinal assessment of 3 years. Interestingly, this effect remained significant even after controlling for potentially confounding variables including the BDNF Val66Met genotype, τ, and β-amyloid. This study demonstrates that CSF levels of BDNF are a marker of AD and cognitive dysfunction and that these correlations occur independently of the BDNF genotype.
The research findings from humans, monkeys, and rodents suggest an important link between BDNF protein and age-related memory impairment. However, an important question remaining from this research was whether serum BDNF levels would be associated with memory function and hippocampal atrophy in aged humans. One recent study set out to assess these associations. In 142 older adults without AD, the size of the hippocampus was acquired using an automated segmentation algorithm from magnetic resonance images (Erickson, Prakash, and others 2010). Serum BDNF levels were obtained using an ELISA and memory function evaluated using a spatial memory paradigm known to be sensitive to hippocampal atrophy. In this study, serum BDNF levels declined with advancing age and mediated age-related decrements in spatial memory performance. Furthermore, hippocampal volumes declined with age, but higher BDNF levels were associated with larger hippocampal volumes (Fig. 6). These results demonstrate that BDNF plays a pivotal role in age-related memory impairments and provide an important link between age-related atrophy of the hippocampus and serum levels of BDNF.
Figure 6.
Serum brain-derived neurotrophic factor is positively correlated with hippocampus volume in older adults. Although this association is a relatively small effect, it is significant (P < 0.05) and remains so after controlling for potentially confounding factors such as sex and years of education (data adapted from Erickson, Prakash, and others 2010).
Overall, this literature unequivocally argues that BDNF plays an important role in memory impairment and hippocampal atrophy, although very few studies have tested a direct causal role for BDNF in age-related memory impairment (Tapia-Arancibia and others 2008). As discussed above, there might be several paths by which disruptions in BDNF signaling could mediate age-related impairments, including factors that moderate BDNF signaling such as environmental and hormonal factors. In the next sections, we describe results that focus on the role of depression and exercise on hippocampal atrophy in late adulthood and the evidence that BDNF plays a pivotal role in these associations.
BDNF, Depression, and Hippocampal Atrophy
Medical illness, physical pain, bereavement, financial insecurity, and death anxiety have all been associated with increased risk for geriatric depression. Furthermore, the interaction of depression and mild cognitive impairment is considered a risk factor for AD (Butters and others 2008), but whether depression is more likely to precede, follow, or covary with nascent dementia remains a matter of debate. Among the multiple paths by which depression may influence the risk for AD (Butters and others 2008), changes in BDNF pathways and hippocampal deterioration are two routes by which geriatric depression and age-related cognitive impairment might be linked.
Major depressive disorder is associated with smaller hippocampal volumes in both young and older populations (Steffens and others 2000), a finding similar to the outcomes of research reviewed above showing an increased risk for dementia due to smaller hippocampal volume. Smaller hippocampal volumes are also predictive of poorer long-term outcomes of antidepressant treatment such as increased relapse rates (Hsieh and others 2002). Associations between depression and decreased hippocampal volume, reviewed below, are especially disconcerting because hippocampal deterioration and resultant age-related memory impairment might exacerbate depression and vice versa.
There are several lines of evidence that link BDNF to the hippocampal atrophy seen in depression. First, postmortem examinations of depressed patients and suicide victims have found decreased levels of BDNF protein in several brain regions including the hippocampus, but individuals receiving antidepressant therapy do not show the same reduction in BDNF protein levels (Duman and Monteggia 2006). In fact, serum levels of BDNF are lower in depression but can be rescued by antidepressant therapy. Antidepressants appear to block or reverse hippocampal shrinkage associated with depression (Schmidt and Duman 2007). These results suggest a significant degree of hippocampal plasticity that can be manipulated with antidepressants and a possible mediating role for BDNF in that process.
Research with rodents provides a second line of evidence that describes the molecular mechanisms by which BDNF might be linked to depression. Animal models of depression have demonstrated loss of serotonergic fibers and a reduction in dendritic spine density in the hippocampus, yet BDNF works through trkB signaling cascades to enhance the growth and survival of serotonergic neurons (Mattson and others 2004). Interestingly, antidepressant therapy increases hippocampal BDNF mRNA expression and BDNF immunoreactivity in both young and aged rodents (Garza and others 2004). Antidepressant medications also activate trkB receptors, which have downstream effects on serotonergic neurotransmission and neuronal arborization. In short, animal models and postmortem research show that impaired 5-HT and BDNF signaling is central to depression and that the mechanism of action of antidepressants may involve normalizing BDNF activity and reversing hippocampal atrophy.
Finally, a third line of evidence linking depression, BDNF, and hippocampal volume emerges from research examining the association between the BDNF Val66Met polymorphism and risk for depression. Presence of the Met variant has been associated with childhood (Strauss and others 2005) and geriatric depression (Benjamin and others 2010), but any potential moderating effect of the Met variant on hippocampal atrophy in depression has not been firmly established. In one study with younger adults, the Met variant was associated with smaller hippocampal volumes in depressed individuals (Frodl and others 2007), but other studies have failed to demonstrate this association (Benjamin and others 2010). Therefore, the genetic associations between the BDNF Met variant and risk for depression are relatively well established, but the link between BDNF and hippocampal atrophy in the etiology of depression remains questionable. This may be explained by evidence supporting an interaction between BDNF genotype and both hippocampal volume and physical activity in predicting risk for depression (Gonul and others 2010; Lau and others 2010).
In sum, there is growing evidence that changes in BDNF signaling underlie several neurobiological characteristics of depression, including hippocampal shrinkage and the efficacy of antidepressants. Because the causal links between BDNF and depression are difficult to establish in human studies, it is not yet clear if BDNF is a causal factor leading to depression or a correlate of depression and hippocampal volume (Fig. 7A). Nonetheless, the research on the links between BDNF, depression, and hippocampal atrophy are intriguing and could be critical for a greater understanding of the risk factors for both depression and cognitive impairment in late adulthood. Unfortunately, at this point, we can only speculate about the possible mediating role of serum BDNF on hippocampal volume in depressed older adults or the additional links to cognitive impairment and AD (Box 1).
Figure 7.
Hypothetical mediation models for (A) the association between brain-derived neurotrophic factor (BDNF) signaling, serotonin fibers and neurotransmission, hippocampal volume, and depressive symptoms. Depressive symptoms are modeled here as a latent cluster of symptoms. In this model, BDNF signaling has a reciprocal relationship with serotonin such that reductions in both lead to decreased hippocampal volume. There is a circular path between decreased hippocampal volume, increased depressive symptoms, and decreased serotonin fibers and neurotransmission. Thus, in this model, it appears as if decreased hippocampal volume precedes depressive symptoms, but increased depressive symptoms might have compounding effects on decreased hippocampal volume by magnifying the loss of serotonergic fibers. In model (B), we hypothesize that increased aerobic exercise leads to increased production of BDNF, which in turn leads to increased hippocampal volume (e.g., dendritic branching and cell proliferation) and improved memory function. However, similar to model (A), we predict that exercise influences hippocampal volume through several pathways, BDNF being only one of them. Furthermore, exercise could also improve memory function by influencing several different molecules and brain networks in addition to the hippocampus.
Exercise and Hippocampal Atrophy
It is clear that age-related changes in the BDNF system might play an important mediating role in hippocampal atrophy. However, if BDNF levels could be manipulated over time and hippocampal atrophy and memory function assessed in relation to changes in BDNF, we could be more convinced about the causal pathways linking BDNF to hippocampal atrophy. There are several factors shown to influence BDNF including caloric restriction, environmental enrichment, and estrogen administration, all of which are challenging to manipulate for extended periods in human populations. However, aerobic exercise has been shown to elevate BDNF levels in the hippocampus and other brain regions and can be more easily manipulated and measured in a laboratory setting in both humans and rodents. In this section, we will briefly describe the evidence linking aerobic exercise to hippocampal function and the putative role of BDNF in this association.
Research in rodents suggests that exercise is capable of improving hippocampal function, even in aged animals. For example, aged mice that had voluntary access to a running wheel for 45 days demonstrated greater cell proliferation in the dentate gyrus than age-matched sedentary controls, but the genesis of new cells was attenuated relative to the levels reached by younger animals (van Praag and others 2005). Furthermore, cell proliferation in the dentate gyrus was associated with enhanced learning rates on the Morris water maze, suggesting an important behavioral link to enhanced cell proliferation and survival. This effect was similar to another study that found that voluntary exercise reversed age-related decline in precursor cell activity but failed to maintain cell proliferation at the level of younger animals (Kronenberg and others 2006). These results suggest that exercise increases neural growth and survival in the hippocampus of both young and adult rodents but that there might be limitations to the degree to which exercise can reverse age-related losses.
In humans, aerobic fitness has also been associated with hippocampal volume in both children and older adults. For example, in one study with 165 older adults without dementia, greater aerobic fitness levels were associated with larger hippocampal volumes even after controlling for potentially confounding factors including age, sex, and years of education (Fig. 8). Furthermore, hippocampal volume mediated fitness-related performance on a spatial memory task (Erickson and others 2009). These results in humans are consistent with research in rodents and suggest that aerobic exercise might be an effective method for enhancing or reversing hippocampal volume in late adulthood (Box 2).
Figure 8.
Greater mean hippocampus volume (across left and right hemispheres) is associated with higher cardiorespiratory fitness levels as quantified by VO2 peak, the gold standard measure of aerobic fitness, in 165 older adults without dementia (data adapted from Erickson and others 2009).
Exercise unequivocally influences the morphology and physiology of the hippocampus in both young and old animals. However, to what extent do changes in the BDNF system mediate the effect of exercise on the hippocampus? Could exercise enhance hippocampal function by increasing the production of BDNF? In fact, many studies in rodents find that exercise consistently increases BDNF expression in several regions including the hippocampal formation and cerebral cortex (Cotman and Berchtold 2002). Interestingly, the amount of voluntary running is positively associated with the amount of BDNF detected in the hippocampus, suggesting a dose-dependent effect of exercise on BDNF production. In an important set of studies, BDNF mediated the exercise-enhancing effect on Morris water maze acquisition, suggesting a pivotal role of BDNF in hippocampal-dependent learning and memory (Vaynman and others 2004). Exercise has also been shown to enhance cell signaling by increasing the expression of trkB receptors. For example, in AD transgenic mice, APOE 4 mice had 50% fewer trkB receptors than APOE e3 mice, but exercise increased the expression of trkB to those levels shown by APOE 3 mice (Nichol and others 2009). In short, increased BDNF and trkB expression is one of the most consistent effects of exercise on brain function. It is clear from this research that not only is exercise very effective at enhancing neuroplasticity especially in the hippocampus but also that BDNF is critically involved in this process.
Recent evidence suggests that exercise might also be effective at increasing BDNF levels in humans. For example, moderate-intensity endurance training increases BDNF in serum and plasma (Zoladz and others 2008). However, other studies have reported inverse associations between basal serum BDNF and cardiorespiratory fitness or have failed to detect changes in BDNF after exercise or strength training (Goekint and others 2010). There are several reasons for this variation, including differences between acute versus long-term exercise, cross-sectional versus randomized designs, the length and intensity of exercise trials, the timing and methods by which BDNF is assessed, and the specific population being studied. In fact, few studies have measured BDNF after exercise in older adults (Baker and others 2010), and no studies to date have examined the association between exercise, hippocampal atrophy, and BDNF in an aged sample (Fig. 7B).
In sum, exercise improves hippocampal function and is associated with decreased hippocampal atrophy, which in turn might be mediated by increased BDNF. Convincing evidence for these associations emerges from animal research, but more research is needed in humans to confirm or refute these claims.
Conclusions and Future Directions
The prefrontal cortex and hippocampus deteriorate in late adulthood, preceding and leading to deficits in executive and memory function. We examined in this review the evidence that age-related changes in BDNF might at least partially explain hippocampal atrophy and increased risk for memory impairment. We can conclude that 1) decreases in BDNF protein expression are associated with poorer hippocampal function and increased rates of geriatric depression and AD. 2) Hippocampal atrophy occurs independently from the BDNF gene. That is, the BDNF Val66Met polymorphism appears to have inconsistent and weak effects on hippocampal size in late adulthood and may exert its effects only as a moderator of other polymorphisms, diseases, or environmental factors. 3) Aerobic exercise enhances executive and memory function and reduces hippocampal atrophy in late adulthood, and this may be partially mediated through a BDNF pathway.
Despite these conclusions, there is also an important limitation that has been alluded to throughout this review. Only studies in rodents have manipulated BDNF and examined the effect that the manipulation has on memory and hippocampal physiology in aged animals. This research has convincingly shown that BDNF plays an important role in age-related hippocampal decline. However, in humans, determining whether BDNF is simply a biomarker for cognitive decline and hippocampal atrophy or instead is causally associated with hippocampal atrophy is methodologically difficult, if not impossible (Fig. 10). The conceptual difference between a biomarker and a mediator is critical for understanding the role that BDNF has on hippocampal morphology and physiology. For example, both AD and depression are associated with reductions in BDNF protein expression in the hippocampus, but we cannot conclude from these associations that decreases in BDNF cause hippocampal atrophy or symptoms of AD or depression. It may be that changes in some other set of molecules or cellular structures (e.g., dendritic branches) are causing both depleted BDNF levels and increased memory impairment. Therefore, at this point, we can argue that there is an association between hippocampal atrophy and BDNF, but we cannot currently differentiate between a biomarker model of BDNF and a causal mechanism for BDNF in age-related hippocampal atrophy. Nonetheless, animal research has shown that BDNF and its corresponding trkB receptor and signaling cascades are fundamentally involved with AD, memory impairment, hippocampal atrophy, depression, and the ameliorating effect of exercise. Similar associations in humans provide translational support for a fundamental role of BDNF in these associations.
Figure 10.
Hypothetical biomarker models of brain-derived neurotrophic factor (BDNF) signaling, hippocampal volume, and memory function. In model (A), increased age is associated with decreased BDNF signaling and decreased hippocampal volume, but these paths run parallel with each other such that reductions in BDNF signaling are viewed as a liability for decreased hippocampal volume but not a mechanistic pathway. Similarly, in model (B), reciprocal connections between BDNF and serotonin exist, and reductions in BDNF signaling only indirectly influence the risk for depressive symptoms. In this model, changes in BDNF and hippocampal volume would be correlated with depressive symptoms but would not by themselves be sufficient for causing depressive symptoms. In model (C), an increase in the amount of exercise results in improvements in memory but does so independently of changes in BDNF or hippocampal volume. Again, BDNF and hippocampal volume might be correlated with exercise-related improvements in memory function but would not be causally related.
Major depressive disorder is considered a risk factor for AD and memory impairment and is associated with less BDNF and greater hippocampal atrophy, possibly through a BDNF pathway. On the other hand, exercise is an effective treatment for geriatric depression, increases BDNF levels, increases serotonergic fibers, is associated with greater hippocampal volumes, and reduces the risk for AD. Is it coincidental that depression and exercise have opposing effects on the aging hippocampus and serotonergic system and are both at least partially mediated by BDNF? We can speculate that aerobic exercise could be a treatment for depression by increasing BDNF and hippocampal volume. In fact, consistent with this hypothesis, two recent studies found that exercise increased serum BDNF in elderly women with remitted major depression (Laske, Banschbach, and others 2010) and in patients with panic disorder (Strohle and others 2010). However, although BDNF is a possible mechanistic pathway for the therapeutic effects of exercise on depression, it is also likely that exercise works through alternative paths (e.g., hypothalamic-pituitary-adrenal axis) as well. In short, more research is needed to determine the ways in which exercise exerts its therapeutic effects on hippocampal morphology and depressive symptoms.
In summary, we conclude from this review that there is mounting evidence that BDNF protein expression plays an important role in age-related hippocampal atrophy and that geriatric depression magnifies hippocampal atrophy and risk for AD through BDNF pathways while exercise enhances hippocampal morphology and physiology by elevating the production of BDNF. Future research is needed to determine the extent to which BDNF mediates hippocampal atrophy and memory impairment or is a biomarker for other cellular and molecular changes.
Box 1. Depression and Exercise.
Epidemiological studies have reported that a greater amount of physical activity is associated with reduced depressive symptoms in older adults (Lampinen and others 2000). However, observational studies are inherently limited in determining causality between activity and depression. Therefore, several interventions have been conducted that more conclusively demonstrate that physical activity is effective at treating depressive symptoms. In these interventions, older adults with major depressive disorder are assigned to receive monitored regimens of moderate-intensity exercise for several weeks to several months, whereas control groups receive antidepressant therapy, social contact, or educational treatments. These studies have consistently shown therapeutic effects of exercise on depressive symptoms. In fact, one study conducted over a 16-week period demonstrated that moderate-intensity exercise was as effective as antidepressant medication at reducing depressive symptoms and was more effective at reducing relapse (Blumenthal and others 1999). Exercise is also effective at reducing depressive symptoms in medically ill older adults (Emery and others 1998). Although intriguing, the mechanisms by which exercise influences mood and depressive symptoms remain unknown. Given the role of brain-derived neurotrophic factor (BDNF) in both depression and exercise, it is possible that exercise reduces depressive symptoms through BDNF pathways (Laske, Banschbach, and others 2010).
Box 2. Long-Term Effects of Physical Activity.
The effect of physical activity on hippocampal volume might endure for several years and be predictive of subsequent conversion to dementia according to a recent longitudinal study (Erickson, Raji, and others 2010). In this study, physical activity was quantified by the total number of blocks walked per week in 299 adults without dementia. After a span of nine years, magnetic resonance imaging scans were taken, and voxel-based morphometry was used to estimate tissue volume. After controlling for several potentially confounding factors (e.g., mobility, age, sex, years of education), individuals reporting greater amounts of physical activity nine years earlier had greater gray matter volume in several regions, including the hippocampus. Interestingly, this effect was dependent on the amount of physical activity obtained. Those walking at least 72 blocks per week, or roughly one mile per day, were the only group to show significant sparing of brain tissue after nine years (Fig. 9). Furthermore, after a follow-up period of four more years, greater brain volume in the hippocampus, inferior frontal gyrus, and supplementary motor area was associated with a two-fold reduced risk for cognitive impairment. These results are important in that they link, for the first time, physical activity patterns earlier in life and brain volume and cognitive impairment later in life.
Figure 9.
(A) Over a nine-year period, greater amounts of physical activity in the form of walking are associated with greater gray matter volume in several regions including prefrontal, temporal, and hippocampal areas. (B) The effect of walking on greater gray matter volume was dependent on the amount of walking. Those individuals walking at least 72 blocks or roughly 6 to 9 miles per week showed the greatest amount of gray matter volume. Given the association between exercise and brain-derived neurotrophic factor (BDNF), it is possible that these effects are at least partially mediated by BDNF (data adapted from Erickson, Raji, and others 2010).
Acknowledgments
We would like to thank the following people for their support: Andrea Weinstein, Robin Brown, Stephanie Akl, and Sarah Banducci.
Financial Disclosure/Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: K.I.E. was supported by a Junior Scholar Award from the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827) and a seed grant awarded through the University of Pittsburgh Alzheimer's Disease Research Center (P50 AG005133).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–9. doi: 10.1001/archneurol.2009.307. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin S, McQuoid DR, Potter GG, Payne ME, MacFall JR, Steffens DC. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am J Geriatr Psychiatry. 2010;18:323–31. doi: 10.1097/JGP.0b013e3181cabd2b. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak M, Moore K, Craighead WE, Herman S, Khatri P. Effects of exercise training on older adults with major depression. Arch Intern Med. 1999;159:2349–56. doi: 10.1001/archinte.159.19.2349. others. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–94. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubietta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–5. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–57. doi: 10.31887/DCNS.2008.10.3/mabutters. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarros O, Infante J, Llorca J, Berciano J. Polymorphism at codon 66 of the brain-derived neurotrophic factor gene is not associated with sporadic Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;18:55–8. doi: 10.1159/000077736. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicot JH, Goldberg TE, Kolachana BS, Bertolino A. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. others. [DOI] [PubMed] [Google Scholar]
- Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17:232–40. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci. 2008;2:11. doi: 10.3389/neuro.09.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB. Physical activity predicts gray matter volume in late adulthood: the cardiovascular health study. Neurology. 2010;75:1415–22. doi: 10.1212/WNL.0b013e3181f88359. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–31. doi: 10.1523/JNEUROSCI.3252-09.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D. Brain atrophy is healthy aging is related to CSF levels of Ab1-42. Cereb Cortex. 2010;20:2069–79. doi: 10.1093/cercor/bhp279. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–6. doi: 10.1001/archpsyc.64.4.410. others. [DOI] [PubMed] [Google Scholar]
- Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai SJ, Matsuchita S. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer's disease: new data and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:235–42. doi: 10.1002/ajmg.b.30986. others. [DOI] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–20. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol. 2010;110:285–93. doi: 10.1007/s00421-010-1461-3. others. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Kitis O, Eker MC, Eker OD, Ozan E, Coburn K. Association of the brain-derived neurotrophic factor Val66Met polymorphism with hippocampus volumes in drug-free depressed patients. World J Biol Psychiatry. 2010 doi: 10.3109/15622975.2010.507786. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related changes in reasoning skills. Mol Psychiatry. 2006;11:505–13. doi: 10.1038/sj.mp.4001799. others. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–25. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Peterson RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–9. doi: 10.1212/wnl.51.4.993. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik MS, Wang L, Barch DM, Morris JC, Csemansky JG. BDNF polymorphism rs6265 and hippocampal structure and memory performance in healthy control subjects. Psychiatry Res. 2010;178:425–9. doi: 10.1016/j.psychres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Land SJ, Raz N. BDNF Val66Met polymorphism influences age differences in microstructure of the corpus callosum. Fron Hum Neurosci. 2009;3:19. doi: 10.3389/neuro.09.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Lampinen P, Heikkinen R, Ruoppila I. Changes in intensity of physical exercise as predictors of depressive symptoms among older adults: an eight year follow up. Prev Med. 2000;30:371–80. doi: 10.1006/pmed.2000.0641. [DOI] [PubMed] [Google Scholar]
- Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010;13:595–602. doi: 10.1017/S1461145709991234. others. [DOI] [PubMed] [Google Scholar]
- Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 2010 doi: 10.1017/S1461145710001008. others Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113:1217–24. doi: 10.1007/s00702-005-0397-y. others. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53(3):952–61. doi: 10.1016/j.neuroimage.2009.11.026. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Peskind ER, Millard SP, Chi P, Sokal I, Yu CE. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Chicherio C, Nyberg L, von Oertzen T, Nagel IE, Papenberg G. Ebbinghaus revisited: influences of the BDNF Val66Met polymorphism on backward serial recall are modulated by human aging. J Cogn Neurosci. 2010;22:2164–73. doi: 10.1162/jocn.2009.21374. others. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Aging Neurosci. 2008;2:2. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–23. doi: 10.1016/j.neurobiolaging.2004.03.002. others. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature Rev Neurosci. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurode-generative disorders. Trends Neurosci. 2004;27:589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–7. doi: 10.1111/j.1601-183X.2007.00363.x. others. [DOI] [PubMed] [Google Scholar]
- Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience. 1999;88:1015–32. doi: 10.1016/s0306-4522(98)00219-x. others. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–7. doi: 10.1038/nm.1912. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci. 2008;2:1. doi: 10.3389/neuro.09.001.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neurosci Lett. 2006;397:25–9. doi: 10.1016/j.neulet.2005.11.067. others. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–7. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, Jacobs BS. Brain-derived neurotrophic factor Val66Met and blood glucose: a synergistic effect on memory. Front Hum Neurosci. 2008;2:12. doi: 10.3389/neuro.09.012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–16. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–85. doi: 10.1152/jn.00336.2006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–91. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Maurice T, Tapia-Arancibia L. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience. 2007;146:962–73. doi: 10.1016/j.neuroscience.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–24. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–9. doi: 10.1016/s0006-3223(00)00829-5. others. [DOI] [PubMed] [Google Scholar]
- Strauss J, Barr CL, George CJ, Devlin B, Vetro A, Kiss E. Brain-derived neurotrophic factor variants are associated with childhood-onset mood disorder: confirmation in a Hungarian sample. Mol Psychiatry. 2005;10:861–7. doi: 10.1038/sj.mp.4001685. others. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Graetz B, Scheel M, Wittmann A, Gallinat J. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010;35:364–8. doi: 10.1016/j.psyneuen.2009.07.013. others. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–20. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Von Bohlen Halbach OB. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2010;13:2. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbruggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D. Serum neurotrophins—a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–45. doi: 10.1016/j.neurobiolaging.2006.06.011. others. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59:119–32. [PubMed] [Google Scholar]