Abstract
This study examined competing substantive hypotheses about dynamic (i.e., time-ordered) links between memory and functional limitations in old age. We applied the Bivariate Dual Change Score Model to 13-year longitudinal data from the Asset and Health Dynamics Among the Oldest Old Study (AHEAD; N = 6,990; ages 70 – 95). Results revealed that better memory predicted shallower increases in functional limitations. Little evidence was found for the opposite direction that functional limitations predict ensuing changes in memory. Spline models indicated that dynamic associations between memory and functional limitations were substantively similar between participants aged 70–79 and those aged 80–95. Potential covariates (gender, education, health conditions, and depressive symptoms) did not account for these differential lead–lag associations. Applying a multivariate approach, our results suggest that late-life developments in two key components of successful aging are intrinsically interrelated. Our discussion focuses on possible mechanisms why cognitive functioning may serve as a source of age-related changes in health both among the young-old and the old-old.
Keywords: Cognitive functioning, Health, Dynamic models, Oldest Old, Health and Retirement Study
Lifespan psychological research has long been interested in examining intraindividual changes within and structural relations between domains of functioning (Baltes & Nesselroade, 1979; Brandtstädter & Lerner, 1999; Magnusson & Cairns, 1996). Following this tenet, the current study explores dynamic (i.e., time-ordered) links between age-related changes in two key domains of functioning in old age, namely cognition and health. Several conceptual accounts outline the importance of across-domain associations between cognition and health for development in old age (Bäckman, Small, & Wahlin, 2001; Hertzog, Kramer, Wilson, & Lindenberger, 2009; Rowe & Kahn, 1987; Schaie, 2005; Willis et al., 2006). However, it is an open question whether level and change in cognitive functioning precedes and predicts subsequent changes in the health domain (Gottfredson, 2004; Stuck et al., 1999) or whether vice versa health represents a risk factor for or a protective factor against cognitive decline (Anstey & Christensen, 2000). Our study examines unidirectional, bidirectional, and third-variable accounts of across-domain associations between cognition and health in old age. To do so, we apply the Bivariate Dual Change Score Model (BDCSM; McArdle & Hamagami, 2001) to longitudinal age-based data from the Asset and Health Dynamics Among the Oldest Old Study (AHEAD) and examine dynamic links between memory and functional limitations in old age. Furthermore, we explore whether such time-ordered associations differ between the young-old and old-old and are independent of possible confounds, including gender, education, health conditions, and depressive symptoms.
Dynamics of Cognition and Health in Old Age
Cognitive abilities and health constitute integral components of the functional systems in old and very old age. We view cognitive functioning as a general-purpose mechanism for adaptation and a resource to draw upon in the face of obstacles (Baltes, Lindenberger, & Staudinger, 2006). Health comprises physical functioning and the incidence of diseases that may undermine one’s ability to complete activities of daily living (Steinhagen-Thiessen & Borchelt, 1999; Verbrugge & Jette, 1994). Our objective in this study is to investigate the time-ordered dynamics between indicators of these two key components. Earlier empirical reports provide initial evidence that these domains may indeed shape one another reciprocally. For example, several epidemiological studies have shown that cognitive impairment, as measured by global indicators of cognition (e.g., MMSE), is associated with an increased risk for reporting functional limitations in everyday activities (Liang et al., 2003; Moritz, Kasl, & Berkman, 1995; Seeman et al., 1994). Reports from the Framingham Heart Study and Whitehall II Study suggest that individuals with cognitive impairment were more likely to experience disability and accrue functional limitations over time (Elovainio et al., 2009; Kelly-Hayes, Jette, Wolf, D’Agostino, & Odell, 1992). Similar findings have also been reported from a more psychological perspective, focusing on specific cognitive facets such as memory and processing speed. For example, previous work using the AHEAD study suggests that participants with poorer performance on tests of episodic memory, working memory, and mental processing had a greater risk of functional decline over a period of two years (Mehta, Yaffe, & Covinsky, 2002). Similarly, Wahl and colleagues (2010) found that slower processing speed was a risk factor for declines in functional ability.
Previous research shows that health is a leading indicator of changes in cognition, particularly when the focus is on health conditions. For example, individuals suffering from cardiovascular disease, diabetes, hypertension, or stroke have all been reported to show lower performance and experience steeper declines in both global and specific facets of cognitive functioning (Anstey & Christensen, 2000; Bäckman, Jones, Small, Aguero-Torres, & Fratiglioni, 2003; Hertzog, Schaie, & Gribbin, 1978; Verhaeghen, Borchelt, & Smith, 2003). In the present study, we instead focus on functional limitations, for which similar associations with cognitive abilities have been reported. Specifically, fewer difficulties with activities of daily living were associated with better performance on multiple measures of fluid intelligence (e.g., the Digit Symbol Substitution test: Christensen et al., 1994). Participants with more daily physical activities performed better on various cognitive tests such as semantic memory, episodic memory, or working memory (Buchman, Wilson, & Bennett, 2008). Similarly, participants who reported more frequent physical and strenuous activities around the house showed shallower cognitive declines and reduced odds ratios for developing dementia over time (Albert et al., 1995; Andel et al., 2008).
Cognition and health are also involved in bidirectional, possibly reciprocal associations. For example, the cognition and health domains can be conceptualized as inter-related constituent areas of successful aging that both show substantial age-related changes and are intrinsically linked with one another (Rowe & Kahn, 1987; Ryff & Singer, 1998; Small, Dixon, & McArdle, in press; Zarit et al., 1995). Empirically, initial evidence suggests that global cognitive impairments may serve as a risk for substantial functional declines, and at the same time disability may increase the risks for subsequent cognitive declines (Black & Rush, 2002).
Finally, an underlying third-variable or common cause may contribute to cognition-health associations in old age. Potential candidate factors that are known to relate to either of the two domains include gender, education, health conditions, and depressive symptoms. To begin with, gender-linked inequalities in both cognitive abilities and health have long been acknowledged in the lifespan and gerontological literature (see Moen, 1996; Smith & Baltes, 1998). It is well documented, for example, that women typically experience more functional limitations than do men (Crimmins, 2001). Similarly, more years of education are often associated with resources and strategies that individuals can utilize to buffer normative age-related declines in cognition and health (Adler et al., 1994; Rönnlund, Nyberg, Bäckman, & Nilsson, 2005). Furthermore, health conditions may undermine both one’s cognitive functioning and the ability to successfully perform tasks of everyday living (Wahlin, MacDonald, deFrias, Nilsson, & Dixon, 2006). Finally, research suggests that compromised well-being may be a risk factor for lower levels or steeper declines of cognitive abilities and health (Gerstorf, Lövdén, Röcke, Smith, & Lindenberger, 2007; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). Taken together, cognition and health have several common correlates that need to be taken into account when examining how facets of cognition and health are interrelated in old age.
Dynamics of Cognition and Health Among the Young-Old vs. Old-Old
Conceptual and empirical work alike suggest that dynamic links between indicators of cognition and health may differ between the young-old and old-old (Baltes & Smith, 2003; Suzman, Manton, & Willis, 1992). Specifically, it has been argued that the young-old are often characterized by relative cognitive and physical fitness, social embeddedness, and comparatively few severe illnesses (Smith & Baltes, 1997). It also appears as if the young-old typically have the adaptive capacities and resources necessary to adjust to losses occurring in circumscribed domains (Jopp & Smith, 2006). In contrast, old-old individuals are frequently confronted with more severe and distinctive challenges, including broad-based dysfunctions and losses in many key domains such as cognitive, physical, and social functions (Gerstorf, Smith, & Baltes, 2006; Jopp & Rott, 2006).
Following this general line of reasoning, we argue that differences between the young-old and old-old may also have bearings on the nature of cognition–health dynamics. We expect that associations between cognition and health are stronger in the old-old, because of increases in the frequency and severity of decline in either domain as well as diminished resources and adaptive capacities to adjust to those challenges (Baltes & Smith, 2003). In a similar vein, notable age-related changes in physiological functioning (e.g., immune system; Kiecolt-Glaser & Glaser, 2001) may make individuals more vulnerable to diseases and health risks, thereby increasing interdependencies amongst domains to sustain overall functioning. Also, we note that determining cut-offs for age groups is an arbitrary endeavor. Due to continuous demographic shifts in average life expectancies, it is often difficult to maintain a steady criterion for distinguishing the young-old from the old-old. In our analyses, we follow conceptual work that defines the shift from young-old to old-old to be around age 80, which approximates current life expectancies in developed countries (see Baltes & Smith, 2003; Vaupel et al., 1998). Methodologically, this approach allowed the added benefit of having relatively similar frequencies of observations in the two age groups to ensure enough statistical power in our age-differential analyses.
The Present Study
In our study, we examine dynamic links between specific facets of cognition and health in old age, operationally defined by memory and functional limitations. We selected these two domains to represent major components of individuals’ daily functioning in old age. Specifically, remembering events, places, people, and procedures is a cornerstone of being able to plan, execute, and complete important tasks of everyday life. Similarly, functional limitations involve basic tasks of daily functioning relevant for maintaining independent living. These refer to limitations in performing instrumental everyday activities such as cooking, taking medications, using the phone, managing money, and walking flights of stairs. Examining whether dynamic links exist between these important domains of everyday functioning thus carries the potential to inform cognitive and health interventions.
To determine the existence, direction, and nature of time-ordered associations between memory and functional limitations, we utilize the BDCSM (McArdle & Hamagami, 2001). The BDCSM is a statistical method of analysis that has successfully been applied to test competing substantive hypotheses regarding dynamic associations both within and between domains of functioning (Ferrer & McArdle, 2004; Finkel, Reynolds, McArdle, & Pedersen, 2007; Gerstorf et al., 2007; Gerstorf, Hoppmann, Anstey, & Luszcz, 2009; Ghisletta & Lindenberger, 2003; Ghisletta, Bickel, & Lövdén, 2006; Lövdén et al., 2005; McArdle et al., 2004; Small, Dixon, McArdle, & Grimm, 2008). Limitations in statistical power and substantial sample attrition over time often required these studies to model dynamic associations over a time-in-study metric (for notable exceptions, see Ferrer & McArdle, 2004; Ghisletta & Lindenberger, 2003; Grimm, An, McArdle, Zonderman, & Resnick, 2011; McArdle et al., 2004). Using data from the large-scale long-term longitudinal AHEAD study, in contrast, allowed us to apply the BDCSM over chronological age and directly model the age-based structural dynamics between memory and functional limitations we are interested in.
Taken together, conceptual and empirical work both suggest that across-domain associations exist between facets of cognition and health. Our objective is to expand upon those insights by targeting questions about their developmental ordering in old age and possible underlying variables. First, we apply the BDCSM to longitudinal age-based data from the AHEAD study and examine dynamic links between memory and functional limitations in old age. Second, we explore whether dynamic links differ between the young-old and old-old. Third, we examine the role of between-person differences in gender, education, health conditions, and depressive symptoms for associations between memory and functional limitations in old age.
Method
In the present study, we applied the BDCSM to seven waves (covering 13 years) of longitudinal data from the national AHEAD study. Descriptions of the larger AHEAD study, its design, participants, variables, and assessment procedures are published elsewhere (McArdle, Fisher, & Kadlec, 2007; Soldo, Hurd, Rodgers, & Wallace, 1997). Select details relevant to our report are given below.
Participants and Procedure
The AHEAD study is a nationally representative probability sample of households in the contiguous United States of non-institutionalized adults aged 70 years and older (n = 8,222). The measures assessed cover a wide range of economic, sociological, psychological, and physical health information. If more than one member of a sample household was born pre-1924, one of them was randomly selected; if that individual was married or living with a partner, the spouse or partner was also selected regardless of the age of that person. Participants in the AHEAD study were visited biennially, and beginning in 1998 data collection was conducted at the same time as the Health and Retirement Study.
In our report, we utilized data from seven waves (1993, 1995, 1998, 2000, 2002, 2004, and 2006) and selected those participants who (1) provided valid data on both memory and functional limitations in 1993, and (2) provided valid data on our covariates for one measurement occasion or more (n = 6,990). Relative to those participants not included because of missing data, our subsample was younger in 1993 (M = 76.17, SD = 6.44 vs. M = 79.55, SD = 7.69; F [1, 7,984] = 227.54, p < .01), received more years of education (M = 11.04, SD = 3.62 vs. M = 9.07, SD = 4.08; F [1, 7,984] = 251.12, p < .01), and included more women (65% vs. 53%; χ2 [1, 7,984] = 52.69, p < .01).
Data were collected via face-to-face and telephone interviewing. Interviews for participants aged 70 – 79 were primarily conducted via telephone, whereas those aged 80 and older were generally interviewed in person. Comparing telephone and face-to-face interview approaches have found very few systematic differences between modes of assessment (Herzog & Rodgers, 1988).
Measures
Episodic memory was measured using a unit-weight composite of performances on the immediate and delayed free-recall tests. The immediate recall test was typically given during the first interview quarter and asked participants to recall as many nouns as possible from a list of 10 nouns selected from four lists. For the delayed recall test, interviewers asked participants after a period of about 5 minutes again to recall as many nouns as possible out of the original word list. We used the number of words correctly remembered from both tests, ranging from 0 to 20, with higher scores representing more words remembered or better memory. On average, participants contributed 4.09 (SD = 2.22) episodic memory observations, with n = 5,849 or 74% providing two or more repeated measures. Details of the cognitive measures in the AHEAD study and their measurement properties are published in Ofstedal, Fisher, and Herzog (2005).
Functional limitations were measured with a composite sum across items that asked participants if they have any difficulty carrying out each of 10 instrumental activities of daily living (coded as 1) or not (coded as 0). Activities included using the phone, managing money, taking medications, shopping groceries, preparing hot meals, walking several blocks, climbing one flight of stairs, lifting or carrying 10 lb (4.53 kg) of weight, picking up a dime, and pushing or pulling large objects. Higher scores indicate poorer levels of physical functioning or a greater number of functional limitations. On average, participants contributed 4.53 (SD = 2.16) functional limitations observations, with n = 6,242 or 89% providing two or more repeated measures. Details of the functional limitations measures in the AHEAD study and their measurement properties are published in Rodgers and Miller (1997).
Covariates
To control for potential confounds, we included gender, education, health conditions, and depressive symptoms as covariates into our models. 65% of our sample or 4,531 participants were women. Education was measured as the number of years in school (M = 11.04, SD = 3.62). Health conditions were assessed with a sum index of the number self-reported physician-diagnosed medical conditions, including high blood pressure, diabetes, cancer, lung disease, heart condition, stroke, psychiatric problems, and arthritis. To use participants’ poorest level of health (i.e., highest number of health conditions) recorded throughout the course of the study, we computed a composite sum index for each of the seven waves and included in our analyses the highest total for each participant (M = 3.15, SD = 1.59). Depressive symptoms were assessed with eight items selected from the CES-D scale (Radloff, 1977). Items asked participants whether they had experienced the following symptoms “much of the time during the past week” (coded as 1) or not (coded as 0): feeling depressed, everything was an effort, restless sleep, was (not) happy, felt lonely, (did not) enjoy life, felt sad, and could not get going. Positively valenced items were reverse coded and higher scores indicated more depressive symptoms. Again, to use participants’ highest number of depressive symptoms recorded throughout the course of the study, we computed a composite sum index at each wave and included the respondent’s highest total into our analyses (M = 2.94, SD = 2.25).
Data preparation
To facilitate interpretation and to ensure a common metric across measures, scores for memory and functional limitations were standardized to the T metric (M = 50, SD = 10) using the T1 AHEAD sample as a reference group (n = 6,990). To model associations between memory and functional limitations over chronological age (ages 70 – 95), we created age-based memory and functional limitations variables based on the age of the participants at each of the seven waves. This procedure allowed us to model across-domain associations between memory and functional limitations over a span of 25 years, equivalent to 26 possible ages. Table 1 shows the means, standard deviations, and number of observations for memory and functional limitations by age of assessment. It can be seen that most observations are available from ages 70 to 90. We note that although the starting T1 sample included a few participants younger than age 70, we only included observations beginning at age 70. This procedure ensured more observations at the beginning of the time series, thereby facilitating model convergence and trustworthiness of the parameter estimates. Descriptively, Table 1 shows that with advancing age, memory declines and functional limitations increase.
Table 1.
Descriptive Statistics for Memory and Functional Limitations over Chronological Age
| Memory |
Functional limitations |
|||||
|---|---|---|---|---|---|---|
| Age | Number of Observations |
M | SD | Number of Observations |
M | SD |
| 70 | 675 | 55.05 | 9.82 | 677 | 47.35 | 8.43 |
| 71 | 823 | 53.85 | 9.52 | 836 | 48.68 | 9.93 |
| 72 | 1144 | 53.79 | 9.74 | 1170 | 48.98 | 9.70 |
| 73 | 1185 | 53.31 | 9.54 | 1223 | 49.08 | 9.87 |
| 74 | 1397 | 53.37 | 9.32 | 1442 | 49.28 | 10.15 |
| 75 | 1478 | 52.77 | 9.52 | 1535 | 49.59 | 10.22 |
| 76 | 1553 | 52.12 | 9.20 | 1622 | 50.35 | 10.84 |
| 77 | 1626 | 51.77 | 9.48 | 1727 | 50.96 | 11.17 |
| 78 | 1678 | 51.08 | 9.06 | 1800 | 52.03 | 11.92 |
| 79 | 1654 | 50.14 | 9.09 | 1759 | 52.17 | 11.80 |
| 80 | 1711 | 50.04 | 9.05 | 1859 | 53.37 | 12.33 |
| 81 | 1678 | 49.52 | 8.82 | 1819 | 53.45 | 12.28 |
| 82 | 1614 | 49.20 | 8.68 | 1772 | 54.92 | 13.07 |
| 83 | 1515 | 48.06 | 8.57 | 1688 | 56.19 | 13.44 |
| 84 | 1413 | 47.86 | 8.73 | 1592 | 56.32 | 13.70 |
| 85 | 1191 | 47.06 | 8.37 | 1379 | 58.21 | 14.13 |
| 86 | 1024 | 46.82 | 8.52 | 1232 | 59.26 | 14.57 |
| 87 | 857 | 45.97 | 8.13 | 1045 | 61.00 | 14.93 |
| 88 | 710 | 45.65 | 8.41 | 888 | 61.44 | 14.69 |
| 89 | 575 | 44.90 | 7.96 | 735 | 63.19 | 14.81 |
| 90 | 506 | 44.06 | 8.12 | 623 | 63.46 | 14.17 |
| 91 | 348 | 45.02 | 8.52 | 475 | 65.94 | 15.24 |
| 92 | 291 | 43.05 | 7.58 | 389 | 65.78 | 14.91 |
| 93 | 205 | 44.12 | 8.13 | 287 | 67.66 | 14.88 |
| 94 | 146 | 42.64 | 6.97 | 222 | 68.89 | 15.13 |
| 95 | 100 | 41.87 | 7.30 | 174 | 71.71 | 14.94 |
Note. Memory and functional limitations were standardized to a T metric (M = 50, SD = 10) using the T1 AHEAD sample as the reference (N = 6,990).
Statistical Analyses
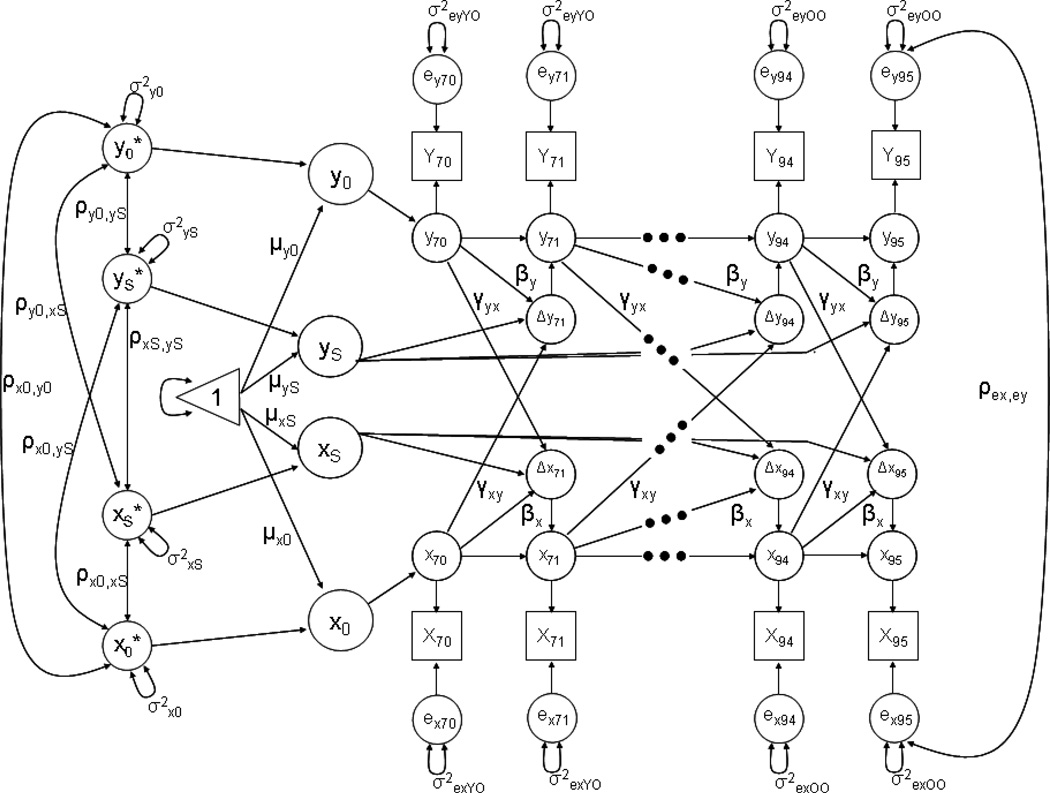
To examine our research questions, we use the BDCSM that combines aspects of the latent growth curve model (Meredith & Tisak, 1990) and the cross-lagged regression approach (Rogosa, 1980) to examine whether time-ordered associations exist between the variables of interest. The model is presented in Figure 1 (for extensive discussions, see Ferrer & McArdle, 2004, 2010; McArdle & Hamagami, 2001). The diagram shows manifest variables as squares, unobserved (latent) variables as circles, fixed model parameters as one-headed arrows, and random parameters as two-headed arrows; the triangle is used to allow the inclusion of means and intercepts. Unlabeled paths are fixed to 1. The X[70], X[71] and X[94], X[95] and Y[70], Y[71] and Y[94] and Y[95] represent the various measurements of X and Y over chronological age, respectively. The ∆x[71] thru ∆x[95] and ∆y[71] thru ∆y[95] represent the reliable change score of X and Y at each age between 70 and 95 years, respectively. The intercept (x0, y0) and slope factors (xS, yS) are supposed to account for the time series of both variables X and Y. Intercepts denote scores for memory and functional limitations at age 70, and the slope factors relate to the change scores ∆x[age] and ∆y[age] with a constant loading of 1, thus denoting linear one-year age-related changes in the present setup. Intercepts and slopes are estimated at the population level (µx0, µxS; µy0, µyS); they are allowed to vary (σx0, σxS; σy0, σyS) and covary (ρx0xS, ρy0yS, ρx0yS, ρxSyS, ρx0yS, ρy0xS). The separately estimated error terms (ex, ey) are assumed to be normally distributed with a mean of zero and allowed to covary between memory and functional limitations within each chronological age, but not across age (ρex,ey). For our analyses, we estimate separate error terms for the young-old (aged 70 – 79) and old-old (aged 80 – 95)1.
Figure 1.
Graphical representation of the Bivariate Dual Change Score model as applied in the current study. Observed variables are represented by squares, latent variables by circles, regression weights by one-headed arrows, and variances and covariance by two-headed arrows. The triangle represents a constant indicating means and intercepts. Unlabeled paths are set to 1. Residual variances are estimated separately for the young-old and old-old.
Of particular interest are the latent change scores, ∆x[age] and ∆y[age], which represent the reliable change between adjacent ages. These change scores are affected by three sets of influence: (i) the linear component of change within a given variable; (ii) the auto-proportion parameter β indicating the effect that level of functioning on variable Y at [age] has on subsequent change at [age + 1] of this same variable Y; and (iii) the inter-variable, cross-lagged coupling γ representing the effect of the other variable X at [age] on subsequent change in the focus variable Y at [age + 1]. Both β’s and γ’s are assumed age-invariant, except in our spline model that explored age differences in these parameters. With β and γ parameters set to zero, the BDCSM is equivalent to a (bivariate) linear latent growth curve model. The major focus of this study is on the inter-variable, coupling parameters γ that allows for direct empirical testing of competing substantive hypotheses regarding dynamic links between memory and functional limitations in old age.
Models for examining differences between the young-old vs. old-old
To examine whether associations of memory and functional limitations differ between the young-old and old-old, we followed procedures applied in research on developmental dynamics in childhood and adolescence (Ferrer & McArdle, 2004; Ferrer et al., 2007) and modeled a BDCSM with “splines”. This model permitted modeling age trajectories continuously over chronological age, but allowed the auto-proportion β and coupling γ parameters to be estimated differently across specified age periods: Young-old (aged 70 – 79) and old-old (aged 80 – 95). This model will yield, as before, one intercept and one slope for each variable, but different dynamic parameters across the two age segments. Analyses were conducted using MPlus (Muthén & Muthén, 1998–2007). We applied full information maximum likelihood estimation to all data points available, which allowed treating incomplete data as missing at random (Little & Rubin, 1987) and adjusting for unbalanced data structures (Singer, 1998).
Results
Our results are organized in three sections. First, we report results from nested model comparisons examining competing hypotheses about time-ordered links between memory and functional limitations. Second, we explored whether these developmental dynamics differed between the young-old and old-old. Third, we included possible confounds (gender, education, health conditions, and depressive symptoms) as covariates into our models to determine whether the observed dynamics were independent of between-person differences in these constructs.
Dynamics of Cognition and Health in Old Age
Results of a series of nested model comparisons are shown in Table 2. It can be seen that a Full Coupling model, which allowed both the γMemory → Functional limitations and γFunctional limitations → Memory coupling parameters to be freely estimated, provided a reasonably good fit to our data (e.g., CFI larger than .900 and RMSEA below .050). This model was the most complex model tested and served as reference by which to judge all other models that estimated fewer parameters. We first estimated two models that constrained the two coupling parameters to be of the same size (Equal Coupling) or to be zero (No Coupling). Both models resulted in significant losses in model fit (e.g., No Coupling model: Δχ2 = 880.24, df = 1, p < .01), suggesting that we can reject the hypotheses that lead-lag associations between memory and functional limitations were of equal size or would not exist. We then proceeded to estimate unidirectional models that fixed either of the two coupling parameters to zero. Specifically, not allowing for memory to predict changes in functional limitations (Coupling γMemory → FL = 0), resulted in a significant loss in model fit (Δ χ2 = 879.82 df = 1, p < .01), suggesting that we cannot reject the unidirectional account that memory precedes subsequent changes in functional limitations. Similarly, not allowing for functional limitations to predict changes in memory (Coupling γFL → memory = 0), resulted in a significant loss in model fit (Δ χ2 = 212.87, df = 1, p < .01), suggesting that functional limitations may also predict changes in memory. Taken together, results from our nested model comparisons revealed that the Full Coupling model is the most parsimonious model that provided the best description to the AHEAD data. It thus appears as if memory and functional limitations are bi-directionally linked in old age.
Table 2.
Goodness-of-Fit Model Comparison Among Alternative Bivariate Dual Change Score Models of Memory and Functional Limitations over Chronological Age in the AHEAD Study
| Model | χ2 | df | Δ χ2/df | CFI | RMSEA |
|---|---|---|---|---|---|
| Zero-order model | |||||
| Bidirectional | |||||
| Full Coupling | 2,401.01 | 1,100 | – | .947 | .013 |
| Equal Coupling | 3,203.44 | 1,101 | 802.43 (1)* | .914 | .017 |
| No direction | |||||
| No Coupling | 3,281.25 | 1,102 | 880.24 (2)* | .911 | .017 |
| Unidirectional | |||||
| Coupling γmemory → FL = 0 | 3,280.83 | 1,101 | 879.82 (1)* | .911 | .017 |
| Coupling γFL → memory = 0 | 2,613.88 | 1,101 | 212.87 (1)* | .938 | .014 |
| Gender, education, health conditions, and depressive symptoms included | |||||
| Bidirectional | |||||
| Full Coupling | 2,709.70 | 1,292 | – | .948 | .013 |
| Equal Coupling | 3,556.85 | 1,293 | 847.15 (1)* | .918 | .016 |
| No direction | |||||
| No Coupling | 3,637.95 | 1,294 | 928.25 (2)* | .915 | .016 |
| Unidirectional | |||||
| Coupling γmemory → FL = 0 | 3,636.99 | 1,293 | 927.29 (1)* | .915 | .016 |
| Coupling γFL → memory = 0 | 2,947.12 | 1,293 | 237.42 (1)* | .940 | .014 |
Note. N = 6,990. FL = Functional Limitations. CFI = Comparative Fit Index. RMSEA = Root Mean Square Error of Approximation.
p < .01.
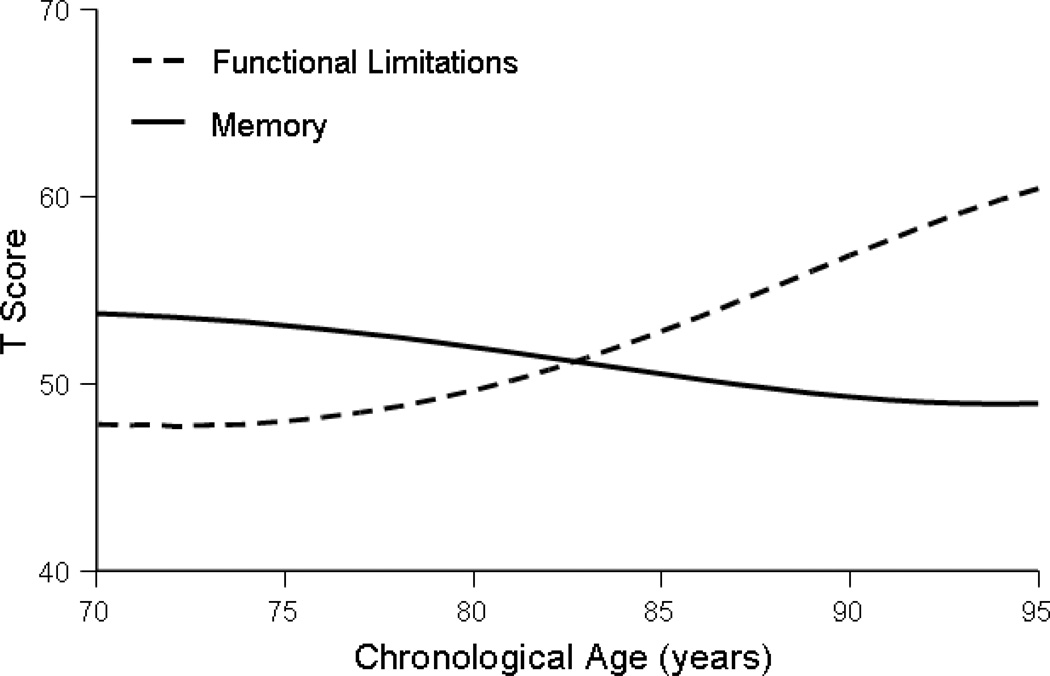
To provide the background for understanding how the cross-lagged dynamics parameters are embedded in other components of change, we first plotted the model-implied means from the Full Coupling model (parameter estimates are reported in Table 3). Model-implied means are a conjoint product of the various components of change modeled by the BDCSM (i.e., linear change, auto-proportion parameter, and coupling parameter) and were produced by the formulas, x[age] = 1 * Xs + (1 + βx) * x [age – 1]+ γyx * y [age – 1] and y[age] = 1 * Ys + (1 + βy) * y [age – 1] + γxy * x [age – 1]. Corroborating the general pattern seen in Table 1, Figure 2 shows that model-implied means for memory decline with advancing age, whereas functional limitations increase.
Table 3.
Bivariate Dual Change Score Model of Memory and Functional Limitations over Chronological Age in the AHEAD Study: Parameter Estimates from a Zero-Order Model
| Memory |
Functional Limitations |
|||
|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE |
| Fixed effects | ||||
| Initial mean µ0 | 53.76* | 0.15 | 47.85* | 0.19 |
| Slope mean µS | −15.14* | 1.09 | 30.88* | 1.84 |
| Proportion β | 0.20* | 0.02 | −0.13* | 0.01 |
| Coupling γ | −0.46* | 0.02 | 0.09* | 0.01 |
| Random effects | ||||
| Initial variance σ20 | 42.61* | 1.29 | 50.59* | 2.58 |
| Slope variance σ2S | 1.63* | 0.23 | 7.48* | 0.83 |
| Residual variance Young-old σ2e | 43.91* | 0.66 | 36.71* | 0.63 |
| Residual variance Old-old σ2e | 34.26* | 0.52 | 59.48* | 0.91 |
| Covariance | ||||
| Memory initial ↔ slope | −7.31* | 0.48 | ||
| Memory initial ↔ initial | −11.96* | 1.33 | ||
| Memory initial ↔ slope | 15.87* | 0.88 | ||
| Memory slope ↔ initial | −1.41* | 0.36 | ||
| Memory slope ↔ slope | −3.47* | 0.42 | ||
| Functional Limitations initial ↔ slope | 2.01* | 0.69 | ||
Note. Memory and functional limitations were standardized to a T metric (M = 50, SD = 10) using the T1 AHEAD sample as the reference (N = 6,990). N = 6,990. Model Fit Statistics: χ2 = 2,401.01, df = 1,100, CFI = .947, RMSEA = .013.
p < .01
Figure 2.
Model-implied means for memory and functional limitations over chronological age. Parameter estimates taken from the Full Coupling model at the zero-order level.
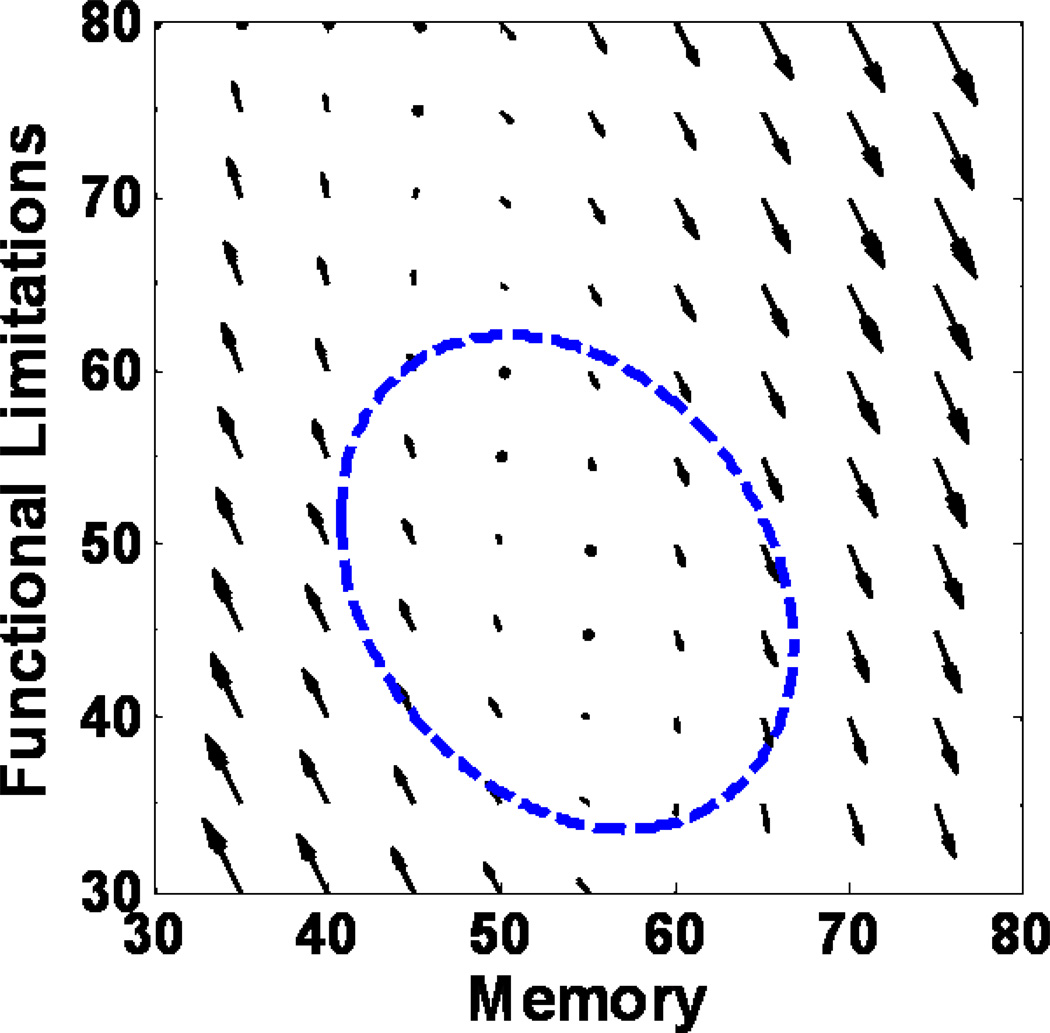
To illustrate the magnitude and direction of the observed dynamics between memory and functional limitations, we followed earlier applications of dynamic models (see Boker & McArdle, 1995; Ferrer & McArdle, 2004; McArdle et al., 2004) and used a vector field that visualizes the dynamic relationship between memory and functional limitations. In Figure 3, the vector field represents the expected yearly changes in the two variables as a function of current states. For a given pair of memory and functional limitations scores, the arrow indicates the size and direction in which these variables are expected to change at the next occasion (i.e., next age). The figure illustrates that memory predicts changes in functional limitation (γMemory → Functional limitations = − 0.46, SE = 0.02, p < .01), whereas the effect in the opposite direction is only marginal (γFunctional limitations → Memory = 0.09, SE = 0.01, p < .01): The direction and size of arrows (i.e., predictive effects) differ within each row (i.e., exerted by memory), but not within each column (i.e., exerted by limitations). For example, the plot shows that functional limitations tend to decline at higher memory scores, whereas functional limitations tend to increase at lower memory scores. The ellipsoid encompasses the location of 95% of the data.
Figure 3.
Vector field illustrating the magnitude and direction of the observed dynamics between memory and functional limitations by showing the expected yearly changes as a function of current states. For a given pair of memory and functional limitations scores, the arrow indicates the size and direction in which these variables are expected to change at the next occasion (i.e., next age). The figure illustrates that memory predicts changes in functional limitation, whereas the effect in the opposite direction is only marginal: The direction and size of arrows (i.e., predictive effects) differ within each row (i.e., exerted by memory), but not within each column (i.e., exerted by limitations). For example, the plot shows that functional limitations tend to decline at higher memory scores, whereas functional limitations tend to increase at lower memory scores. The ellipsoid encompasses the location of 95% of the data.
Dynamics of Cognition and Health among the Young-Old vs. Old-Old
Our second research question was whether the dynamic links between memory and functional limitations presented above differed between the young-old and old-old. To examine this question, we estimated a Full Coupling model that allowed the auto-proportion β and coupling γ parameters to be different across the young-old (aged 70 – 79) and old-old (aged 80 – 95; see Ferrer & McArdle, 2004; Ferrer et al., 2007).
Table 4 shows the parameter estimates from such a spline model. We first note that the spline model provides a better fit to the data than the previously examined models with constant coupling parameters over chronological age (Δ χ2 = 262.93, df = 4, p < .01). Importantly, the two coupling parameters estimated separately for the young-old and old-old were reliably different from zero and showed only slight nominal differences between the two age groups. For example, the γMemory → Functional limitations coupling parameter is slightly larger for the old-old compared to the young-old (−0.68, SE = 0.04, p < .01 vs. −0.66, SE = 0.03, p < .01). In contrast, the γFunctional limitations → Memory coupling parameter is slightly larger in the young-old compared to the old-old (0.12, SE = 0.01, p < .01 vs. 0.11, SE = 0.01, p < .01). Our results from the spline model suggest that dynamic links between memory and functional limitations are substantively similar in size for both the young-old and old-old.
Table 4.
Bivariate Dual Change Score Model of Memory and Functional Limitations over Chronological Age in the AHEAD Study: Parameter Estimates from a Spline Model for Young-old and Old-old Participants
| Memory |
Functional Limitations |
|||
|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE |
| Fixed effects | ||||
| Initial mean µ0 | 53.68* | 0.14 | 47.85* | 0.19 |
| Slope mean µS | −17.73* | 1.38 | 44.46* | 2.66 |
| Proportion β Young-old | 0.22* | 0.02 | −0.20* | 0.02 |
| Proportion β Old-old | 0.22* | 0.02 | −0.19* | 0.02 |
| Coupling γ Young-old | −0.66* | 0.03 | 0.12* | 0.01 |
| Coupling γ Old-old | −0.68* | 0.04 | 0.11* | 0.01 |
| Random effects | ||||
| Initial variance σ20 | 41.35* | 1.27 | 48.42* | 2.32 |
| Slope variance σ2S | 2.10* | 0.33 | 15.38* | 1.73 |
| Residual variance Young-old σ2e | 43.75* | 0.66 | 34.89* | 0.61 |
| Residual variance Old-old σ2e | 33.79* | 0.51 | 56.78* | 0.90 |
| Covariance | ||||
| Memory initial ↔ slope | −7.39* | 0.62 | ||
| Memory initial ↔ initial | −8.92* | 1.27 | ||
| Memory initial ↔ slope | 22.72* | 1.27 | ||
| Memory slope ↔ initial | −3.72* | 0.50 | ||
| Memory slope ↔ slope | −5.46* | 0.74 | ||
| Functional Limitations initial ↔ slope | 4.61* | 1.06 | ||
Note. Memory and functional limitations were standardized to a T metric (M = 50, SD = 10) using the T1 AHEAD sample as the reference (N = 6,990). Young-old (aged 70 – 79); Old-old (aged 80 – 95). N = 6,990. Model Fit Statistics: χ2 = 2,138.08, df = 1,096, CFI = .957, RMSEA = .012.
p < .01
The Role of Covariates
We finally included gender, education, health conditions, and depressive symptoms as covariates into our models to residualize the coupling parameters (and all other model parameters) for individual differences in each of these possible confounds.
Most important for the question under study, including the covariates did not alter the dynamic associations reported above. As the bottom portion of Table 2 indicates, the Full Coupling model again provided the most parsimonious fit to the data, suggesting that dynamic links exist between memory and functional limitations in both directions. As before, both the γMemory → Functional limitations (−0.48, SE = 0.03, p < .01) and γFunctional limitations → Memory (0.09, SE = 0.01, p < .01) coupling parameters were reliably different from zero, suggesting that the observed dynamics cannot be reduced to between-person differences in the covariates examined.
Discussion
Our objective in this study was to examine the direction, nature, and correlates of time-ordered links between age-related changes in memory and functional limitations in old age. Applying a BDCSM to seven occasions of longitudinal age-based data from the AHEAD study, our analyses first revealed that better memory predicted shallower age-related increases in functional limitations. Little evidence was found that functional limitations were predictive of ensuing changes in memory. Second, applying spline-model extensions of the BDCSM indicated that dynamic links between memory and functional limitations were substantively similar between the young-old and old-old. Third, potential confounds and third-variable factors included in our models (gender, education, health conditions, and depressive symptoms) did not account for the differential lead–lag associations observed. These results suggest that late-life developments in two key components of successful aging are intrinsically interrelated. We discuss possible mechanisms why cognitive functioning may serve as a source for aging-related changes in health both among the young-old and the old-old and consider further steps to substantiate our findings.
Dynamics of Cognition and Health in Old Age
Addressing our first research question, we found that memory precedes and predicts subsequent changes in functional limitations, whereas little evidence emerged for the reversed direction. Our results corroborate and extend previous work from epidemiologic and psychological perspectives reporting that individuals performing better on tests of global cognitive functioning and specific abilities such as memory and processing speed were more likely to report better physical functioning and to remain disability-free (Black & Rush, 2002; Elovainio et al., 2009; Kelly-Hayes et al., 1992; Moritz et al., 1995; Mehta et al., 2002; Wahl et al., 2010). Our results add to those reports by utilizing longitudinal data from a national study in the US and applying contemporary methods of analysis to simultaneously test competing substantive hypotheses regarding the time-ordered nature of late-life developments in these domains. Our results are relatively clear-cut in suggesting that memory is a leading indicator of changes in functional limitations. It remains an open question, however, if memory serves as a risk factor for or a protective factor against subsequent increases in functional limitations or whether both mechanisms are operating at the same time. Individuals with superior memory may be better able to remember, plan, and execute everyday activities such as taking medications, managing finances, and cooking. Alternatively, poor memory may leave individuals with fewer mental resources for remembering ways to execute or plan actions needed to perform everyday activities. For example, declines in cognitive function may play an integral role in the execution of most physical tasks and the maintenance of physical functioning and health (Tabbarah, Crimmins, & Seeman, 2002; Vaughan & Giovanello, 2010).
Our findings are consistent with notions suggesting that cognitive functioning serves as a general-purpose mechanism for adaptation and a resource that people can draw upon in the face of obstacles (Baltes et al., 2006). As a corollary, our findings provide impetus for the utility of cognitive interventions aimed at alleviating age-related cognitive declines through numerous training exercises and strategies (Ball et al., 2002; Schaie & Willis, 1986; for discussion, see Hertzog et al., 2009; Salthouse, 2006). Recent work has shown that the positive effects of interventions in the cognitive domain may indeed transfer to the health domain. For example, the ACTIVE Study found that individuals in various training groups reported more favorable five-year changes in physical functioning (Willis et al., 2006). Our findings, together with cognitive intervention studies, corroborate that cognitive functioning has pivotal implications for changes in the health domain. More work exploring links between other cognitive abilities (e.g., executive functioning, processing speed, and vocabulary) and health is of course warranted before more definitive inferences can be drawn.
We found very little evidence that functional limitations predict subsequent changes in memory in old age. This pattern is somewhat at odds with previous reports that more functional limitations were associated with poorer cognitive functioning (Christensen et al., 1994) or that a related construct, social participation, was associated with subsequent changes in perceptual speed (Lövdén et al., 2005). We note that social participation can be construed as a more complex, higher-order construct that is built on and necessitates that functional limitations, as measured here, do not exist or are of minor severity. In contrast, if (severe) functional limitations exist, predictive effects of activities for cognitive change may be weaker because individuals may not be able anymore to be actively engaged or involved in social network opportunities and cognitively stimulating activities. It is also possible that functional limitations do not necessarily shape cognitive functioning and change directly, but represent a proxy variable that reflects the operation of other factors, including chronic illnesses and physical activities (Spiro & Brady, 2008; Verbrugge & Jette, 1994; Verhaeghen et al., 2003). To begin with, chronic illnesses such as cardiovascular disease, diabetes, stroke, and hypertension are known risk factors for cognitive impairments and decline. In a similar vein, chronic illnesses and physiological declines often restrict one’s ability to successfully complete everyday tasks both at the instrumental level (e.g., managing one’s bank account, shopping grocery) and more basic levels (e.g., bathing, dressing). Although we covaried for some of those variables in our models (chronic illness), we note that the gamma parameters of functional limitations onto memory in our BDCSM analyses may indeed only be small, indirect, and not entirely comparable to the opposite direction of memory predicting functional limitations. Detailed future studies are thus needed to pinpoint the time-ordered associations of cognitive trajectories with those of specific diseases and physical activities.
Dynamics of Cognition and Health among the Young-Old vs. Old-Old
Our second research question revolved around the potentially age-differential nature of structural dynamics between memory and functional limitations. We had argued that increased risks for more frequent and severe constraints in either of these domains along with diminished resources and adaptive capacities should make the old-old more vulnerable for cognition–health associations. Our analyses, however, revealed that such dynamics were substantively similar for both the young-old and old-old. One interpretation is that these associations prevail independent of considerable differences in level of overall functioning.
For both the young-old and old-old, cognitive functioning is a vital domain that serves as a resource to buffer against accruing functional limitations that may limit one’s ability to carry out everyday tasks. It is conceivable that cognition–health dynamics may differ when change is considered along a different time metric, for example, how secondary or tertiary aging processes accumulate and unfold over time (e.g., Birren & Cunningham, 1985; Ram, Gerstorf, Fauth, Zarit, & Malmberg, 2010). To illustrate, if change in either or both physical and cognitive functioning is tied to the accumulation of pathology (i.e., secondary aging), stronger overall declines and accompanying across-domain dynamics may emerge. For example, suffering from chronic illnesses such as heart disease or arthritis may reduce one’s ability to perform everyday tasks, which in turn limit opportunities for cognitively stimulating activities. Similarly, increasing pathology is treated with medication, which in turn often causes side effects that undermine physical and cognitive functioning. If the young-old indeed have more self-regulatory and adaptive resources available to buffer further health declines, such associations may then be less pronounced relative to the old-old.
The Role of Covariates
In our third research question, we examined the role of gender and education as well as the highest recorded frequency of health conditions and depressive symptoms in cognition-health links. None of these common correlates of memory and functional limitations accounted for the differential lead–lag associations observed. It is unclear then, what mechanisms may underlie associations between memory and functional limitations. Conceptual notions often highlight the role of health behaviors, compensatory strategies, and stressor reactivity in cognition-health associations. As a first pathway, individuals with better cognitive functioning may be more likely to perform health-promoting and health-sustaining behaviors. For example, cognitive fitness may aid in understanding the importance of performing health behaviors such as eating a balanced diet, exercising regularly, avoiding drug abuse, and keeping up with continually changing health behavior recommendations (Singh-Manoux et al., 2009). In a similar vein, older adults with better memory may have greater health literacy and be better able to adhere to medical regimens such as complex medication schedules (Gottfredson & Deary, 2004; Insel, Morrow, Brewer, & Figueredo, 2006; Reyna, Nelson, Han, & Dieckmann, 2009).
As a second pathway, older adults with higher levels of cognitive functioning may be more effective at using adaptive and compensatory strategies for dealing with the challenges of old age. As a result, health conditions and other ailments may not necessarily result in physical limitations or functional decline (Stuck et al., 1999; Willis et al., 2006). As a third pathway, better cognitive functioning has also been associated with resiliency to stressors. For example, despite reporting greater exposure to stressors, individuals with higher levels of fluid cognitive abilities are suggested to experience smaller emotional reactivity to reported daily stressors (Stawski, Almeida, Lachman, Tun, & Rosnick, 2010). Preserved cognitive fitness may be related to increased mental resiliency for coping with stressors and exhibiting a less severe emotional and physiological response. It was beyond the scope and possibilities of the present study to address such speculations. More mechanism-oriented research is certainly needed to specifically pinpoint these and other etiological questions (for discussion, see Spiro & Brady, 2008).
Rather than reflecting the operation of specific mechanisms, it is possible that more broad-based changes associated with old age act as an underlying third variable for cognition–health associations. One candidate factor may be wide-ranging declines over age in the structure and synaptic plasticity of the brain (Bäckman et al., 2000; Li, Lindenberger, & Sikström, 2001; Raz, Ghisletta, Rodrigue, Kennedy, & Lindenberger, 2010). Such changes may alter the strength and fidelity of neurological connections between brain areas associated with cognitive and physical functioning. Empirically, there is evidence to suggest that text and spatial abilities show moderate associations with sensorimotor functioning (Li, Aggen, Nesselroade, & Baltes, 2001). A second set of candidate factors encompasses social relationships and participation. For example, social networks characterized by intellectual engagement, support, and leisure pursuits often relate to higher levels of and more favorable changes in cognitive abilities (Hertzog et al., 2009; Hultsch, Hertzog, Small, & Dixon, 1999; Kramer, Bherer, Colcombe, Dong, & Greenough, 2004). Similarly, social support and integration are known protective factors against disability and functional limitations (Berkman, Glass, Brissette, & Seeman, 2000; House, Landis, & Umberson, 1988; Uchino, 2006).
Methodological Considerations
To thoroughly test our research questions, we have applied dynamic models that overcome major limitations of related techniques typically used to examine competing substantive hypotheses about lead-lag associations between two sets of variables (e.g., cross-lagged regressions; Rogosa, 1980). For example, the BDCSM accounts for differential reliabilities and stabilities of the variables examined and also separates intra-variable from inter-variable dynamics (cf., Ghisletta & Lindenberger, 2003; Lövdén et al., 2005). In the context of these strengths, we highlight several methodological caveats.
First, as is true in many other studies, our findings are contingent upon a number of untested statistical assumptions, including sample homogeneity (e.g., regarding the dynamics parameters) and data missing at random (cf., Lövdén, Li, Shing, & Lindenberger, 2007). To aid model convergence, for example, we had to estimate the coupling parameters as fixed-effects population parameters with no variance, reflecting the strong assumption that couplings are invariant across participants. Furthermore, by estimating the BDCSM over chronological age, participants have only contributed segmented data portions to the overall time-series. Specifically, our time-series covered 25 years of chronological ages, whereas we utilized data from seven waves covering a total of 13 years of time. We also note that the estimation algorithm gives implicitly more weight to information obtained from those participants who provided the most change (i.e., most occasions) information.
Second, the BDCSM allows for testing across-domain associations at the between-person level of analysis, but inferences cannot be made regarding within-person relations. Specifically, we have examined how between-person differences in one construct (memory) were related to between-person differences in a second construct of interest (functional limitations). It is upon future research to examine ergodicity questions about the equivalence of structural relations based on between-person and within-person variation (for discussion, see Molenaar et al., 2003; Sliwinski & Mogle, 2008). At the within-person level, it is conceivable that different dynamics emerge with functional limitations predicting ensuing changes in memory. For example, Li and colleagues (2001) reported that within-person fluctuation in walking steps uniquely predicted text and spatial memory, over and above level of performance.
Finally, we note that despite the capabilities of the BDCSM, our sample size and large number of observations may have contributed to non-substantial effects attaining statistical significance (e.g., more functional limitations were associated with shallower declines in memory). More generally, targeted simulation studies are needed that focus on the validity of (dynamic) models under varying conditions, including density and completeness of data and measurement properties of the variables under scrutiny (Ferrer & McArdle, 2010; Hertzog, Lindenberger, Ghisletta, & von Oertzen, 2006).
Limitations and Outlook
In closing, we note several limitations in our study. As a first limitation, cognition and health are multidimensional constructs, whereas we focused on memory and functional limitations only. For example, if data for crystallized abilities rather than memory had been available, different associations with functional limitations may have emerged because such limitations often implicate constraints onto active engagement with one’s network and allow for fewer opportunities to practice and maintain one’s knowledge and word vocabulary. In a similar vein, the overlap among objective, subjective, and functional measures of health is typically only of moderate size (Steinhagen-Thiessen & Borchelt, 1999). It would thus be informative to thoroughly test bi-directional associations using other health indicators (e.g., gait variability, self-rated health, or health conditions), some of which have already been reported to predict subsequent changes in memory (Anstey & Christensen, 2000; Bäckman et al., 2003; Hertzog et al., 1978; Verhaeghen et al., 2003).
As a second limitation, we acknowledge that measuring cognitive abilities with only one task and real-life competencies with only self-reports are both not optimal. The direction and size of time-ordered associations found for memory may not necessarily generalize to other cognitive abilities. In a similar vein, it is possible that self-reports of everyday activities may not map onto observed competencies for everyday problem solving (Diehl, Willis, & Schaie, 1995; Owsley, Sloane, McGwin, & Ball, 2002). As a consequence, associations revealed by self-reports of functional limitations may differ from those revealed by observed competencies (Vaughan & Giovanello, 2010). As a third limitation, we note that our study solely focused on old age. It is an open question whether or not our findings generalize to earlier phases of life. For example, midlife is characterized by relatively stable levels of memory and people typically maintain reasonably good health (Spiro, 2001). It is well possible then that cognition-health links do not exist in midlife or are restricted to highly change-sensitive measures such as processing speed or particular physical health symptoms. Finally, we note that our models describe and examine time-ordered associations and cannot be used to draw causal inferences.
In sum, our results highlight the importance of cognitive functioning as it relates to health in old age. Our findings are in line with conceptual notions highlighting that cognitive abilities serve as a resource for both the young-old and old-old to buffer age-related declines in everyday functioning. More broadly, our study adds to theoretical and empirical work highlighting the importance of cognition-health associations for adult development and aging (Anstey & Christensen, 2000; Bäckman et al., 2001; Hertzog et al., 2009; Rowe & Kahn, 1987; Ryan & Smith, 2009; Schaie, 2005; Willis et al., 2006). We take our results to provide further impetus to thoroughly examine the developmental ordering between aspects of cognition and health in old age and the underlying mechanisms linking these key components of old age.
Acknowledgments
Frank J. Infurna and Denis Gerstorf gratefully acknowledge the support provided by the National Institute on Aging (NIA) NIA R21-AG032379 and NIA R21-AG033109. The study of Asset and Health Dynamics Among the Oldest Old was supported by a cooperative agreement (Grant U01 AG09740) between the NIA and the University of Michigan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pag
We ran a series of nested models to estimate the best fitting error structure for our analyses. Results indicate that estimating the variance and residual errors for memory and functional limitations separately for the young-old (aged 70 – 79) and old-old (aged 80 – 95) revealed a significantly better description of the structure of our data than a model that set these to be invariant across ages 70 to 95, Δ χ2 = 558.50 df = 3, p < .01.
Contributor Information
Frank J. Infurna, Email: infurna@psu.edu.
Denis Gerstorf, Email: gerstorf@psu.edu.
Lindsay H. Ryan, Email: linryan@isr.umich.edu.
Jacqui Smith, Email: smitjacq@umich.edu.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, et al. Predictors of cognitive change in older persons: MacArthur Studies of Successful Aging. Psychology and Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish Twins. Journals of Gerontology: Medical Sciences. 2008;63A:62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin TBR, Wahlin Å, Halldin C, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. American Journal of Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Small BJ, Agüero-Torres H, Fratiglioni L. Rate of cognitive decline in preclinical Alzheimer’s disease: The role of comorbidity. Journal of Gerontology: Psychological Sciences. 2003;58B:P228–P236. doi: 10.1093/geronb/58.4.p228. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Wahlin Å. Aging and memory: Cognitive and biological perspectives. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego: Academic Press; 2001. pp. 349–377. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Nesselroade JR. History and rationale of longitudinal research. In: Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. New York, NY: Academic Press; 1979. pp. 1–39. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Life-span theory in developmental psychology. In: Lerner RM, editor. Handbook of child psychology Vol. 1: Theoretical models of human development. 6th ed. New York, NY: Wiley; 2006. pp. 569–664. [Google Scholar]
- Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young-old to the dilemmas of the fourth age. Gerontology. 2003;49:123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Science & Medicine. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Birren JE, Cunningham W. Research on the psychology of aging: Principles, concepts, and theory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 2nd ed. New York, NY: Van Nostrand Reinhold; 1985. pp. 3–34. [Google Scholar]
- Black SA, Rush RD. Cognitive and functional decline in adults aged 75 and older. Journal of American Geriatrics Society. 2002;50:1978–1986. doi: 10.1046/j.1532-5415.2002.50609.x. [DOI] [PubMed] [Google Scholar]
- Boker SM, McArdle JJ. Statistical vector field analysis applied to mixed cross-sectional and longitudinal data. Experimental Aging Research. 1995;21:77–93. doi: 10.1080/03610739508254269. [DOI] [PubMed] [Google Scholar]
- Brandtstädter J, Lerner RM. Action and self development: Theory and research through the lifespan. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. American Journal of Geriatric Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- Christensen H, Jorm AF, Henderson AS, Mackinnon AJ, Korten AE, Scott LR. The relationship between health and cognitive functioning in a sample of elderly people in the community. Age and Ageing. 1994;23:204–212. doi: 10.1093/ageing/23.3.204. [DOI] [PubMed] [Google Scholar]
- Crimmins EM. Mortality and health in human life spans. Experimental Gerontology. 2001;36:885–897. doi: 10.1016/s0531-5565(00)00248-5. [DOI] [PubMed] [Google Scholar]
- Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: Observational assessment and cognitive correlates. Psychology and Aging. 1995;10:478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Kivimäki M, Ferrie JE, Gimeno D, De Vogli R, Virtanen M, et al. Physical and cognitive function in midlife: Reciprocal effects? A 5-year follow-up of the Whitehall II Study. Journal of Epidemiological Community Health. 2009;63:468–473. doi: 10.1136/jech.2008.081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Developmental Psychology. 2004;40:935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. Longitudinal modeling of developmental changes in psychological research. Current Directions in Psychological Science. 2010;19:149–154. [Google Scholar]
- Ferrer E, McArdle JJ, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Longitudinal models of developmental dynamics between reading and cognition from childhood to adolescence. Developmental Psychology. 2007;43:1460–1473. doi: 10.1037/0012-1649.43.6.1460. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Hoppmann CA, Anstey KJ, Luszcz M. Dynamic links of cognitive functioning among married couples: Longitudinal evidence from the Australian Longitudinal Study of Ageing. Psychology and Aging. 2009;24:296–309. doi: 10.1037/a0015069. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology. 2007;43:705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Smith J, Baltes PB. A systemic-wholistic approach to differential aging: Longitudinal findings from the Berlin Aging Study. Psychology and Aging. 2006;21:645–663. doi: 10.1037/0882-7974.21.4.645. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: Direct evidence for ability dedifferentiation in old age. Psychology and Aging. 2003;18:696–713. doi: 10.1037/0882-7974.18.4.696. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Exploring structural dynamics within and between sensory and intellectual functioning in old and very old age: Longitudinal evidence from the Berlin Aging Study. Intelligence. 2005;33:555–587. [Google Scholar]
- Ghisletta P, Bickel J-F, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. Journals of Gerontology: Psychological Sciences. 2006;61B:P253–P261. doi: 10.1093/geronb/61.5.p253. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: Is it the epidemiologists’ elusive 'Fundamental Cause' of social class inequalities in health? Journal of Personality and Social Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Current Directions in Psychological Science. 2004;13:1–4. [Google Scholar]
- Grimm KJ, An Y, McArdle JJ, Zonderman AB, Resnick SM. Recent changes leading to subsequent changes: Extensions of multivariate latent difference score models. Manuscript submitted for publication. 2011 doi: 10.1080/10705511.2012.659627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Lindenberger U, Ghisletta P, von Oertzen T. On the power of multivariate latent growth curve models to detect correlated change. Psychological Methods. 2006;11:244–252. doi: 10.1037/1082-989X.11.3.244. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Schaie KW, Gribbin K. Cardiovascular disease and changes in intellectual functioning from middle to old age. Journal of Gerontology. 1978;33:872–883. doi: 10.1093/geronj/33.6.872. [DOI] [PubMed] [Google Scholar]
- Herzog RA, Rodgers WL. Interviewing older adults: Mode comparison using data from a face-to-face survey and a telephone survey. Public Opinion Quarterly. 1988;52:84–89. [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Insel K, Morrow D, Brewer B, Figueredo A. Executive function, working memory, and medication adherence among older adults. The Journals of Gerontology. 2006;61B:P102–P107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- Jopp D, Rott C. Adaptation in very old age: Exploring the role of resources, beliefs, and attitudes for centenarians’ happiness. Psychology and Aging. 2006;21:266–280. doi: 10.1037/0882-7974.21.2.266. [DOI] [PubMed] [Google Scholar]
- Jopp D, Smith J. Resources and life-management strategies as determinants of successful aging: On the protective effect of selection, optimization, and compensation. Psychology and Aging. 2006;21:253–265. doi: 10.1037/0882-7974.21.2.253. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Jette AM, Wolf PA, D’Agostino RB, Odell PM. Functional limitations and disability among elders in the Framingham Study. American Journal of Public Health. 1992;82:841–845. doi: 10.2105/ajph.82.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Stress and immunity: Age enhances the risks. Current Directions in Psychological Science. 2001;10:18–21. [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. Journal of Gerontology: Medical Sciences. 2004;59A:940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Li S-C, Aggen SH, Nesselroade JR, Baltes PB. Short-term fluctuations in elderly people’s sensorimotor functioning predict text and spatial memory performance: the MacArthur Successful Aging Studies. Gerontology. 2001;47:100–116. doi: 10.1159/000052782. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Liang J, Shaw BA, Krause NM, Bennett JM, Blaum C, Kobayashi E, et al. Changes in functional status among older adults in Japan: Successful and usual aging. Psychology and Aging. 2003;18:684–695. doi: 10.1037/0882-7974.18.4.684. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Li S-C, Shing YL, Lindenberger U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia. 2007;45:2827–2838. doi: 10.1016/j.neuropsychologia.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Magnusson D, Cairns RB. Developmental science: Toward a unified framework. In: Cairns RB, Elder GH, Costello EJ, editors. Developmental science. New York, NY: Cambridge University Press; 1996. pp. 7–30. [Google Scholar]
- McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends from the Health and Retirement Study, 1992–2004. Psychology and Aging. 2007;22:525–545. doi: 10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 137–176. [Google Scholar]
- McArdle JJ, Hamagami F, Jones K, Jolesz F, Kikinis R, Spiro A, III, et al. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the Normative Aging Study. Journal of Gerontology: Psychological Sciences. 2004;59B:P294–P304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. Journal of American Geriatrics Society. 2002;50:1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55:107–122. [Google Scholar]
- Moen P. Gender, age, and the life course. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. 4th ed. San Diego, CA: Academic Press; 1996. pp. 171–187. [Google Scholar]
- Molenaar PCM, Huizenga HM, Nesselroade JR. The relationship between the structure of interindividual and intraindividual variability: A theoretical and empirical vindication of developmental systems theory. In: Staudinger UM, Lindenberger U, editors. Understanding human development. Dialogues with lifespan psychology. Dordrecht, NL: Kluwer; 2003. pp. 339–360. [Google Scholar]
- Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. American Journal of Epidemiology. 1995;141:41–49. doi: 10.1093/oxfordjournals.aje.a117344. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 4th ed. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- Ofstedal MB, Fisher GG, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study (HRS/AHEAD Documentation Report DR-006) Ann Arbor, MI: University of Michigan; 2005. [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr, Ball K. Time instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological measurement. 1977;1:385–401. [Google Scholar]
- Ram N, Gerstorf D, Fauth B, Zarit SH, Malmberg B. Aging, disablement, and dying: using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development. 2010;7:27–44. doi: 10.1080/15427600903578151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. NeuroImage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychological Bulletin. 2009;135:943–973. doi: 10.1037/a0017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers WL, Miller B. A comparative analysis of ADL questions in surveys of older people. The Journals of Gerontology: Psychological Sciences. 1997;52B:21–36. doi: 10.1093/geronb/52b.special_issue.21. [DOI] [PubMed] [Google Scholar]
- Rogosa D. A critique of cross-lagged correlation. Psychological Bulletin. 1980;88:245–258. [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson L-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Ryan LH, Smith J. Cognition and health disparities over the life course: Longevity as an example. In: Antonucci TC, Jackson JS, editors. Annual Review of Gerontology and Geriatrics: Life-Course Perspectives on Late-Life Health Inequalities. Vol 29. New York, NY: Springer; 2009. pp. 131–157. [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9:1–28. [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Schaie KW, Willis SL. Can intellectual decline in the elderly be reversed? Developmental Psychology. 1986;22:223–232. [Google Scholar]
- Seeman TE, Charpentier PA, Berkman LF, Tinetti ME, Guralnik JM, Albert M, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur Studies of Successful Aging. Journal of Gerontology: Medical Sciences. 1994;49:M97–M108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Singh-Manoux A, Sabia S, Kivimaki M, Shipley JJ, Ferrie JE, Marmot MG. Cognition and incident coronary heart disease in late midlife: The Whitehall II Study. Intelligence. 2009;37:529–534. doi: 10.1016/j.intell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski JM, Mogle J. The multiple facets of change: Implications for modeling relationships in cognitive aging research. In: Hofer SM, Alwin DF, editors. Handbook on cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ. Tracking cognition-health changes from 55–95 years of age. Journal of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbq093. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Changes in lifestyle activities in relation to changes in cognitive abilities; Poster presented at the 12th Cognitive Aging Conference; 2008, April; Atlanta, GA. [Google Scholar]
- Smith J, Baltes MM. The role of gender in very old age: Profiles of functioning and everyday life patterns. Psychology and Aging. 1998;13:676–695. doi: 10.1037//0882-7974.13.4.676. [DOI] [PubMed] [Google Scholar]
- Smith J, Baltes PB. Profiles and psychological functioning in the old and oldest old. Psychology and Aging. 1997;12:458–472. doi: 10.1037//0882-7974.12.3.458. [DOI] [PubMed] [Google Scholar]
- Soldo B, Hurd M, Rodgers W, Wallace R. Asset and Health Dynamics Among the Oldest Old: An overview of the AHEAD study. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1997;52:1–20. doi: 10.1093/geronb/52b.special_issue.1. [DOI] [PubMed] [Google Scholar]
- Spiro AIII. Health in midlife: Toward a life-span view. In: Lachman ME, editor. Handbook of midlife development. New York, NY: Wiley; 2001. pp. 156–187. [Google Scholar]
- Spiro AIII, Brady CB. Integrating health into cognitive aging research and theory: Quo vadis? In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage Publications; 2008. pp. 260–283. [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun P, Rosnick CB. Fluid cognitive ability is associated with greater exposure and smaller emotional reactions to daily stressors. Psychology and Aging. 2010;25:330–342. doi: 10.1037/a0018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen-Thiessen E, Borchelt M. Morbidity, medication, and functional limitations in very old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. pp. 131–166. [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Suzman RM, Manton KG, Willis DP. Introducing the oldest old. In: Suzman RM, Willis DP, Manton KG, editors. The oldest old. New York, NY: Oxford University Press; 1992. pp. 3–14. [Google Scholar]
- Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. Journal of Gerontology: Medical Sciences. 2002;57A:M228–M235. doi: 10.1093/gerona/57.4.m228. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin A, Holm NV, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychology. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- Wahl H-W, Schmitt M, Danner D, Coppin A. Is the emergence of functional ability decline in early old age related to change in speed of cognitive processing and also to change in personality? Journal of Aging and Health. 2010;22:691–712. doi: 10.1177/0898264310372410. [DOI] [PubMed] [Google Scholar]
- Wahlin Å, MacDonald SWS, deFrias CM, Nilsson L-G, Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit SH, Johansson B, Malmberg B. Changes in functional competency in the oldest old: A longitudinal study. Journal of Aging Health. 1995;7:3–23. doi: 10.1177/089826439500700101. [DOI] [PubMed] [Google Scholar]