Abstract
Purpose.
We explored the role of myeloid-related protein 8 and 14 (MRP8/14) in Pseudomonas aeruginosa (PA) keratitis.
Methods.
MRP8/14 mRNA levels in human corneal scrapes and mouse corneas infected by PA were tested using real-time PCR. MRP8/14 protein expression in C57BL/6 (B6) corneas was confirmed using Western blot assay and immunohistochemistry. B6 mice were injected subconjunctivally with siRNA for MRP8/14, and then infected with PA. Bacterial plate counts and myeloperoxidase assays were used to determine the bacterial load and polymorphonuclear neutrophil (PMN) infiltration in infected B6 corneas. Pro-inflammatory cytokine levels in vivo and in vitro were examined with PCR and ELISA. In murine macrophage-like RAW264.7 cells, phagocytosis and bacterial killing were assessed using plate count assays, and reactive oxygen species (ROS) and nitric oxide (NO) levels were tested with flow cytometry and Griess assay, respectively.
Results.
MRP8/14 expression levels were increased significantly in human corneal scrapes and B6 corneas after PA infection. Silencing of MRP8/14 in B6 corneas significantly reduced the severity of corneal disease, bacterial clearance, PMN infiltration, and pro-inflammatory cytokine expression after PA infection. In vitro studies demonstrated further that silencing of MRP8/14 suppressed pro-inflammatory cytokine production, bacterial killing, and ROS production, but not phagocytosis or NO production.
Conclusions.
Our study demonstrated a dual role for MRP8/14 in bacterial keratitis. Although MRP8/14 promotes bacterial clearance by enhancing ROS production, it functions more importantly as an inflammatory amplifier at the ocular surface by enhancing pro-inflammatory cytokine expression, thus contributing to the corneal susceptibility.
Our study firstly explores the role of MRP8/14 in microbial keratitis. Although MRP8/14 promotes bacterial clearance by enhancing ROS production, it functions more importantly as an inflammatory amplifier by enhancing pro-inflammatory cytokine expression, thus leading to corneal perforation.
Introduction
Pseudomonas aeruginosa (PA) is a common Gram-negative bacteria associated with microbial keratitis, especially in users of soft contact lenses.1 PA keratitis is a rapidly progressive corneal disease that often causes inflammatory epithelial edema, stromal infiltration, corneal ulceration, tissue destruction, and vision loss.2 Pathogenesis of PA keratitis is an intricate process that depends largely on the interaction between the host and invading pathogens. Apart from bacterial virulence factors, immunopathologic damage resulting from excessive host inflammation is thought to be another major pathogenic mechanism of PA keratitis.3
Innate immunity is the first line of host defense against microbial infection. When pattern-recognition receptors (PRRs) recognize invading microbes, inflammatory cells, including polymorphonuclear neutrophils (PMNs) and monocytes/macrophages, are recruited to the infection sites.4,5 These inflammatory cells engulf bacteria with the help of different phagocytic receptors6 and then kill the bacteria intracellularly using either the oxygen-dependent microbicidal system, consisting of reactive oxygen species (ROS) and reactive nitrogen species (RNS),7,8 or an oxygen-independent system, such as lysozymes and antimicrobial peptides.9 However, persistence of inflammatory cells and sustained production of pro-inflammatory cytokines also may lead to serious pathologic damage,10,11 including corneal ulceration and stromal destruction. Therefore, corneal inflammation must be balanced appropriately to facilitate bacterial clearance and tissue repair; otherwise, excessive inflammation may result in a susceptible disease outcome.
As members of the calcium-binding S100 protein family, MRP8 (S100A8) and MRP14 (S100A9) are expressed mainly in phagocytic myeloid cells, such as PMNs and macrophages, and they often form a functional MRP8/14 heterodimer in various inflammatory and infectious situations,12–14 including cystic fibrosis,15 inflammatory bowel disease,16 and rheumatoid arthritis.17,18 Nonetheless, the role of MRP8/14 in modulating the inflammatory response remains controversial. Several studies have suggested that MRP8/14 functions as an important damage/danger-associated molecular pattern (DAMP) and an inflammatory amplifier in some inflammatory disorders19 by promoting the expression of pro-inflammatory cytokines and adhesion molecules.20,21 However, others have reported that MRP8/14 suppresses indirectly the inflammatory response by neutralizing extracellular pro-inflammatory cytokines in lipopolysaccharide-induced liver injury and autoimmune myocarditis.22,23
Moreover, MRP8/14 possesses a broad spectrum of antimicrobial activities against microorganisms, such as Escherichia coli,24 Candida albicans,25 and Listeria monocytogenes.26 Regarding its bactericidal mechanism, Akerström et al. found that MRP8/14 kills Finegolidia magna by interacting with the cell membrane and lysing the bacteria,27 and Simard et al. demonstrated that MRP14 stimulates PMN microbicidal activity, killing Escherichia coli by elevating phagocytosis.28 In addition, Steinckwich et al. reported that MRP8/14 promotes IgG-opsonized zymosan internalization in PMNs by modulating calcium signaling and phagosomal ROS production.29 Together, these studies indicate that MRP8/14 executes their antimicrobial functions through different mechanisms that might be determined by the species of the pathogen.
The potential role of MRP8/14 in microbial keratitis remains unknown. In our study, we found that MRP8 and MRP14 expression levels were upregulated after PA infection in human corneal scrapes and mouse corneas. In vivo and in vitro knockdown studies indicated that although MRP8/14 promoted bacterial killing of PA, it more importantly enhanced corneal inflammation and, therefore, resulted in corneal susceptibility (perforation). Our study suggested that modulation of MRP8/14 is a potential target for promoting corneal healing and visual recovery during PA keratitis, but further work is required to develop strategies to reduce the concurrent enhanced bacterial burden.
Materials and Methods
Patients and Tissue Specimens
PA keratitis patients at the Zhongshan Ophthalmic Center (Sun Yat-sen University, Guangzhou, China) from January 2010 to December 2010 were included in our study. Inclusive criteria were defined as clinically diagnosed PA keratitis and experimentally confirmed by microbial culture of corneal scrapes. Based upon the infection time, these patients were divided into 3 groups (each with 8 patients) as follows: Group1: 1 to 6 days postinfection (p.i.), including 6 male and 2 female patients 27 to 60 years old; Group 2: 7 to 14 days p.i., including 5 male and 3 female patients 19 to 71 years old; and Group 3: 15 to 30 days p.i., including 5 male and 3 female patients 22 to 76 years old. Corneal scrapes from the patients with PA keratitis were collected before their first treatment in Zhongshan Ophthalmic Center and analyzed using real-time PCR. Controls were normal corneal buttons from transplants. Donor corneas were procured within 6 hours of the donor's death, kept in a special storage reagent at low temperature to maintain biologic activity, and confirmed to be free of any detectable prior pathologic conditions. For the use of these clinical materials for research purposes, the patient's consent and approval from the Institutional Research Ethics Committee were obtained. All research with human subjects adhered to the tenets of the Declaration of Helsinki.
Ocular Infection and Clinical Examination
Female C57BL/6 (B6) mice (Jackson Laboratory, Bar Harbor, ME) 8 weeks old were anesthetized with ether and placed beneath a stereoscopic microscope (Motic SMZ-168 Stereo Zoom Microscope; Motic China Group Co., Ltd., Shenzhen, China) at ×40 magnification. The cornea of the left eye was wounded with three 1-mm incisions using a sterile 25-gauge needle. A 5 μL aliquot containing 1 × 106 CFU of PA (American Type Culture Collection strain 19660; American Type Culture Collection [ATCC], Manassas, VA) was applied topically to the ocular surface. Eyes were examined at 1 day p.i. and/or at times described below to ensure that mice were similarly infected and to monitor the disease. Corneal disease was graded using an established scale30–33: 0, clear or slight opacity, partially or fully covering the pupil; +1, slight opacity, partially or fully covering the anterior segment; +2, dense opacity, partially or fully covering the pupil; +3, dense opacity, covering the entire anterior segment; and +4, corneal perforation or phthisis. A clinical score was recorded for each mouse after infection for statistical comparison of the disease severity, and photography with a slit-lamp was used to illustrate the disease response. Animals were treated humanely and in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cell Culture
Human monocytic THP-1 cells (TIB-202; ATCC) were cultured in 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Murine macrophage-like RAW264.7 cells (TIB-71; ATCC) were cultured in Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were cultured in a standard tissue culture incubator at 37°C, and in an atmosphere of 5% CO2 and 95% room air.
RNA Interference
siRNA for mouse MRP8 and MRP14 (siMRP8/14) or the appropriate scrambled control (siCTL) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Either siMRP8/14 or siCTL was injected subconjunctivally (5 μL/mouse at a concentration of 10 μM) into the left eye of B6 mice (n = 5/group/time) 1 day before infection and then applied topically onto the infected corneas (5 μL/mouse per time at a concentration of 5 μM, once on the day of infection, and twice on 1 and 3 days p.i.). For in vitro silencing, cells were transfected transiently with siMRP8/14 or siCTL using Lipofectamine 2000 (Invitrogen) following the manufacturer's instruction. siRNAs targeting human MRP8 (5′-CCUUGAACUCUAUCGACGUCUA-3′) and human MRP14 (5′-CCAUCAUCAACACCUUCCACCAAUA-3′) were synthesized by Invitrogen.
Real-Time PCR
Total RNA was isolated from individual corneas or cell pellets using TRIzol (Invitrogen) according to the manufacturer's recommendation, and quantitated using a NanoDrop 2000C Spectrophotometers (Thermo Scientific, West Palm Beach, FL). Total RNA (1 μg) was reverse-transcribed to produce cDNA, which was amplified using SYBR Green Master Mix (Takara BIO, Inc., Mountain View, CA) as suggested by the manufacturer. Quantitative real-time PCR reactions were performed using the CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA). Relative mRNA levels were calculated after normalization to β-actin. Primer sequences are listed in the Table.
Table. .
Nucleotide Sequence of the Specific Primers Used in PCR Amplification
|
Gene |
Primer Sequence (5′-3′) |
|
| mβ-actin | GAT TAC TGC TCT GGC TCC TAG C | F |
| GAC TCA TCG TAC TCC TGC TTG C | R | |
| mMRP8 | TCA CCA TGC CCT CTA CAA GAA TGA CTT C | F |
| GCC ACA CCC ACT TTT ATC ACC ATC G | R | |
| mMRP14 | CTG GAC ACA AAC CAG GAC AAT CAG C | F |
| TTC CCA CAG CCT TTG CCA TGA CTG | R | |
| mIL-6 | CAC AAG TCC GGA GAG GAG AC | F |
| CAG AAT TGC CAT TGC ACA AC | R | |
| mIL-1β | CGC AGC AGC ACA TCA ACA AGA GC | F |
| TGT CCT CAT CCT GGA AGG TCC ACG | R | |
| mTNF-α | CAC AGA AAG CAT GAT CCG CGA C | F |
| TGC CAC AAG CAG GAA TGA GAA GAG | R | |
| mMIP-2 | TGT CAA TGC CTG AAG ACC CTG CC | F |
| AAC TTT TTG ACC GCC CTT GAG AGT GG | R | |
| mNOX2 | TCC GTA TTG TGG GAG ACT GG | F |
| AAA GGG CGT GAC TCC AAT C | R | |
| miNOS | CTA AGA GTC ACC AAA ATG GCT CCC | F |
| AGA CCA GAG GCA GCA CAT CAA AGC | R | |
| hβ-actin | GCT CCT CCT GAG CGC AAG | F |
| CAT CTG CTG GAA GGT GGA CA | R | |
| hMRP8 | ACC TGA AGA AAT TGC TAG AGA CCG AGT G | F |
| CCA CGC CCA TCT TTA TCA CCA GAA TGA G | R | |
| hMRP14 | ACC TTC CAC CAA TCA TCT GTG AAG CTG | F |
| GTC CAG GTC CTC CAT GAG TGG TTC TAT G | R | |
| hIL-6 | ATT CGG TAC ATC CTC GAC GGC A | F |
| CAG CCA TCT TTG GAA GGT TCA GGT | R | |
| hIL-1β | CGA TCA CTG AAC TGC ACG CT | F |
| GAG AAC ACC ACT TGT TGC TCC A | R | |
| hTNF-α | GCG TGG AGC TGA GAG ATA ACC A | F |
| GGC TCT TTG ATG GCA GAG AGG A | R | |
| hMIP-2 | ACC GAA GTC ATA GCC ACA CTC A | F |
| AGC CAC CAA TAA GCT TCC TCC T | R |
Western Blot
Whole corneas (n = 5/group/time) from normal uninfected and infected B6 mouse eyes were collected and pooled at 1, 3, and 5 days p.i. Pooled corneas were lysed and homogenized using a tissue lyser (Qiagen, Valencia, CA) in 1× lysis buffer (1 mM EDTA, 20% glycerol, 0.5% NP-40, 1% protease inhibitor cocktail, 1 mM dithiothreitol [DTT], 1 mM phenylmethanesulfonylfluoride [PMSF]). Debris was collected by centrifugation for 5 minutes at 10,000g, and the protein concentration of the supernatant was determined by Bradford protein assay (Bio-Rad). Corneal protein samples (5 μg) were separated on a 15% SDS-PAGE, and then transferred to a supported polyvinylidine difluoride (PVDF) membrane. After blocking in a 5% solution of nonfat dry milk prepared in 1× PBS with 0.05% Tween-20 (PBST), blots were incubated with primary goat-antimouse MRP8 antibody and goat-antimouse MRP8 antibody (0.2 μg/mL; R&D, Minneapolis, MN) at 4°C overnight, washed three times with PBS, incubated in secondary antibody (donkey-antigoat IgG-HRP, 1:1000; R&D) for 1 hour at room temperature, and developed using an ECL kit (KeyGEN, Nanjing, China) following the manufacturer's protocol.
Immunohistochemistry
Normal uninfected and infected eyes from B6 mice were enucleated (n = 3/group/time) at 5 days p.i., immersed in 1 × PBS, embedded in Tissue-Tek OCT compound (Miles, Elkhart, IN), and frozen in liquid nitrogen. Then, 8 μm thick sections were cut and mounted to polylysine-coated glass slides. Immunohistochemical staining was performed with the UltraSensitive SP Immunodetection Kit (Maixin, Inc., Fuzhou, China) according to the manufacturer's instructions. Primary antibodies (goat-antimouse MRP8 or goat-antimouse MRP14) were purchased from R&D. Controls were similarly treated, but the primary antibody was replaced with isotype-matched goat IgG. All sections were visualized with a Zeiss microscope (Zeiss microscope AXIO Imager A1; Carl Zeiss, Inc., Oberkochen, Germany).
ELISA
Corneas from siMRP8/14- or siCTL-treated B6 mice were collected at 1 and 5 days p.i. (n = 5/group/time), and the protein levels of MIP-2, TNF-α, IL-6, and IL-1β were tested using ELISA kits (R&D). The reported sensitivity of these assays is <1.5 pg/mL for MIP-2, <5.1 pg/mL for TNF-α, 1.3 to 1.8 pg/mL for IL-6, and <3.0 pg/mL for IL-1α.
Myeloperoxidase (MPO) Assay
Corneas from siMRP8/14- or siCTL-treated B6 mice were collected at 1 and 5 days p.i. (n = 5/group/time), and homogenized in PBS. Then, the MPO concentration in each sample was assayed by using the Zen Myeloperoxidase ELISA Kit (Invitrogen) according to the manufacturer's recommendations.
Bacterial Plate Counts
Corneas from siMRP8/14- or siCTL-treated B6 mice were collected at 1 and 5 days p.i. (n = 5/group/time), and individual corneas were homogenized in sterilized water containing 0.85% (wt/vol) NaCl and 0.25% BSA. Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar (BD Biosciences, Sparks, MD) in triplicate, and the plates were incubated overnight at 37°C. The results are reported as 105 CFU per cornea ± SEM.
Phagocytosis Assay
Phagocytosis was assayed by flow cytometry as described previously.34 In brief, PA was incubated with Filmtracer Green Biofilm (FTGB, 1:50; Invitrogen) at room temperature for 30 minutes protected from light, and then rinsed gently with sterilized water. RAW264.7 cells were transfected with siMRP8/14 or siCTL for 24 hours, and then challenged with FTGB-stained PA at a multiplicity of infection (MOI) of 25. After 1 hour of incubation, the cells were washed three times with cold PBS to remove nonadherent bacteria. Extracellular fluorescence was quenched by the addition of 0.1% trypan blue (Sigma, St. Louis, MO) in PBS for 15 minutes. Cells were collected and analyzed using a Beckman Coulter EPICS XL/MCL (Beckman Coulter, Inc., Fullerton, CA) instrument.
Intracellular Bacterial Killing Assay
Intracellular bacterial killing was assessed by plate count as described previously.35 In brief, RAW264.7 cells were transfected with siMRP8/14 or siCTL for 24 hours, and then challenged with PA at an MOI of 25. After 1 hour, cells were treated with gentamicin (Sigma) at 300 μg/mL for 30 minutes to kill the extracellular bacteria, and then washed with PBS for three times. Afterwards, one of the duplicate wells was lysed with 0.1% Triton-X, and the duplicate well was incubated at 37°C for an additional 1 hour and then lysed with 0.1% Triton-X. Serial 10-fold dilutions of each sample were plated on Pseudomonas isolation agar in triplicate and incubated overnight at 37°C. For intracellular bacterial killing, the efficiency was calculated using the following equation:
ROS Measurement by Flow Cytometry
RAW264.7 cells were transfected with siMRP8/14 or siCTL for 24 hours. After PA challenge, cells were incubated with a ROS-sensitive probe, 2′,7′-dichlorofluorecscin diacetate (H2DCFDA; Invitrogen), at a final concentration of 10 μM. Then, cells were collected and analyzed using a Beckman Coulter EPICS XL/MCL (Beckman Coulter, Inc.). ROS levels were determined by the fluorescence of dichlorofluorecscin (DCF), the deacetylated and oxidized product of H2DCFDA.
Griess Assay
RAW264.7 cells were transfected with siMRP8/14 or siCTL, and the supernatant of each sample was collected at 6, 18, and 36 hours after PA challenge. Nitric oxide (NO) levels were determined by measuring the levels of the stable end product, nitrite, using a Griess reagent (Sigma). The results were expressed as the mean micromoles of nitrite per sample ± SEM.
Statistical Analysis
The difference in clinical score between siMRP8/14- and siCTL-treated B6 corneas at 1, 3, and 5 days after PA infection was determined using the Mann-Whitney U test. An unpaired, two-tailed Student's t-test was used to determine the significance of other assays. Data were considered significant at P < 0.05.
Results
MRP8 and MRP14 Expression Was Elevated after PA Infection
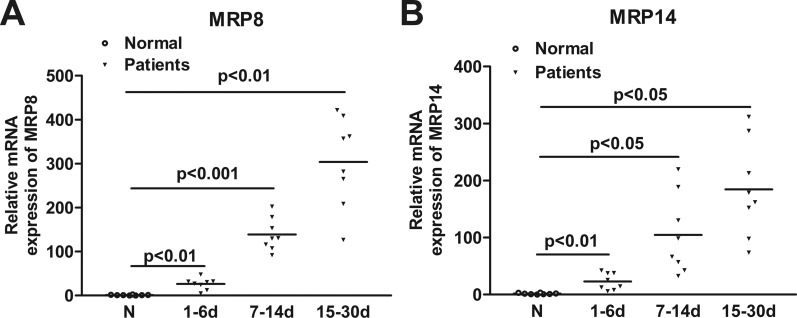
To determine the relevance of MRP8/14 to PA keratitis, mRNA levels of MRP8 and MRP14 were examined in PA-infected human corneal scrapes. PCR data showed that MRP8 and MRP14 mRNA levels were significantly upregulated in human corneal scrapes after PA infection (Figs. 1A, 1B). When compared to normal corneal buttons from transplants, MRP8 expression levels in PA-infected corneas were approximately 30-fold higher at 1 to 6 days p.i., 100-fold higher at 7 to 14 days p.i., and 300-fold higher at 15 to 30 days p.i. (P < 0.01, P < 0.001, P < 0.01, respectively), and MRP14 expression levels were approximately 30-fold higher at 1 to 6 days p.i., 100-fold higher at 7 to 14 days p.i., and 200-fold higher at 15 to 30 days p.i. (P < 0.01, P < 0.05, P < 0.05, respectively), indicating that MRP8 and MRP14 expression dynamically follows the disease progression of PA keratitis.
Figure 1. .
Expression of MRP8 and MRP14 in human corneas. MRP8 (A) and MRP14 (B) mRNA levels were examined in normal and PA-infected human corneas at 1 to 6 days p.i. (Group 1), 7 to 14 days p.i. (Group 2), and 15 to 30 days p.i. (Group 3). Data are the mean ± SEM with 8 patients/group.
Furthermore, a well-characterized murine model of PA keratitis was used to mimic the clinical ocular infection. Western blot data (Fig. 2A) indicated that the levels of MRP8 and MRP14 protein in B6 corneas were significantly upregulated in a time-dependent manner, peaking at 5 days p.i. The integrated density value of MRP8 and MRP14 was shown in Figure 2B. In addition, the MRP8 and MRP14 distribution pattern in the B6 cornea before and after PA infection was confirmed further by immunohistochemistry (Fig. 2C). Higher levels of positive staining for MRP8 and MRP14 (depicted as brown dots) were detected in the infected B6 corneas at 3 days p.i., localizing in the corneal epithelium and stroma. Controls, in which the primary antibody was replaced by isotype-matched goat IgG, were negative for staining in normal and infected corneas.
Figure 2. .
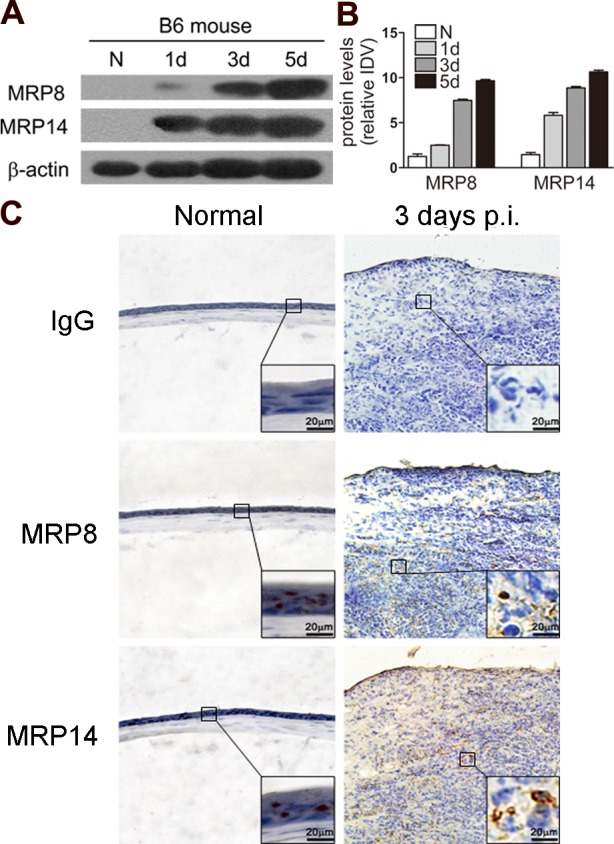
Expression of MRP8 and MRP14 in mouse corneas. (A) MRP8 and MRP14 protein levels in B6 corneas were examined using Western blot before and after PA infection. Equal quantities of protein (5 μg) were loaded in each lane. The band intensity of MRP8 and MRP14 (B) was quantitated and normalized to β-actin. Data shown represent one of three individual experiments, each using 5 pooled corneas/time. (C) MRP8 and MRP14 protein expression also was determined by using immunohistochemistry in normal uninfected and infected B6 corneas at 3 days p.i. Magnifications: ×40 (low magnification), ×100 (large inset).
Silencing of MRP8/14 Promoted Host Resistance to PA Corneal Infection
Since MRP8 and MRP14 expression levels were upregulated significantly in B6 corneas after PA infection, the next series of in vivo studies were designed to determine if MRP8 and MRP14 had a role in PA keratitis. B6 mice were injected subconjunctivally and treated topically with siMRP8/14 or siCTL, and they then were given the ocular infection. Clinical score data (Fig. 3A) showed that mice treated with siMRP8/14 compared to siCTL exhibited a reduced disease level at 3 and 5 days p.i. (P < 0.01 at both time points), but no differences were detected between the two groups at 1 day p.i. Figure 3B shows representative photographs of infected corneas from siMRP8/14- and siCTL-treated mice at 5 days p.i. Most of the MRP8/14-silenced corneas presented with decreased disease severity, whereas the control-treated corneas were perforated. These results demonstrated clearly that silencing of MRP8/14 shifted B6 mice from a susceptible to a resistant phenotype in response to PA corneal infection. The expression level of MRP8 and MRP14 in the infected B6 corneas was tested by PCR and Western blot, and the data demonstrated that MRP8 and MRP14 mRNA (Fig. 3C), and protein levels (Fig. 3D) were greatly reduced after treatment with siMRP8/14, confirming the efficacy of silencing.
Figure 3. .
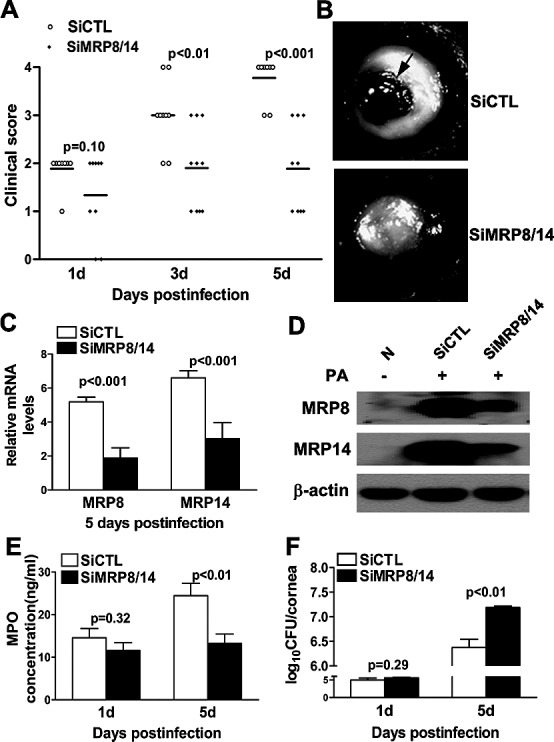
In vivo knockdown studies of MRP8 and MRP14. Clinical scores (A) showed statistically significant differences in siMRP8/14- and siCTL-treated corneas at 3 and 5 days p.i. (P < 0.01 for time points). The horizontal lines among the open and filled dots represent the means of indicated clinical scores. Three individual experiments were performed, each with 10 animals per group each time. Data were generated from one representative experiment. (B) Slit-lamp photographs of PA-infected eyes at 5 days p.i. displayed reduced disease severity in siMRP8/14-treated compared to siCTL-treated mice. Most of the siCTL-treated corneas were perforated at 5 days p.i. (black arrow highlights the perforation). PCR (C) and Western blot (D) data confirmed the effect of silencing MRP8 and MRP14 in the B6 corneas at 5 days p.i. MRP8/14 silencing decreased PMN infiltration as detected by MPO activity (E) and increased viable bacterial numbers (F) at 5 days p.i. Data are the mean ± SEM and represent three individual experiments, each with five animals per group per time per assay.
MRP8/14 Silencing Reduced PMN Infiltration and Bacterial Clearance after PA Infection
To explore the role of MRP8/14 further in PA keratitis, we assessed further the effect of MRP8/14 silencing on PMN infiltration and bacterial clearance. PMN infiltration was detected by MPO activity (Fig. 3E) and was reduced in siMRP8/14-treated compared to siCTL-treated B6 corneas at 5 days p.i. (P < 0.01), whereas no changes were observed between the two groups at 1 day p.i. Meanwhile, bacterial plate count (Fig. 3F) indicated that MRP8/14 silencing elevated the bacterial load at 5 days p.i. (P < 0.01), suggesting that MRP8/14 is required for the bacterial clearance of PA.
MRP8/14 Elevated PA-Induced Pro-Inflammatory Cytokine Production
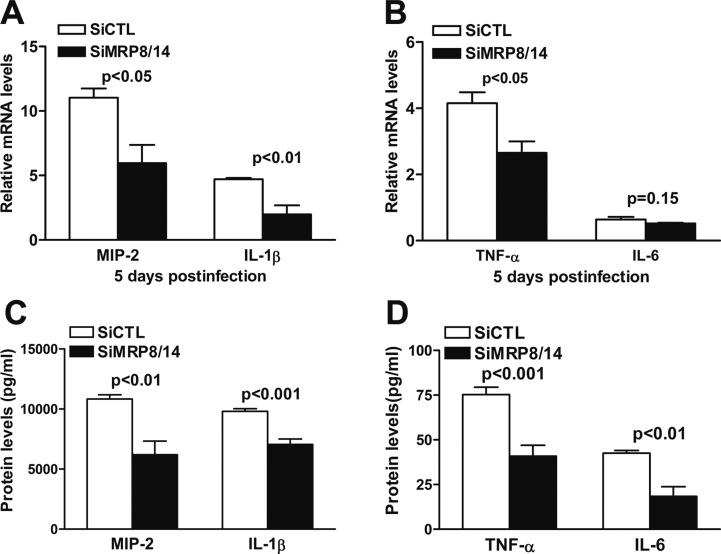
To ascertain the mechanisms by which MRP8/14 modulates corneal inflammation, the mRNA expression levels of several pro-inflammatory cytokines were analyzed using real-time PCR on samples from siMRP8/14- or siCTL-treated B6 corneas at 5 days p.i. The results indicated that silencing MRP8/14 significantly decreased the mRNA levels of MIP-2, IL-1β, and TNF-α at 5 days p.i. (P < 0.05, P < 0.01, P < 0.05, respectively), whereas IL-6 mRNA expression was comparable between the two groups (Figs. 4A, 4B). Moreover, ELISA data demonstrated that treatment with siMRP8/14 significantly downregulated the protein expression levels of MIP-2 and IL-1β (Fig. 4C, P < 0.01, P < 0.001), and TNF-α and IL-6 (Fig. 4D, P < 0.001, P < 0.01) at 5 days p.i.
Figure 4. .
MRP8/14 silencing reduced pro-inflammatory cytokine production in vivo. mRNA expression levels of MIP-2, IL-1β, TNF-α, and IL-6 (A, B) in siMRP8/14- and siCTL-treated corneas were tested using real-time PCR at 5 days p.i. The protein expression levels of MIP-2, IL-1β, TNF-α, and IL-6 (C, D) in siMRP8/14- and siCTL-treated corneas were tested by ELISA at 5 days p.i. Data are the mean ± SEM and represent three individual experiments with five animals per group each time.
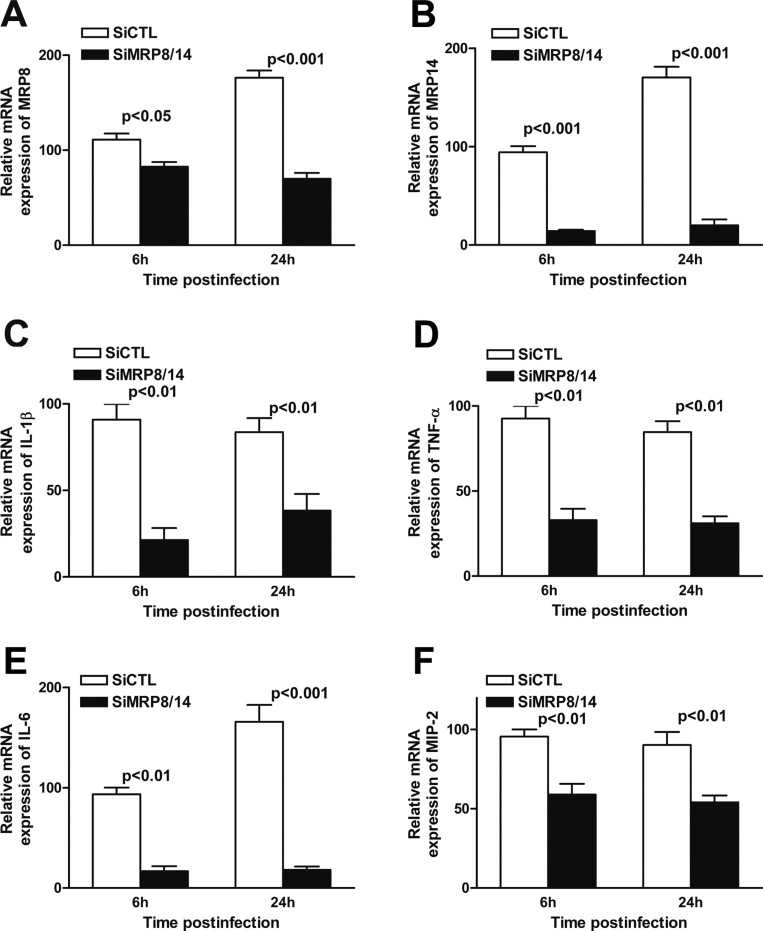
We tested further the effect of MRP8/14 in the PA-induced inflammatory response by using human monocytic THP-1 cells. After transient transfection of siMRP8/14, mRNA levels of MRP8 and MRP14 were reduced significantly in THP-1 cells at 6 and 24 hours p.i. (Figs. 5A, 5B; MRP8 P < 0.05, P < 0.001 at 6 and 24 hours p.i., respectively; MRP14 P < 0.001 at both time points), when compared to siCTL treatment, confirming the efficiency of silencing. Moreover, the mRNA expression levels of MIP-2 (Fig. 5C), IL-1β (Fig. 5D), TNF-α (Fig. 5E), and IL-6 (Fig. 5F) were significantly downregulated in siMRP8/14-treated compared to siCTL-treated THP-1 cells at 6 and 24 hours p.i. (MIP-2, IL-1β, and TNF-α, P < 0.01 at both time points; for IL-6, P < 0.01, P < 0.001 at 6 and 24 hours p.i., respectively). Together, our in vivo and in vitro data suggested that MRP8/14 elevates PA-induced pro-inflammatory cytokine production.
Figure 5. .
MRP8/14 silencing reduced pro-inflammatory cytokine production in vitro. Human monocytic THP-1 cells were transfected with siMRP8/14 or siCTL for 24 hours and then challenged with PA. mRNA expression levels of MRP8 (A) and MRP14 (B) as well as pro-inflammatory cytokines, including MIP-2 (C), IL-1β (D), TNF-α (E), and IL-6 (F) were tested using real-time PCR at 6 and 24 hours p.i. Data are the mean ± SEM and represent three individual experiments.
MRP8/14 Enhanced Intracellular Bacterial Killing but Not Phagocytosis in PA-Challenged Macrophages
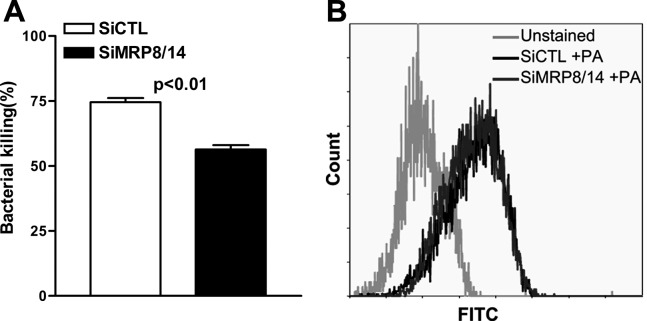
Because in vivo silencing of MRP8/14 enhanced bacterial load in the mouse cornea, we assessed further whether MRP8/14 modulates the process of bacterial clearance. Murine macrophage-like RAW264.7 cells were transfected with siMRP8/14 or siCTL, challenged with PA at an MOI of 25, and then bacterial clearance was assessed using a bacterial killing assay based on plate counts and a phagocytosis assay based on flow cytometry. The results showed that silencing of MRP8/14 significantly reduced intracellular bacterial killing in RAW264.7 cells (Fig. 6A, P < 0.01), although the uptake of PA by RAW264.7 cells was not affected (Fig. 6B). These data indicated that MRP8/14 enhances macrophage-mediated intracellular killing of PA, but was not involved in the process of phagocytosis.
Figure 6. .
MRP8/14 promoted intracellular bacterial killing but not phagocytosis in PA-challenged macrophages. RAW264.7 cells were transfected with siMRP8/14 or siCTL and then challenged with PA. The killing assay by plate count (A) showed that the killing efficiency of PA was decreased after silencing MRP8/14, whereas phagocytosis of PA was comparable in siMRP8/14- and siCTL-treated cells when detected by the phagocytosis assay with flow cytometry (B). Data represent three individual experiments.
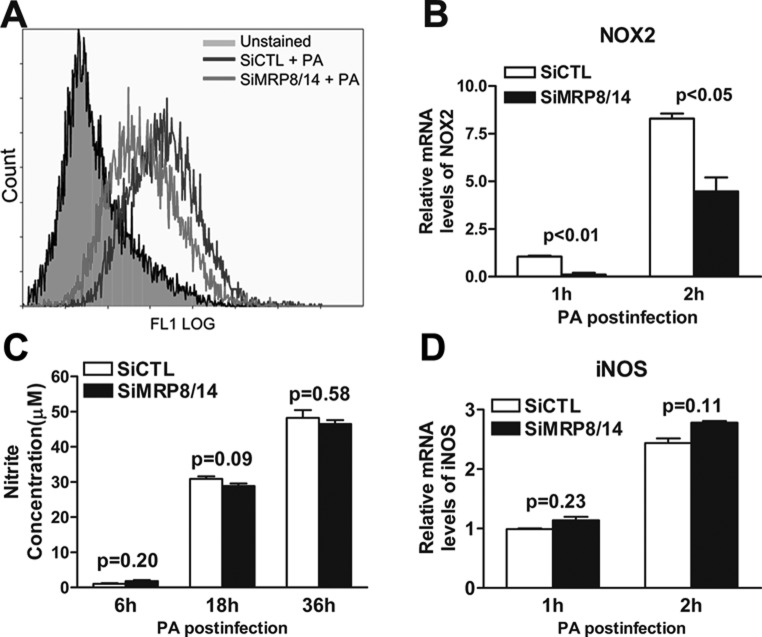
MRP8/14 Promoted Macrophage-Mediated Bacterial Killing by Elevating ROS Levels but Not NO Production
To explore further the microbicidal mechanisms involved during PA infection, ROS and NO production were measured in siMRP8/14- and siCTL-treated RAW264.7 cells after PA challenge. The results demonstrated that the PA-induced ROS production (calculated by the percentage of DCF-positive cells using flow cytometry) was reduced significantly after silencing MRP8 and MRP14 (Fig. 7A). Moreover, mRNA expression levels of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2), an important enzyme for ROS production, also were downregulated in siMRP8/14-treated compared to siCTL-treated RAW264.7 cells at 1 and 2 hours p.i. (Fig. 7B, P < 0.01, P < 0.05, respectively). However, the NO levels (determined by the stable end product nitrite) were comparable between the siMRP8/14- and siCTL-treated groups in PA-challenged RAW264.7 cells at 6, 18, and 36 hours p.i. (Fig. 7C), and inducible NO synthase (iNOS) expression was the same between the two groups (Fig. 7D). These data indicated that MRP8/14 promoted macrophage-mediated intracellular killing of PA by elevating ROS, but not NO production.
Figure 7. .
MRP8/14 promoted macrophage-mediated bacterial killing of PA by enhancing ROS, but not NO production. RAW264.7 cells were transfected with siMRP8/14 or siCTL and then challenged with PA. (A) ROS production (as calculated by the percentage of DCF positive cells) was determined by flow cytometry in siMRP8/14- and siCTL-treated cells after PA infection. (C) NO production (as indicated by the nitrite concentration) was tested using the Griess assay in siMRP8/14- and siCTL-treated cells at 6, 18, and 36 hours p.i. mRNA expression levels of NOX2 (B) and iNOS (D) also were tested using real-time PCR at 1 and 2 hours p.i. Data represent three individual experiments.
Discussion
MRP8/14 (also known as calprotectin) is a functional heterodimer released by myeloid-derived cells in response to cell damage, infection, or inflammation.12,13 However, the role of MRP8/14 in ocular infection remains unclear. Thus, our study was designed to explore the function of MRP8/14 on corneal inflammation and bacterial clearance in response to PA-induced corneal infection.
Our data demonstrated that MRP8 and MRP14 mRNA levels were enhanced in corneal scrapes (which mainly consist of infiltrated inflammatory cells and bacteria) from patients with PA keratitis. Despite the sex and age variations, the increase in MRP8 and MRP14 expression was associated closely with the infection time and disease severity. Moreover, MRP8 and MRP14 expression levels were also upregulated in PA-infected mouse corneas, which is consistent with our clinical data. Immunohistochemistry indicated that most of the positive staining for MRP8 and MRP14 was located in the corneal stroma, either within or outside the infiltrated inflammatory cells. These findings support other reports demonstrating that MRP8/14 is elevated strongly by bacterial infection in the inflammatory cells,36 and nonsecreted and secreted forms are expressed to execute intracellular and extracellular activities.13 The PA-induced MRP8 and MRP14 expression on human and mouse ocular surface indicates the potential association of MRP8/14 with PA keratitis. Our in vivo study revealed that silencing of MRP8/14 in B6 mice promoted host resistance to PA infection, suggesting that MRP8/14 accelerates the disease progression of PA keratitis.
Our results also demonstrated that MRP8/14 enhanced corneal inflammation by promoting PMN infiltration, and the expression of IL-1β, IL-6, TNF-α, and MIP-2. As the hallmark of acute inflammation,2 PMNs migrate quickly to infection sites within minutes along the MIP-2 chemotactic gradient.37–39 Despite potent antibacterial activities, PMNs release a large amount of pro-inflammatory cytokines, which may lead to tissue damage due to excessive inflammation.40 Moreover, studies have demonstrated that MRP8/14 can be induced by IL-1β, IL-6, and TNF-α, and in turn promotes pro-inflammatory cytokine expression, thereby forming a positive feedback loop of inflammatory response.41,42 Thus, we hypothesize that in PA keratitis, the elevated MRP8/14 and pro-inflammatory cytokines also might form a positive feedback loop to amplify the ocular inflammatory responses.
Furthermore, we observed that in vivo silencing of MRP8/14 enhanced bacterial load in the mouse cornea, which is consistent with other reports showing that MRP8/14 has antimicrobial activity.24–26 Different antimicrobial mechanisms for MRP8/14 have been reported, such as interacting with bacterial surface proteins to lyse bacterial cells,27 or enhancing the phagocytic and phagosomal oxidative activity of phagocytes, such as PMNs.28 Our study indicated that MRP8/14 promotes macrophage-mediated intracellular killing of PA, but has no effect on the process of internalization. It is reported that MRP8/14 is required for zymosan internalization in HL-60 cells, which were differentiated into the neutrophil lineage.29 Since different phagocytic receptors may be required to facilitate phagocytosis,6 the different role of MRP8/14 on bacterial phagocytosis between our observation and others may be explained partially by the specificity of phagocytes and pathogens.
In addition, our data demonstrated that silencing MRP8/14 reduced ROS and NOX2 levels in RAW264.7 cells, suggesting that MRP8/14 may promote macrophage-mediated bacterial killing of PA by enhancing ROS production. This is supported by others showing that MRP8/14 increases NOX activity and ROS production in HaCaT keratinocytes.43 However, in our study, NO production and iNOS expression were comparable between MRP8/14-silenced and control-treated RAW264.7 cells at each time point postinfection, suggesting that NO may not be involved in MRP8/14-mediated bacterial killing. Pouliot et al. reported that MRP8/14 induces NO production in macrophages to control infectious agents,44 while De Lorenzo et al. found that MRP14 inhibits NO production and suppresses the activation of macrophages.45 These data together suggest that the role of MRP8/14 on NO production likely is context-dependent.
Host inflammation and bacterial virulence contribute to corneal susceptibility, but treating bacterial infection with antibiotics commonly does not prevent the ocular pathology.46,47 Our data demonstrated that silencing of MRP8/14 minimizes corneal damage by limiting the host inflammatory responses, which may provide a potential candidate for promoting corneal healing and visual recovery. It is worthwhile to note that the bacterial load enhanced by siMRP8/14 also is harmful to the cornea. Therefore, further work is required to develop strategies to reduce the concurrent enhanced bacterial burden.
Footnotes
Supported by grants from the National Natural Science Foundation of China (U0832006, 81261160323, 81172811, 31200662), Guangdong Innovative Research Team Program (2009010058), Specialized Research Fund for the Doctoral Program of Higher Education of China (20100171110047), Guangdong Natural Science Foundation (10251008901000013, S2012040006680), National Science and Technology Key Projects for Major Infectious Diseases (2013ZX10003001), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2009), and NIH R01 EY019021, P30 EY04068.
Disclosure: Q. Deng, None; M. Sun, None; K. Yang, None; M. Zhu, None; K. Chen, None; J. Yuan, None; M. Wu, None; X. Huang, None
References
- 1. Rattanatam T, Heng WJ, Rapuano CJ, Laibson PR, Cohen EJ. Trends in contact lens-related corneal ulcers. Cornea. 2001; 20: 290–294 [DOI] [PubMed] [Google Scholar]
- 2. Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004; 23: 1–30 [DOI] [PubMed] [Google Scholar]
- 3. Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002; 85: 271–278 [DOI] [PubMed] [Google Scholar]
- 4. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34: 637–650 [DOI] [PubMed] [Google Scholar]
- 5. Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006; 90: 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melendez AJ, Tay HK. Phagocytosis: a repertoire of receptors and Ca(2+) as a key second messenger. Biosci Rep. 2008; 28: 287–298 [DOI] [PubMed] [Google Scholar]
- 7. Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001; 22: 189–216 [DOI] [PubMed] [Google Scholar]
- 8. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2: 907–916 [DOI] [PubMed] [Google Scholar]
- 9. Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004; 76: 909–925 [DOI] [PubMed] [Google Scholar]
- 10. Kernacki KA, Barrett RP, Hobden JA, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000; 164: 1037–1045 [DOI] [PubMed] [Google Scholar]
- 11. Rudner XL, Kernacki KA, Barrett RP, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000; 164: 6576–6582 [DOI] [PubMed] [Google Scholar]
- 12. Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007; 81: 28–37 [DOI] [PubMed] [Google Scholar]
- 13. Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003; 60: 540–551 [DOI] [PubMed] [Google Scholar]
- 14. Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983; 134: 1–6 [DOI] [PubMed] [Google Scholar]
- 15. Dorin JR, Novak M, Hill RE, Brock DJ, Secher DS, van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987; 326: 614–617 [DOI] [PubMed] [Google Scholar]
- 16. Lugering N, Stoll R, Kucharzik T, et al. Immunohistochemical distribution and serum levels of the Ca(2+)-binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn's disease. Digestion. 1995; 56: 406–414 [DOI] [PubMed] [Google Scholar]
- 17. Frosch M, Strey A, Vogl T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000; 43: 628–637 [DOI] [PubMed] [Google Scholar]
- 18. Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol. 2010; 88: 41–49 [DOI] [PubMed] [Google Scholar]
- 19. Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007; 13: 1042–1049 [DOI] [PubMed] [Google Scholar]
- 20. Sunahori K, Yamamura M, Yamana J, et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006; 8: R69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ehlermann P, Eggers K, Bierhaus A, et al. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006; 5: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007; 376: 197–204 [DOI] [PubMed] [Google Scholar]
- 23. Otsuka K, Terasaki F, Ikemoto M, et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. 2009; 11: 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sohnle PG, Collins-Lech C, Wiessner JH. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991; 163: 187–192 [DOI] [PubMed] [Google Scholar]
- 25. Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993; 151: 6291–6301 [PubMed] [Google Scholar]
- 26. Zaia AA, Sappington KJ, Nisapakultorn K, et al. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009; 2: 43–53 [DOI] [PubMed] [Google Scholar]
- 27. Akerstrom B, Bjorck L. Bacterial surface protein L binds and inactivates neutrophil proteins S100A8/A9. J Immunol. 2009; 183: 4583–4592 [DOI] [PubMed] [Google Scholar]
- 28. Simard JC, Simon MM, Tessier PA, Girard D. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol. 2011; 186: 3622–3631 [DOI] [PubMed] [Google Scholar]
- 29. Steinckwich N, Schenten V, Melchior C, Brechard S, Tschirhart EJ. An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcgammaR-mediated phagosomal oxidative activity. J Immunol. 2011; 186: 2182–2191 [DOI] [PubMed] [Google Scholar]
- 30. Wu M, Peng A, Sun M, et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating Toll-like receptor signaling and Th1/Th2-type immune responses. Infect Immun. 2011; 79: 2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang X, Du W, Barrett RP, Hazlett LD. ST2 is essential for Th2 responsiveness and resistance to pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007; 48: 4626–4633 [DOI] [PubMed] [Google Scholar]
- 32. Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol. 2009; 182: 1609–1616 [DOI] [PubMed] [Google Scholar]
- 33. Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006; 177: 548–556 [DOI] [PubMed] [Google Scholar]
- 34. Mariencheck WI, Savov J, Dong Q, Tino MJ, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of P. aeruginosa. Am J Physiol. 1999; 277: L777–L786 [DOI] [PubMed] [Google Scholar]
- 35. Kannan S, Audet A, Knittel J, Mullegama S, Gao GF, Wu M. Src kinase Lyn is crucial for Pseudomonas aeruginosa internalization into lung cells. Eur J Immunol. 2006; 36: 1739–1752 [DOI] [PubMed] [Google Scholar]
- 36. Pechkovsky DV, Zalutskaya OM, Ivanov GI, Misuno NI. Calprotectin (MRP8/14 protein complex) release during mycobacterial infection in vitro and in vivo. FEMS Immunol Med Microbiol. 2000; 29: 27–33 [DOI] [PubMed] [Google Scholar]
- 37. Gupta S, Feng L, Yoshimura T, Redick J, Fu SM, Rose CE Jr. Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am J Respir Cell Mol Biol. 1996; 15: 656–663 [DOI] [PubMed] [Google Scholar]
- 38. Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997; 65: 3847–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W, Tang Q, Hendricks RL. Ex vivo model of leukocyte migration into herpes simplex virus-infected mouse corneas. J Leukoc Biol. 1996; 60: 167–173 [DOI] [PubMed] [Google Scholar]
- 40. Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998; 39: 1854–1862 [PubMed] [Google Scholar]
- 41. Koenders MI, Marijnissen RJ, Devesa I, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011; 63: 2329–2339 [DOI] [PubMed] [Google Scholar]
- 42. Rahimi F, Hsu K, Endoh Y, Geczy CL. FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of S100A8. FEBS J. 2005; 272: 2811–2827 [DOI] [PubMed] [Google Scholar]
- 43. Benedyk M, Sopalla C, Nacken W, et al. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J Invest Dermatol. 2007; 127: 2001–2011 [DOI] [PubMed] [Google Scholar]
- 44. Pouliot P, Plante I, Raquil MA, Tessier PA, Olivier M. Myeloid-related proteins rapidly modulate macrophage nitric oxide production during innate immune response. J Immunol. 2008; 181: 3595–3601 [DOI] [PubMed] [Google Scholar]
- 45. De Lorenzo BH, Godoy LC, Novaes e Brito RR, et al. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2010; 215: 341–347 [DOI] [PubMed] [Google Scholar]
- 46. Allan BD, Dart JK. Strategies for the management of microbial keratitis. Br J Ophthalmol. 1995; 79: 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones DB. Emerging antibiotic resistance: real and relative. Arch Ophthalmol. 1996; 114: 91–92 [DOI] [PubMed] [Google Scholar]