Summary
In extensive burns it becomes difficult for fibroblasts to migrate from the periphery of the healthy tissue and colonize the injured area. Even under such circumstances healing takes place, and this is attributed to the differentiation of circulating fibrocytes which enter the wound site. This normal cell type is identified in keloid fibroblasts: it expresses fibrocyte markers and secretes extra cellular matrix proteins. In-vitro collagen contraction assay reveals that fibrocytes contract collagen gels with an efficacy similar to normal fibroblasts. The contribution of fibrocytes to the formation of keloid fibroblasts in post-burn healing is discussed.
Keywords: fibrocytes, keloid fibroblasts, collagen gel
Abstract
Quand le patient est atteint de brûlures de grande extension, pour les fibroblastes il devient difficile de migrer de la périphérie du tissu sain pour coloniser la zone lésée. Même dans ces conditions la guérison a lieu, ce qui est attribué à la différenciation des fibrocytes en circulation qui entrent dans la plaie. Ce type normal de cellule a été identifié dans les fibroblastes chéloïdaux: il exprime des marqueurs des fibrocytes et sécrète des protéines de la matrice extracellulaire. Les épreuves in vitro sur la contraction du collagène révèlent que les fibrocytes contractent le gel de collagène avec une efficacité similaire à celle des fibroblastes normaux. Les Auteurs concluent avec une discussion sur la contribution des fibrocytes à la formation des fibroblastes chéloïdaux dans la phase finale de la guérison des brûlures.
Introduction
The process of wound healing after an acute injury, regardless of the cause, recapitulates the major events associated with embryogenesis. Various stages of wound healing are characterized by a tremendous amount of cellular migration, proliferation and phenotypic differentiation.1,2 All these stages of wound healing are well orchestrated with any disturbance leading to improper wound healing, resulting in either ulcers or fibrosis. When such a disturbance leading to fibrosis occurs in the skin, keloid lesions form as a result in many patients. These lesions occur due to excessive deposition of collagen in the dermal matrix. The dermis of keloid lesions is characterized not only by excess matrix deposition but also by its improper alignment and unstimulated overexpression of some the key signaling molecules.3 The extracellular matrix (ECM) of the keloid dermis has been extensively studied; however, knowledge of the origin of keloid fibroblast is still obscure.
When extensive areas of skin are burned, it may be difficult or sometimes impossible for fibroblasts to migrate from the edges of healthy tissue and colonize the injured area. Even these acute burns heal, and healing of such extensive burns often leads to the formation of keloids and hypertrophic scars. Bucala et al. (1994)4 identified a novel population of cells while studying the acute responses to wound healing. This population of cells was identified as a subpopulation of leukocytes. These cells had fibroblast- like properties and therefore they were called fibrocytes. Peripheral blood fibrocytes can rapidly enter the site of injury along with the circulating inflammatory cells. Their morphological appearance is similar to fibroblasts, and they can produce ECM proteins - like collagen type I & III and fibronectin - along with the expression of CD34, the marker for cells of haematopoietic origin. Subsequent research by several groups5,6 has clearly demonstrated that circulating fibrocytes are an important source of fibroblast and of other dermal cells for healing of large burn wounds.
After extensive study of the research conducted by other groups and analysis of our observations, we hypothesize that the origin of keloid fibroblast probably lies in fibrocyte- like cells. The fibroblast-like properties of fibrocytes and expression of fibrocyte-like markers by keloid and normal skin fibroblasts have been investigated.
Materials and methods
Chemicals and reagents
Unless otherwise mentioned all chemicals and reagents for analysis were purchased from Sigma-Aldrich Co. LLC, USA. All reagents, media, serum and enzymes for animal tissue culture were purchased from Invitrogen, Life Technologies, USA. All antibodies used for immunofluorescence were purchased from Cell Signaling Technologies Inc., USA.
Analytical instruments
Microscopy and image analysis was done using Leica DM IRB Microscope (Leica- Microsystems, Germany) with fluorescence attachment having Leica Application Suite imaging software (Leica-Microsystems, Germany) for image analysis. Spectrophotometry was done using UV/Vis Spectrophotometer (PerkinElmer Inc., USA).
The Institutional Review Board and Ethics Committee approved this study.
Collection of samples
Tissue biopsies of keloid lesions and normal skin were collected in sterile saline for isolation and culture of fibroblasts. Normal healthy subjects were requested to donate blood for isolation and development of fibrocyte cultures.
Isolation of peripheral blood fibrocytes
Fibrocytes were isolated as described by Yang et al.7 Briefly, 25 ml of peripheral blood were collected in heparinised tube. Total blood mononuclear cells (PBMC) were analyzed by density gradient sedimentation on Histopaque 1077 using manufacturer’s protocol. The isolated PBMC were cultured in 6 well plates at 5x105 cells/well in DMEM containing 10% FCS and antibiotics. After 3 days, non-adherent cells were removed and the medium was changed. After 10 days, the cells were harvested by incubation with 0.05% EDTA in PBS and gentle scraping with rubber policeman. Cells were counted, checked for their purity using CD34 as a marker, and used for further experiments. Fibrocyte purity was confirmed by immunofluorescence.
Isolation of normal and keloid fibroblasts
Fibroblasts were isolated as described by Meenakshi J. et al.8 Briefly, the portions of biopsied tissues preserved in sterile saline were washed thoroughly in sterile PBS (0.01 M, pH 7.2) and treated with 0.5% trypsin 20 mM EDTA solution for 16 h at 4 °C. After removing the epidermis, the dermis was finely chopped and treated with 200 units/ml of collagenase in the presence of DMEM and 10% FCS at 37 °C for 24 h. The digested tissue was centrifuged at 2000 rpm for 5 min, and the cell-containing pellet was placed in a tissue culture flask containing sufficient quantity of DMEM and 10% FCS. The plated cells were allowed to adhere and grow to confluence, and were then sub-cultured. For immunofluorescence the cells in the first passage were used, in order to avoid behavioral medications in the fibroblast induced by prolonged exposure to tissue culture media.
Purification of collagen type I from bovine dermis
Bovine dermal collagen type I was purified by soaking, mincing, and grinding the cleaned bovine dermis in 0.5M acetic acid. The acid soluble portion was subjected to salt precipitation in 0.7M NaCl to isolate type I collagen. The salted out collagen was desalted by dialysis against 0.05M acetic acid. The dialysis collagen was lyophilized and stored at -80°C.
Normal skin fibroblast/fibrocytes adherence to collagen films
Type I bovine collagen was purified as mentioned above. A suitable amount of collagen was dissolved in 0.05M acetic acid, reconstituted by gradual neutralization, and cast on polypropylene boats. The boats were air dried in sterile chambers. On complete drying, the collagen films were cut into suitable sizes and sterilized using ethylene oxide treatment.
Sterile films were placed in multiwall culture dishes and fibroblast/fibrocytes were seeded on them. As a control, both types of cells were seeded on empty wells. After 48 hrs all the cells were washed and fixed in 4% paraformaldehyde. The fixed cells were washed in PBS and the cells were stained with propidium iodide and visualized using fluorescence microscope.
Collagen gel contraction assay
Sterilized bovine collagen type I was dissolved in low molarity acetic acid and mixed with normal skin fibroblasts or fibrocytes. Very quickly, the pH was adjusted to near neutral, poured on 35 mm tissue culture dishes, and allowed to gel at 37°C in a CO2 incubator. The gel was observed periodically and its diameter measured. Approximately on the 7th day, contraction was observed. At this stage the gel was fixed using 4% paraformaldehyde and subjected to histological processing. The sections obtained were stained with hematoxylin and eosin to understand cell distribution in the collagen matrix.
Screening of fibrocyte markers on keloid fibroblasts
Screening for fibrocyte markers on keloid fibroblasts was done by immunofluorescence. For this purpose, cells were grown on cover slips placed in multi-well tissue culture dishes. The cover slips containing the cells were washed with sterile PBS and fixed with chilled methanol. Fixed cells were again washed thoroughly with PBS containing 0.05% Tween-20 (PBST). Blocking was done with 3% BSA prepared in PBST for 1 h at 37°C. Suitable dilution of antibodies was prepared in PBST, added to the cover slips and incubated for 1hr at 37°C. Washed cover slips were incubated in a suitable dilution of secondary antibody labeled with fluorescence for 1hr at 37°C. The cover slips were washed rigorously and mounted in antifade containing aqueous mountant. The fluorescence staining was visualized with the help of fluorescence microscope. The intensity of staining was analyzed using the Leica image analysis software mentioned earlier.
Results
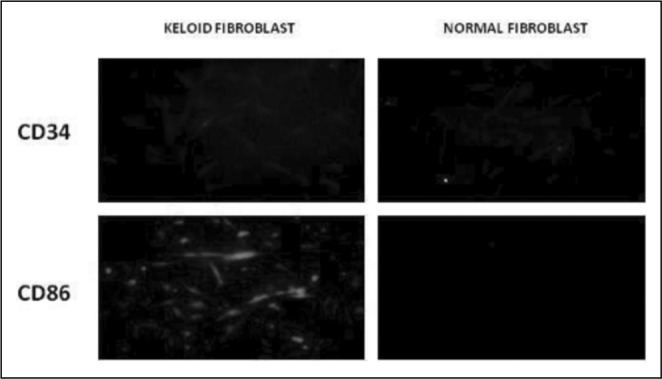
Keloid and normal skin fibroblasts were grown on cover glasses and stained for CD34 and CD86. The cells were visualized and graded using immunofluorescence. The markers studied are over expressed in keloid fibroblasts (Fig. 1A).
Fig. 1A.
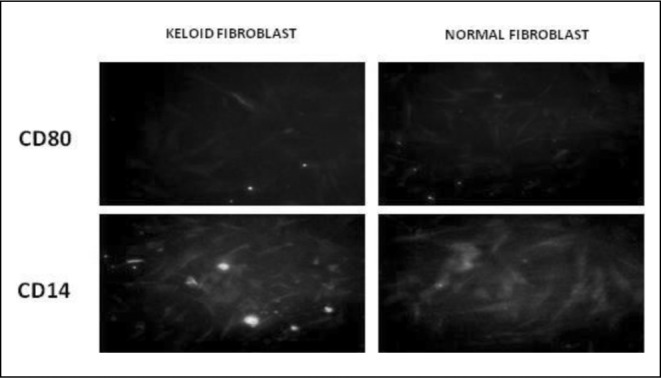
Keloid and normal skin fibroblasts were grown on cover glasses and stained for CD80 and CD14. The cells were visualized and graded using immunofluorescence. The markers studied are expressed more or less equally in both types of cells (Fig. 1B).
Fig. 1B.
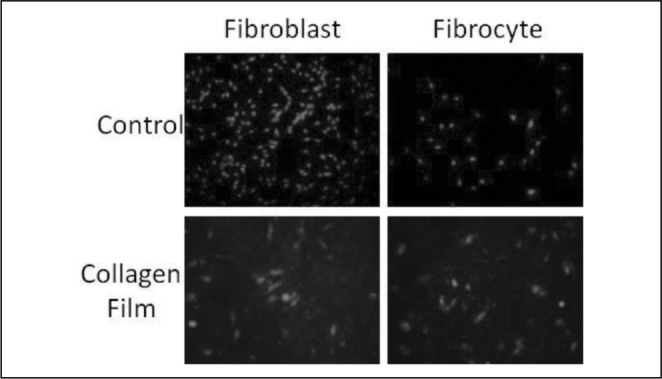
Fibroblasts and fibrocytes were cultured on multiwell culture dishes with and without collagen films as indicated in the figure. The cells were visualized using Propidium Iodide fluorescence staining. Fibrocytes adhere to collagen films as efficiently as fibroblasts (Fig. 2A).
Fig. 2A.
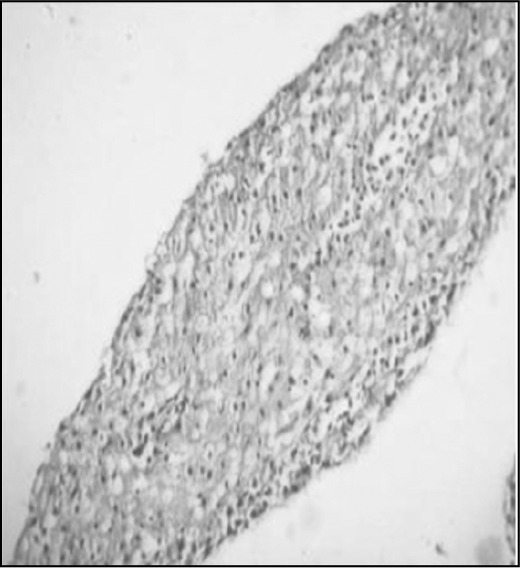
Fibroblasts and fibrocytes were impregnated in collagen gels. Contracted gels were fixed and subjected to histological sectioning. This figure is a representation of cells embedded in collagen matrix. Both cell types contracted collagen gels with similar efficacy (Fig. 2B).
Fig. 2B.
Discussion
Post burn hypertrophic scars and keloids develop as a result of dysregulated wound healing. Hypertrophic scars regress and do not recur on excision, whereas keloids do not regress and recur on excision.9 Therapeutic management of keloids by various methods has not solved this problem yet. Hence, a greater understanding of the signalling mechanism inducing keloid formation is essential. Circulating fibrocytes, first identified in 1994 in the context of wound repair, are unique bone marrow-derived mesenchymal progenitor cells defined by their growth characteristics and surface phenotype: they express markers of leukocytes, haematopoietic progenitor cells, and fibroblasts, as well as a number of other markers including chemokine receptors and adhesion molecules. Fibrocytes participate in tissue remodeling by producing extracellular matrix proteins (collagen I, collagen III, and vimentin) and by secreting matrix metalloproteinases. Moreover, fibrocytes are an important intercellular signal locally within the tissue. Thus, the word fibrocyte (a term combining fibroblast and leukocyte) was coined for this circulating fibroblast progenitor that produced collagen and expressed the haematopoietic marker CD34.
In our experiments the cultured keloid fibroblasts expressed both CD34 and CD86 markers that are present in fibrocytes. These are haematopoietic stem cell markers and MHC class II markers, respectively. Normal skin fibroblasts did not express these markers. Normal skin as well as keloid fibroblasts expressed CD14 and CD80, which are fibroblast markers. This clearly shows the fibrocyte origin of the keloid fibroblasts. We successfully cultured fibrocytes from PBMC, and when they were seeded on collagen gel fabricated with bovine Type I collagen, they were able to contract the gel. This is an inherent property of normal skin as well as of keloid fibroblasts, and this mechanism takes place during wound healing.10
On reaching the wound site, the circulating fibrocytes undergo differentiation into fibroblasts. It has been demonstrated that TGFβ-1 is an important fibrogenic and growth regulating cytokine involved in wound healing and increasing the differentiation and functional activity of fibrocytes. We are interested in investigating signaling cascade responsible for the differentiation of fibrocytes into fibroblasts to manifest keloid lesions. Additional data is needed from basic research as well as clinical studies to further elucidate the fibrotic mechanisms and to ultimately translate the findings into normal, therapeutic tools for selective manipulation of fibrosis in keloid states.
References
- 1.Kumar V, Abbas AK, Fausto N. In: “Pathologic Basis of Disease”. Kumar V, Abbas AK, editors. Elsevier Saunders; Philadelphia: 2005. Tissue renewal and repair: regeneration, healing and fibrosis. pp. 87–118. [Google Scholar]
- 2.Davidson JM. In: Wound Repair. 2nd ed. Gallin JI, Goldstein IM, Snyderman R, editors. Raven Press; New York: 1992. Inflammation: basic principles and clinical correlates. pp. 809–19. [Google Scholar]
- 3.Meenakshi J, Jayaraman V, Ramakrishnan KM, et al. Keloids and hypertrophic scars: a review. Indian J Plast Surg. 2005;38:175–9. [Google Scholar]
- 4.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 6.Ellen CK, Borna M, Robert MS. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010;42:535–42. doi: 10.1016/j.biocel.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Scott PG, Giruffre J, et al. Peripheral blood fibrocytes from burn patients; identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 8.Meenakshi J, Jayaraman V, Ramakrishnan KM, et al. Ultrastructural differentiation of abnormal scars. Ann Burns Fire Disasters. 2005;18:83–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Bishara SA, Michel C, Shady NH. Keloid or hypertrophic scar - the controversy: review of the literature. Ann Plast Surg. 2005;54:676–80. doi: 10.1097/01.sap.0000164538.72375.93. [DOI] [PubMed] [Google Scholar]
- 10.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exper Cell Research. 1999;250:273–83. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]


