Abstract
Drosophila has been the key model system for studies on Planar Cell Polarity (PCP). The rich morphology of the insect exoskeleton contains many structures that display PCP. Among these are the trichomes (cuticular hairs) that cover much of the exoskeleton, sensory bristles and ommatidia. Many genes have been identified that must function for the development of normal PCP. Among these are the genes that comprise the frizzled/starry night (fz/stan) and dachsous/fat pathways. The mechanisms that underlie the function of the fz/stan pathway are best understood. All of the protein products of these genes accumulate asymmetrically in wing cells and there is good evidence that this involves local intercellular signaling between protein complexes on the distal edge of one cell and the juxtaposed proximal edge of its neighbor. It is thought that a feedback system, directed transport and stabilizing protein-protein interactions mediate the formation of distal and proximal protein complexes. These complexes appear to recruit downstream proteins that function to spatially restrict the activation of the cytoskeleton in wing cells. This leads to the formation of the array of distally pointing hairs found on wings.
Keywords: Drosophila, Planar Cell Polarity, frizzled pathway, wing
1. Introduction to Drosophila Planar Cell Polarity
The cuticular surface of insects has a rich morphology that led them to be the first systems where the development of planar cell polarity (PCP) was studied in depth (see for example (Lawrence, 1966). For the last 30 years genetic studies using Drosophila have provided many of the most important insights that resulted in the blossoming of the field (Gubb and Garcia-Bellido, 1982; Held et al., 1986; Wong and Adler, 1993). These included the identification of many of the genes that play key roles in PCP (Adler et al., 1998; Chae et al., 1999; Collier and Gubb, 1997; Collier et al., 2005; Feiguin et al., 2001; Gubb and Garcia-Bellido, 1982; Klingensmith et al., 1994; Strutt and Warrington, 2008; Taylor et al., 1998; Theisen et al., 1994; Usui et al., 1999; Vinson et al., 1989; Wolff and Rubin, 1998; Yan et al., 2008; Yang et al., 2002; Zeidler et al., 1999), that the protein products of these genes accumulate asymmetrically in cells (Adler et al., 2004; Axelrod, 2001; Bastock et al., 2003; Feiguin et al., 2001; Shimada et al., 2001; Strutt, 2001; Strutt and Warrington, 2008; Tree et al., 2002; Usui et al., 1999; Yan et al., 2008), that many of the proteins interact physically (Bastock et al., 2003; Das et al., 2004; Jenny et al., 2003; Jenny et al., 2005; Wong et al., 2003) (Lu et al., 2010) and the evidence for feedback systems (Tree et al., 2002) that are a key for the functioning of the system. As has proved to be true for so much in Biology the genes that function in PCP in flies have similar functions in a wide range of animals (see (Goodrich and Strutt, 2011; Wang and Nathans, 2007) and other articles in this volume).
2. The cellular basis for PCP
Three types of adult structures have been the primary focus of experiments on PCP in Drosophila (Adler, 2002). The most important are the cuticular hairs found over much of the flies body. The wing, which is flat and where each cell elaborates a relatively long distally pointing hair has been the principal tissue for studies on hairs (Fig 1) (Gubb and Garcia-Bellido, 1982; Vinson and Adler, 1987; Wong and Adler, 1993), although they have also been studied in depth on the abdomen (Casal et al., 2006).
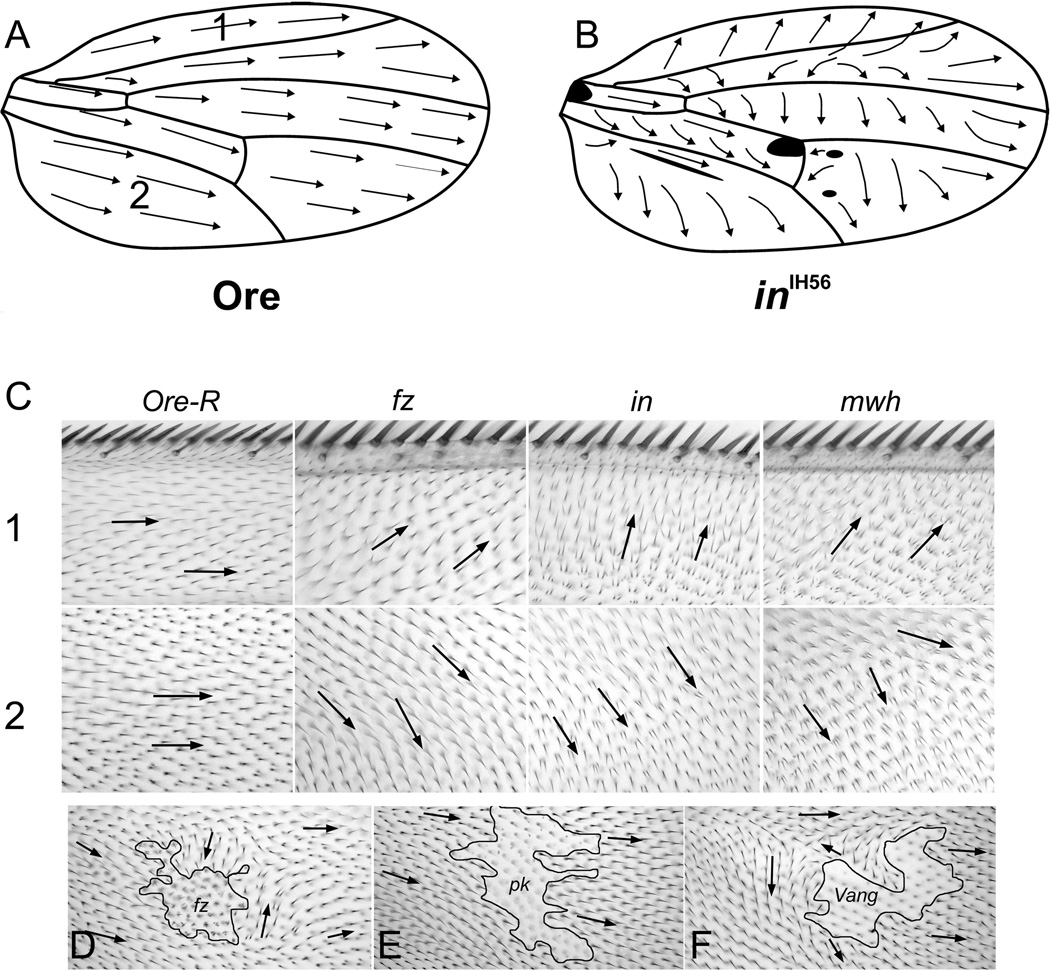
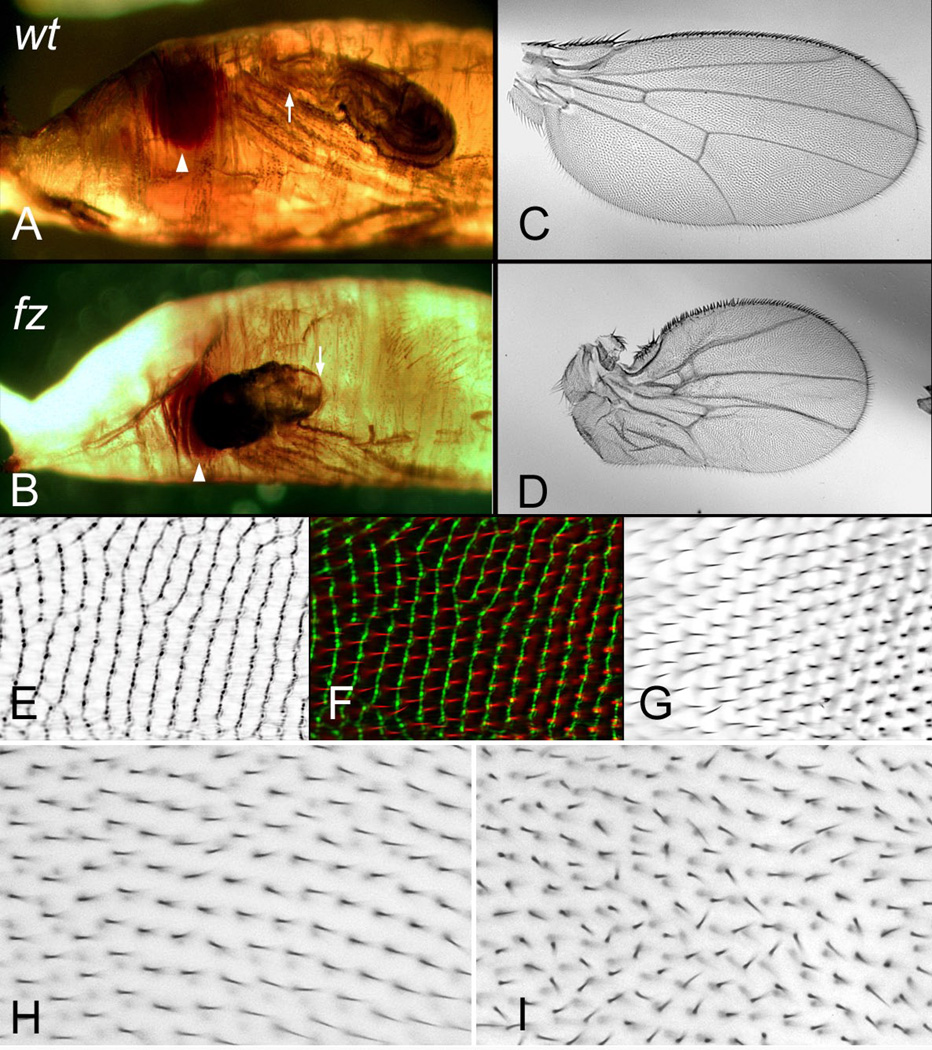
Figure 1.
Hair Polarity on the fly wing. Panel A shows the polarity of hairs on the dorsal surface of a wild type wing. Panel B shows the stereotypic pattern seen on the dorsal surface of an inturned wing. The blackened area is where the polarity was too variable to draw a consensus vector. Panel C shows images of wings from two regions (noted in panel A) of Oregon R, fz, in and mwh mutant wings. Note the relative similarity of the polarity patterns. The downstream genes ofetn show a slightly stronger polarity disruption, but with similar directionality. Panel D shows a fz clone marked by the cell marker strb (causes short deformed and multipled hairs). Panel E a pk clone marked by sha (causes a loss of hairs) and Panel F a Vang clone marked by sha. Arrows show local hair polarity.
2.1 Epidermal hairs and the fly wing
The frizzled (fz) pathway (also called the starry night (stan) pathway (Casal et al., 2006) controls PCP by restricting the site of hair initiation to the distal most part of wing cells (Wong and Adler, 1993) (Fig 2). The hairs grow out away from the distal cell periphery resulting in a distally pointing hair (Wong and Adler, 1993). Mutations in fz/stan pathway genes lead to hairs forming at abnormal subcellular locations and having abnormal polarity (Figs 1, 2). In this review I will focus on the wing with an emphasis on the fz/stan pathway. The simple cell biology and flat shape of the wing make it an ideal tissue for studies on Planar Cell Polarity and the principles deduced from studying it have for the most part proved to be conserved in other tissues in the fly and in other organisms.
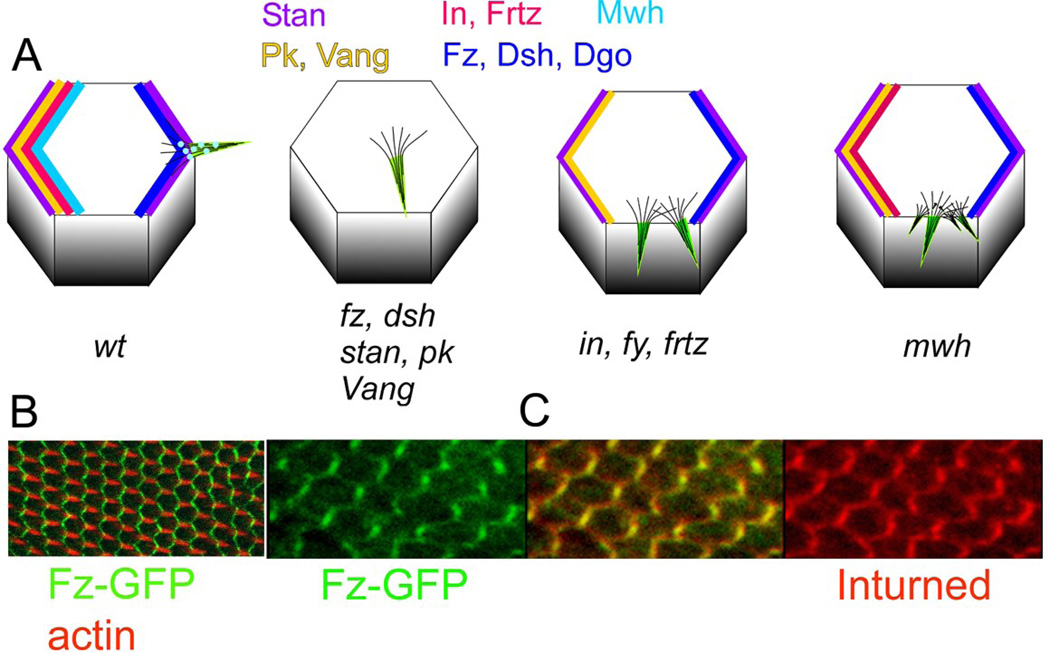
Figure 2.
Polarized protein accumulation and mutant phenotypes. Panel A shows cartoons of wild type and mutant cells. In the wild type cell all of the fz/stan pathway proteins accumulate asymmetrically and hairs are formed at the distal vertex. In cells mutant for fz or other core genes hairs form at a relatively central location and no proteins accumulate asymmetrically. In cells mutant for in or any of the other PPE genes multiple hairs form at an abnormal location on the cell periphery. The core proteins accumulate asymmetrically but the PPE proteins and Mwh do not. In cells mutant for mwh genes multiple hairs form at an abnormal location on the cell periphery. The core and PPE proteins accumulate asymmetrically but Mwh does not. Panel B shows the accumulation of Fz-GFP by direct imaging opf GFP (green) (no immunostaining) and actin in red (hairs). Panel C shows the coordinate asymmetric accumulation of Fz (green) and In (red).
2.2 Sensory Bristles
The cuticular surface of the fly is also decorated with many bristle sense organs. On the dorsal thorax and abdomen the bristle shaft points posteriorly and on appendages they point distally (Adler, 2002) (Fig 3AC). In other body regions (e.g. the head) bristles are locally aligned and show a reproducible polarity. The fz pathway regulates bristle polarity by controlling the orientation of the spindle during the determinative cell divisions that give rise to the cells of the sense organ (Gho and Schweisguth, 1998; Lu et al., 1999)(Fig 3B). On the leg some bristle sense organs signal to a neighboring epidermal cell to form a bract (Fig 3C). This involves the EGFR pathway and this system displays planar polarity as the bract is routinely proximal to the bristle (Held, 2002)(Fig 3). Most leg bristles do not show strongly abnormal polarity in fz/stan pathway mutants but in those that do the bract is coordinately positioned "upsteam" (Fig 3D).
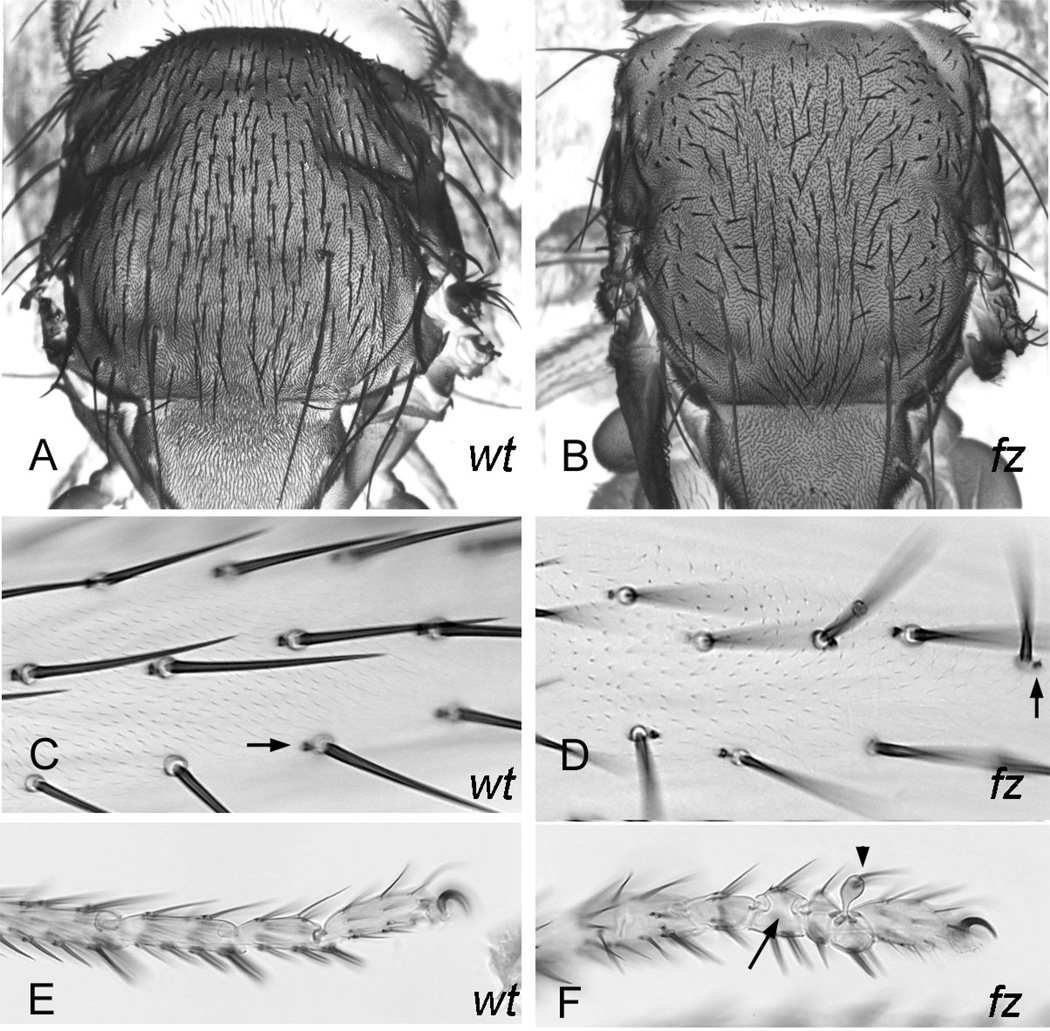
Figure 3.
Bristles and PCP. Panel A is an SEM of a wild type thorax and panel B a fz thorax. Panel C is a wild type femur and panel D a fz femur. Arrows point to bracts. Panel E is a wild type tarsus and panel F a fz tarsus. The arrow points to a segment with a mirror image duplication and the arrowhead to a bulge.
2.3 The eye
The third developmental unit that has been extensively studied with respect to PCP are the ommatidia of the compound eye (Zheng et al., 1995). Each ommatidia is comprised of about 20 cells including 8 photoreceptor cells. The arrangement of the photoreceptor cells is asymmetric and chiral giving each ommatidia and the eye as a whole a polarity (Fig 4A). As each ommatidia develops there is a stepwise recruitment of photoreceptor cells (for a recent review of this see (Kumar). The R3 and R4 cells are recruited at the same time. The cell located closer to the equator becomes R3 and the other R4 (Fig 4A). The ommatidia undergo a subsequent rotation and this is guided by the position of the R3 and R4 cells (Fig 4B). The fz pathway controls PCP by regulating the R3/R4 cell fate decision (Zheng et al., 1995). This is based on the R3 cell having higher Fz activity and R4 higher Vang (Strutt et al., 2002b). This leads to the Delta dependent activation of the Notch (N) receptor in R4, which controls cells fate (Cooper and Bray, 1999; Fanto and Mlodzik, 1999; Tomlinson and Struhl, 1999). There is an argument in the literature with regard to the mechanism involved in N activation and how this regulates cell fate (del Alamo and Mlodzik, 2006; Fanto and Mlodzik, 1999; Strutt et al., 2002b; Weber et al., 2000). Recent data argues that the Ral GTPase also plays a role in R3//R4 cell fate (Cho and Fischer, 2011).
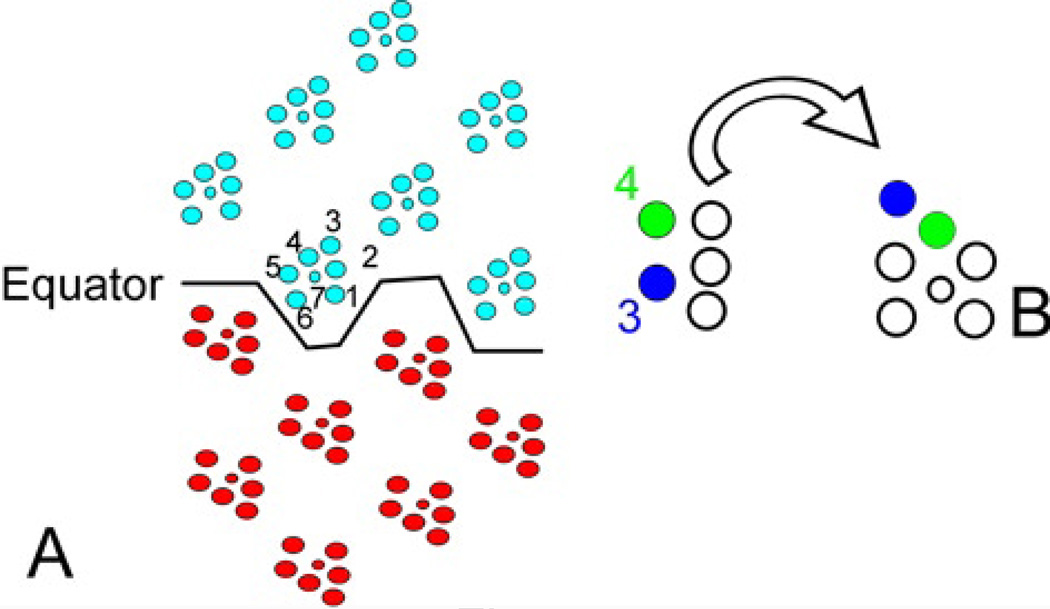
Figure 4.
The eye and PCP. Panel A is a cartoon of the adult retina with Northern (blue) and Southern (red) hemisphere ommatidia in mirror image across the equator. Panel B shows how the pattern in the adult retina is derived by rotation of the developing retina. The crucial R3 (blue) and R4 (green) cells are marked.
2.4 Other manifestations of PCP
There are several other cell types/structures/tissues where fz/stan based PCP has been studied. Collier and colleagues discovered that the fly wing has parallel cuticular ridges that and that their orientation is under the control of the fz/stan and ds/ft pathways (Fig 5EFG) (Valentine and Collier, 2011). Interestingly, the ridges are oriented differently in the anterior and posterior parts of the wing and this appears to be related to differences between two phases of fz signaling that utilize different pk isoforms (Doyle et al., 2008; Hogan et al., 2011; Valentine and Collier, 2011). Their data also argues that the early phase is dependent on ds/ft while the latter is not. The lateral side branches of the arista are another body part where the fz pathway has been found to regulate PCP (He and Adler, 2002). The laterals are produced by polyploid cells and share features of both epidermal hairs and bristles. As is the case for epidermal hairs the fz pathway controls the subcellular location for lateral outgrowth although in this cell type the outgrowth is not juxtaposed to the distal most edge of the cell in wild type (He and Adler, 2002).
Figure 5.
Less well known PCP related phenotypes. Panels A and B show wild type and fz pupae respectively. In a modest fraction of fz pupae the wing everts forward instead of posteriorly (as shown in B). Arrowheads point to the eye and arrows to the wing hinge region. Panel C is a wild type wing and Panel D is a distorted wing that results from the abnormal eversion as shown in B. Panel EFG are wings imaged by Cuticle Reflection microscopy (E) and normal bright field microscopy (G) and a merged image (F). These panels were generously provided by S. Collier. Panel H is a wild type wing and panel I the same region of a wing from a fly homozygous for a mutation in Gliotactin. Note the lack of parallel alignment of neighboring hairs in I.
The denticles that are part of the larval cuticle also display PCP and they have been intensively used as markers of pattern formation in embryonic segments. In recent years they have also been used to study PCP (Donoughe and DiNardo, 2011; Price et al., 2006; Repiso et al., 2010). Here as in the adult abdomen the fz/stan and ds/ft systems appears to function in parallel.
The fz/stan pathway, which plays such a key role in PCP has also been found to regulate a number of processes that are not always thought of as being related to PCP. The first of these to be identified was the formation of the leg joints (Held et al., 1986). Mutations in genes such as fz give rise to defective leg joints that often show mirror image symmetry typical of duplications (Fig 3EF). One often also sees "blebs". The cellular basis for this has not been established.
Mutations in genes such as fz also lead to occasional defects in wing evagination so that the resulting wing points anteriorly in the pupal case (Fig 5AB)( Lee and Adler, 2002). Such wings end up shorter and deformed, perhaps due to defects in cell rearrangements. This could be related to the effects of the fz pathway on the movement of sheets of cells in vertebrates (Goodrich and Strutt, 2011). Mutations in genes such as fz also inhibit the repacking of pupal wing cells so that the fraction of hexagonal cells is decreased compared to wild type (Classen et al., 2005). The connection between the altered hair polarity and the effect on the repacking is unclear (Classen et al., 2005; Ma et al., 2008). An interesting and related observation is that cell rearrangements in the pupal wing lead to changes in the anatomical orientation of fz/stan signaling (Aigouy et al., 2011).
Mutations in fz/stan pathway genes have also been found to alter the pattern of dendrites and axons in some Drosophila neurons (Matsubara et al., 2011; Mrkusich et al., 2011). In both cases it remains to be established as to how much of the pathway is conserved in different tissues. A similar very wide range of cell types have been found to utilize fz pathway homologs in vertebrates (Goodrich and Strutt, 2011; Wang and Nathans, 2007).
2.5 Planar Cell Polarity that is not fz/stan pathway dependent
There are also several interesting cases of Planar Cell Polarity in Drosophila that are not dependent on the fz/stan pathway. The most notable being the polarized cell rearrangements that mediate germ band elongation (Bertet et al., 2004; Zallen, 2007; Zallen and Wieschaus, 2004) and the polarized actin cytoskeleton seen in the follicle cells of developing egg chambers (Bateman et al., 2001; Frydman and Spradling, 2001). Interestingly the fat2 gene is required for actin polarization in follicle cells (Viktorinova et al., 2009).
3. PCP Genes
3.1 Early studies on the fz/stan pathway
Several of the genes of the fz/stan pathway were discovered long ago and were used as makers in classical genetic experiments (see Table 1). These include fz, prickle (pk), dishevelled (dsh), inturned (in) and multiple wing hairs (mwh). Other fz/stan pathway genes were discovered in screens designed to identify new PCP genes. These include stan (also known as flamingo)(Chae et al., 1999; Usui et al., 1999), Van Gogh (Vang) (also known as strabismus) (Taylor et al., 1998; Wolff and Rubin, 1998) and fritz (frtz)(Collier et al., 2005). (Note in this review we will use the primary FlyBase name for all Drosophila genes). The first detailed study on the function of these genes in PCP was published about 30 years ago by Gubb and Garcia-Bellido (Gubb and Garcia-Bellido, 1982). Several important conclusions came from this work. One was that the mutant hair polarity patterns seen on wings were not random. Rather, the mutant pattern was stereotypic. Thus, cells in the anterior region of a fz mutant wing routinely point more anteriorly than do wild type (Fig 1C). A second important insight was that altering wing shape (by mutation) did not make substantial differences to the stereotypic fz mutant polarity pattern. Similarly, the loss of part of the wing margin due to mutation did not affect the mutant polarity pattern.
Table 1.
Key Drosophila Planar Cell Polarity Genes
| Drosophila gene | synonyms | Vertebrate | Comments | Key Drosophila References |
|---|---|---|---|---|
| fz | fz (1,2,3,6) | 7 transmembrane domains | (Strutt, 2001; Vinson and Adler, 1987; Vinson et al., 1989)* | |
| dsh | Dvl (1,2,3) | PDZ, Dix domains | (Axelrod, 2001; Klingensmith et al., 1994; Theisen et al., 1994) | |
| dgo | Inversin diversin | Ankyrin repeat | (Feiguin et al., 2001) | |
| stan | fmi | Celsr | Cadherin domains, 7 transmembrane domains | (Chae et al., 1999; Usui et al., 1999) |
| pk | pk (1,2) | Lim domain, PET domain | (Gubb et al., 1999; Tree et al., 2002) | |
| Vang | stbm | Vangl (1,2) | 4 transmembrane domains | (Taylor et al., 1998; Wolff and Rubin, 1998) |
| in | into | PDZ domain | (Adler et al., 2004; Park et al., 1996) | |
| fy | fuz | (Collier and Gubb, 1997; Strutt and Warrington, 2008) | ||
| frtz | frtz | WD40 domain | (Collier et al., 2005) | |
| mwh | none | GBD-FH3 | (Strutt and Warrington, 2008; Yan et al., 2008) | |
| ds | Dchs1 | Cadherin domains | (Adler et al., 1998; Casal et al., 2006; Clark et al., 1995; Ma et al., 2003; Matakatsu and Blair, 2006; Simon, 2004) | |
| ft | Fat | Cadherin domains | (Adler et al., 1998; Casal et al., 2006; Ma et al., 2003; Mahoney et al., 1991; Matakatsu and Blair, 2006; Simon, 2004) | |
| fj | Fj | Golgi kinase | (Simon et al., 2010; Villano and Katz, 1995; Zeidler et al., 1999) | |
| Rho1 | RhoA | GTPase | (Strutt et al., 1997; Yan et al., 2009) |
The insight that a set of genes comprised a regulatory pathway that controlled PCP came from Wong and Adler (Wong and Adler, 1993). They made several key findings. They realized that mutations in all of the fz/stan pathway genes gave rise to similar stereotypic polarity patterns (Fig 1ABC) but that genes could be placed into phenotypic groups by the frequency of extra hairs produced (Fig 2A). The 3 phenotypic groups were also epistasis groups and it is now clear that they represent different levels in the regulatory hierarchy. The upstream genes (often called core genes) consist of fz, dsh, pk, stan, Vang and dgo (note stan (Chae et al., 1999; Usui et al., 1999), Vang (Taylor et al., 1998; Wolff and Rubin, 1998) and dgo (Feiguin et al., 2001) had not been discovered at the time of the paper). Below the core group are in, fy and frtz, which are often referred to as the Planar Polarity Effector (PPE) genes (note frtz had not been discovered at the time of the paper (Collier et al., 2005). The mwh gene was the only member of the third group. They also found that in wild type pupal wing cells the cytoskeleton was activated in the vicinity of the distal most vertex to promote hair outgrowth and this resulted in a hair that grew distally. Further, mutations in all of the fz pathway genes resulted in hairs forming at an alternative subcellular location. An important insight from the analysis of the pupal phenotype was that the fz/stan pathway regulated hair initiation.
The observation that fz pathway proteins accumulated asymmetrically in wing cells was first observed by Uemura and colleagues studying the stan/flamingo gene (Usui et al., 1999) and this breakthrough opened up a new stage in PCP research (Fig 2AB). Over the next few years similar results were obtained for all of the fz/stan pathway proteins (Adler et al., 2004; Axelrod, 2001; Bastock et al., 2003; Feiguin et al., 2001; Shimada et al., 2001; Strutt, 2001; Strutt and Warrington, 2008; Tree et al., 2002; Usui et al., 1999; Yan et al., 2008). Further biochemical experiments established that several pairs of these proteins could interact directly with one another (Bastock et al., 2003; Das et al., 2004; Jenny et al., 2003; Jenny et al., 2005; Wong et al., 2003) (Lu et al., 2010) . A more detailed discussion of the function of these genes and how the asymmetric accumulation of the proteins is achieved is presented later.
The fz/stan pathway functions to limit the region of the cell where the cytoskeleton is activated to form a hair. Not all wing cells are the same size, nor are all epithelial cells in other body regions the same size or shape as wing cells. Further, nutrition and the temperature during development can alter cell size without altering planar polarity. Hence the pathway has the ability to scale and function in a variety of cell geometries. It is clear however that this ability is not infinite. Cells that are much larger than normal can arise from the cells being polyploidy or stretched due to wound healing. Both of these conditions result in the cells often forming multiple hairs that can be of abnormal polarity (Adler et al., 2000b). The fz/stan pathway is still functioning in these large cells but it is unable to regulate the cytoskeleton well enough so that a single distally pointing hair is produced.
3.2 ds/ft pathway
Mutations in several other groups of genes also give rise to PCP phenotypes. The atypical cadherins dachsous and fat and the golgi kinase four jointed were first discovered to result in PCP phenotypes due to their leg joint phenotypes (Held et al., 1986). Later it was established that they were also required for wing and eye PCP (Adler et al., 1998; Matakatsu and Blair, 2004; Simon, 2004; Zeidler et al., 1999). A variety of evidence indicates that Ft and Ds act as a receptor ligand pair and that Fj modulates their activity by phosphorylation (Brittle et al., 2011; Matakatsu and Blair, 2004; Simon, 2004; Simon et al., 2010; Sopko et al., 2009). One observation that does not fit this model is the observation that transgene supplied Ft that lacks the extracellular domain provides rescue of the ft PCP phenotype over most of the wing (Matakatsu and Blair, 2006). How to reconcile these observations is not clear. The Ft and Ds proteins do not appear to accumulate asymmetrically as do fz/stan pathway proteins however they are able to polarize cells. This is seen both by their effect on hair polarity and by their activity leading to the asymmetric accumulation of the atypical myosin Dachs (Mao et al., 2006; Mao et al., 2011). Early studies found that the fz/stan pathway was functional in ds and ft mutants but that it signaled in an anatomically abnormal way (Adler et al., 1998) and subsequently it was proposed that that the ds/ft pathway functioned as a global signal that oriented the activity of the fz/stan pathway with respect to the body as a whole in the wing and eye (Ma et al., 2003; Simon, 2004; Yang et al., 2002). However, studies by Casal, Lawrence and Struhl provided compelling data that in the abdomen that the ds/ft and fz/stan pathways functioned in parallel (Casal et al., 2006). For example, double mutants of one ds/ft and one fz/stan pathway gene had a more severe phenotype than any single mutant or double mutant where only one of the pathways was affected. They also showed that ds/ft could repolarize cells that were mutant for the fz/stan pathway. One interpretation of these observations is that the relationship between the two pathways is different in different tissues. This is not a satisfying explanation, however it may be correct. More recent experiments suggest that ds/ft may alter fz/stan signaling in indirect ways by affecting the axis of cell division, cell rearrangements and the polarization of microtubules that are involved in the transport of Fz containing vesicles (Harumoto et al., 2010; Mao et al., 2011). There is increasing evidence that ds/ft also function in vertebrate PCP (Goodrich and Strutt, 2011).
3.3 Septate Junction proteins
A third set of genes essential for the morphogenesis of normal wing PCP are genes such as Gliotactin, Neuroglian, Coracle and varicose (Moyer and Jacobs, 2008; Venema et al., 2004). These genes encode proteins that are associated with the septate junction and they are required for the alignment of neighboring hairs (Fig 5HI). The septate junction is the invertebrate equivalent of the vertebrate tight junction. These genes appear to act after hair outgrowth and unlike the fz/stan pathway mutations in these genes do not result in cells forming multiple hairs. The asymmetric localization of Dsh is not altered in a Gli mutant and the localization of Coracle is not affected by mutations in fz/stan genes Venema, 2004 #71}. Further, double mutants of septate junction and fz/stan genes show an additive phenotype consistent with their functioning in parallel. How the septate junction genes function in PCP has not been extensively studied. It is also not clear if this set of genes is needed for PCP in other contexts in Drosophila or in other organisms.
3.4 Other genes that impact PCP
In recent years many additional genes have been identified that are required for PCP in the fly. The literature is somewhat inconsistent as to the properties of a gene required to consider it a PCP gene. Some authors require a hair/bristle orientation phenotype (or ommatidia of the incorrect chiral type), while others have simply required cells form multiple hairs (of normal orientation) or result in misrotated ommatidia of the correct chiral type. As is discussed in more detail below many aspects of general cell biology are likely involved in polarizing cells hence it is not surprising that genes that encode a wide variety of protein types and functions have been found to produce PCP phenotypes. These include some that might be thought of as having rather general functions. Not surprisingly, these genes differ from those of the fz/stan pathway components in being much less specific with regard to their mutant phenotypes. The collection of genes include ones that encode proteins that function in intracellular trafficking such as Rab23 (Pataki et al., 2010), Rab5 (Purvanov et al., 2010) and the Rab5 effector Rabenosyn-5 (Mottola et al., 2010). Mutations in genes that encode proteins that function to promote actin filament disassembly (twinstar (cofilin) (Blair et al., 2006) and flare (AIP1) (Ren et al., 2007) have been found to result in PCP phenotypes and disruptions in the normal asymmetric accumulation of fz/stan pathway proteins. Also implicated in PCP are genes that encode transcription regulators such as grainy head (grh) (Lee and Adler, 2004) and atrophin (Fanto et al., 2003), kinases such as misshapened (Paricio et al., 1999), Rho Kinase (Winter et al., 2001), the Abelson tyrosine kinase (Singh et al.), aPKC (Djiane et al., 2005), casein kinase I (Strutt et al., 2006), and the gilgamesh kinase (Gault et al., 2012), the Widerborst phosphatase (Hannus et al., 2002), the approximated Palmitoyltransferase (Matakatsu and Blair, 2008) and small GTPases such as Rho1 (Strutt et al., 1997; Yan et al., 2009) and Go (Purvanov et al., 2010). An interesting and unexpected group of genes that are required for normal PCP function in ion transport including the Na+/H+ exchanger Nhe2 (Simons et al., 2009), a component of the vesicle ATPase (Buechling et al., 2010; Hermle et al., 2010) and an organic anion transporter, oat30B(Mummery-Widmer et al., 2009). The effect of Nhe2 was suggested to be mediated through an effect on the interaction of Dsh and plasma membrane lipids. Many of the genes noted above produce mutant phenotypes that suggest they could function in concert with or affect the function of either the fz/stan or ds/ft pathways. For example, the PCP phenotype that results from a loss of grh function in wing cells was shown to be associated with a loss of stan expression in the mutant cells (Lee and Adler, 2004). A notable and interesting exception was the identification of chascon and jitterbug, which affect notum PCP by impacting the tendons that attach the indirect flight muscles to the notum (Olguin et al., 2011). In this case a loss of the ability of the tissue to respond to the mechanical tension produced by the IFMs appears to be the mechanism responsible for the PCP mutant phenotype.
3.5 Genome wide screen for PCP genes
A genome wide screen based on transgene mediated RNAi that was designed to identify genes that played a role in Notch signaling also identified many genes required for PCP (Mummery-Widmer et al., 2009). In this screen pannier-Gal4 was used to drive transgene expression. pannier is expressed in the central region of the notum, hence it is an ideal driver to use to look for effects on bristle sense organ determination and differentiation. Effects on PCP in this screen were manifested by the misalignment of notum bristles. Satisfyingly this screen identified in order of the strength of phenotype fz, Vang, frtz, pk, stan, fy, in, ft, and ds. Thus most of the fz/stan and ds/ft pathway genes were identified confirming that the screen was very effective. The screen failed to identify dgo, fj and mwh. The failure to identify dgo and mwh is not surprising as null alleles of dgo and mwh do not show a bristle polarity phenotype (Feiguin et al., 2001; Strutt and Warrington, 2008; Yan et al., 2008). This is not the case for fj and the failure to identify fj may simply reflect the transgene not knocking down the expression enough to produce a mutant phenotype. It is unlikely that this screen identified all of the genes that play a role in PCP. Some genes would likely be missed due to the knock down not being effective (as is presumably the case for fj). Other genes would likely be missed due to the knock downs resulting in lethality or a loss of bristles due to effects on the determinative cell divisions. This seems likely to be the case for genes involved in essential basic cellular functions, and indeed genes that are important for bristle morphogenesis such as Rab11 and exocyst components were missed in the genome wide screen for this reason (Nagaraj and Adler, 2012). This screen has already served as a valuable source of candidate PCP genes. For example, VhaM8.9 (Buechling et al., 2010) and jitterbug (Olguin, 2011) were both identified in this screen and subsequently shown in other studies to produce PCP phenotypes. It is interesting to consider that many of the genes that produced the strongest phenotypes in the screen have not yet been the focus of PCP papers. A knockdown of the Organic anion transporting polypeptide 30B produced the strongest PCP phenotype (along with fz). In the wing a knockdown of this gene can reverse hair polarity much as the over expression of the spiny leg isoform of the pk gene can. It will be interesting to see if these two proteins function in an antagonistic way. Among the other genes where knock downs produced very strong phenotypes were d-Cup (a Ca++ binding protein), CG17290 (which contains a gyrase motif), Protein tyrosine phosphatase 69D, CG15649 (a novel protein) and CG18005 (which contains RED domains suggesting a role in RNA splicing). The roles that these genes and their encoded proteins play in PCP can currently only be guessed at. It is notable that even this small set of candidate genes and proteins suggests a wide range of biochemical functions. It seems likely that much remains to be learned about PCP.
4. What is the basis for the asymmetric localization of fz/stan pathway proteins?
All of the fz/stan pathway proteins accumulate asymmetrically in wing cells, with all except Stan accumulating on either the proximal or distal cell membrane (Stan is found on both) (Adler et al., 2004; Axelrod, 2001; Bastock et al., 2003; Feiguin et al., 2001; Shimada et al., 2001; Strutt, 2001; Strutt and Warrington, 2008; Tree et al., 2002; Usui et al., 1999; Yan et al., 2008) (Fig 2A). The accumulation is uneven across these membranes. That is, some locations along these membranes show a higher level of protein than others and all of the fz/stan pathway proteins appear to be enriched at the same foci on the membrane (Fig 2B). This suggests that the proteins accumulate in a synergistic way. Further, all of the core group proteins must be functional for the normal asymmetric accumulation to occur (Fig 2A).
4.1 Interactions of transmembrane proteins
The distal edge of one cell is juxtaposed to the proximal edge of the neighboring cell distal to it (Fig 6). This provides an interface for asymmetric signaling that is likely to be part of a positive feedback mechanism that helps establish the distinct proximal and distal membrane domains. Several groups have proposed that interactions between the three fz/stan pathway proteins that are transmembrane proteins, Fz, Vang and Stan are a key to this process (Chen et al., 2008; Strutt and Strutt, 2008; Wu and Mlodzik, 2008). A variety of data supports these models. These include biochemical evidence for an interaction and in situ protein localization studies. There are however, differences between experimental results and the interpretations reported by these groups so at the current time there is not a consensus for how these proteins interact and provide for polarized signaling. It will be important for these differences to be resolved; however the general conclusion that heterotypic protein interactions provide the basis for the intercellular signaling between cells appears to be on solid ground.
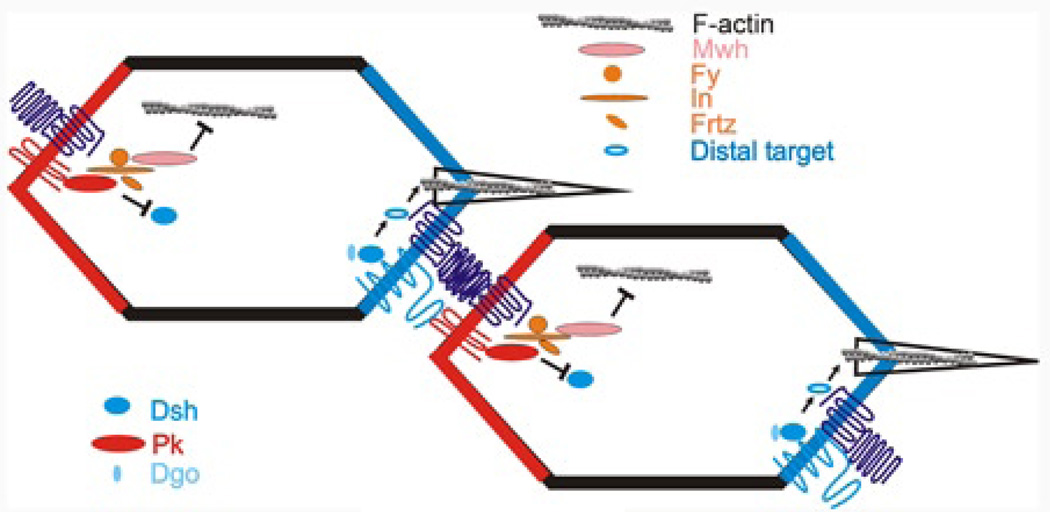
Figure 6.
A possible model for PCP protein action in wing cells. Two neighboring cells are diagrammed. Proteins on the distal membrane of one cell (Fz and Stan) interact with proteins on the proximal membrane of the neighboring cell (Vang and Stan). The core transmembrane proteins recruit the cytoplasmic core proteins (Dsh, Dgo and Pk). The PPE proteins are recruited to the proximal side where In recruits Mwh. Mwh inhibits the actin cytoskeleton in the distal part of the cell. Dsh activates an activator of the actin cytoskeleton distally. For simplicity only one of the feedback interactions between cytoplasmic proteins is drawn (Pk inhibiting Dsh on the distal side).
4.2 Intracellular proteins
It is likely that in addition to intercellular signaling an intracellular feedback system plays an important role in the formation of the proximal and distal protein domains (Amonlirdviman et al., 2005; Tree et al., 2002). Several interactions have been detected between proximal and distal proteins. For example, Dsh and Vang, Dsh and Pk, Dgo and Pk, and Dgo and Vang have been found to interact (Bastock et al., 2003; Das et al., 2004; Jenny et al., 2003; Tree et al., 2002). The interactions between proximal and distal proteins appear to be negative ones. Hence, a negative interaction between Pk and Dsh could prevent Dsh from accumulating on the proximal side and Pk from accumulating on the distal side (Fig 6). Such models suggest the interactions be dependent on cellular location. For example, when Pk and Dsh interact on the proximal side of the cell, Dsh would be lost. A hint as to how this may work comes from experiments on mice where it was found that a Smurf ubiquitin ligase targets Pk for proteosome mediated degradation (Narimatsu et al., 2009). As of yet no equivalent experimental evidence has been published that a similar mechanism works in the fly (e.g. Pk interacting with Dsh results in Dsh being degraded in a proteosomal dependent mechanism), but it is certainly an attractive hypothesis to explain the negative protein interactions.
4.3 Wnts in fly PCP?
The fz gene family gets its name from the PCP phenotype of its founding member, but Fz proteins are very well known as a family of receptors for Wnt ligands (Bhanot et al., 1996). However, in Drosophila studies have failed to detect PCP phenotypes in genotypes where one or multiple Wnts are lost due to mutation (Chen et al., 2008; Lawrence et al., 2002). In vertebrate tissues there is substantial evidence for Wnt ligands functioning in PCP (see (Goodrich and Strutt, 2011; Wang and Nathans, 2007) and other articles in this volume).
4.4 Mechanisms for the formation of PCP protein complexes
There are several possible mechanisms that could be responsible for the asymmetric accumulation of PCP proteins on the proximal or distal membranes. One would be for the proteins to be preferentially synthesized at those locations (Berleth et al., 1988; Davis and Ish-Horowicz, 1991; Macdonald and Struhl, 1988). A second would be the directed trafficking of the proteins to the correct side of the cell (Shimada et al., 2006; Strutt et al., 2011). A third would be trapping of proteins to a domain by binding to a target (Strutt et al., 2011). A fourth possibility would be for the rate of diffusion of the protein to be regulated spatially across the cell (Griffin et al., 2011).
4.5 mRNA localization?
The data do not support the hypothesis for localized synthesis as in situ hybridization experiments have failed to show evidence for mRNA localization. Further, in other systems the localization of a mRNA is usually mediated by sequences outside of the coding region (see for example (Ferrandon et al., 1994; Macdonald and Struhl, 1988). However, in fly PCP fully functional transgenes encode mRNAs that lack most of the 5' and 3' untranslated regions (see for example (Krasnow and Adler, 1994).
4.6 Directional trafficking and trapping
There is evidence supporting the involvement of both directed transport and trapping in the asymmetric accumulation of PCP proteins. Uemura and colleagues have used in vivo confocal imaging to determine that Fz and Stan preferentially traffic along the proximal distal axis of wing cells as opposed to along the anterior-posterior axis (Shimada et al., 2006). They also provided evidence that the trafficking is along the web of apical microtubules that show a proximal/distal bias (Turner and Adler, 1998). This bias in the orientation of microtubules appears to be at least partially mediated by the action of the ds/ft pathway. This may provide an explanation for the ability of the ds/ft pathway to bias fz/stan activity (Harumoto et al., 2010). It is not clear however if the bias for movement along the proximal distal axis is great enough to explain everything. Further, the in vivo observations showed that Fz containing puncta could fuse with accumulations on the membrane and that this appeared to be reversible (Shimada et al., 2006; Strutt et al., 2011). Recent results of Strutt and colleagues (Strutt et al., 2011) obtained by FRAP experiments established that the 3 transmembrane proteins Fz, Stan and Vang are all required for the stable asymmetric accumulation of PCP protein complexes and that the cytoplasmic proteins Dsh, Pk and Dgo are needed for the formation of large puncta. The cytoplasmic proteins could be recruited to the proximal and distal domains by interacting with the complexes of transmembrane domains leading to further stabilization and an increase in the size of the complexes. Consistent with that possibility Dsh has been shown to bind to Fz (Wong et al., 2003) and Pk has been shown to bind to Vang (Bastock et al., 2003; Jenny et al., 2003). The negative protein interactions between cytoplasmic proteins noted above could also serve to remove mislocalized proteins and hence to increase the asymmetry.
Taken together with earlier observations one is lead to a model where directed transport leads to a modest bias in the localization of the 3 transmembrane proteins. Direct protein interactions across the juxtaposed distal and proximal membrane domains would stabilize proximally localized Vang and distally localized Fz and hence increase the bias in protein localization. The binding of the cytoplasmic proteins would further stabilize and increase the size of the protein complexes. Thus, the formation of the asymmetric protein complexes would involve several layers of mechanisms.
5. Directional cell non-autonomy
One striking property of fz and Vang mutant cells is the directional domineering nonautonomy displayed by clones (Taylor et al., 1998; Vinson and Adler, 1987)(Fig 1DF). This differs from the essentially cell autonomous activity of other fz pathway genes (Fig 1E)(these other genes show very limited cell nonautonomy (rare or limited to neighboring cells) (Chae et al., 1999; Collier and Gubb, 1997; Collier et al., 2005; Gubb and Garcia-Bellido, 1982; Lee and Adler, 2002; Park et al., 1996). The polarity of wild type cells distal to fz clones and proximal to Vang clones are affected. This is due to hairs pointing toward cells of lower Fz activity and toward higher Vang activity (Adler et al., 2000a; Adler et al., 1997; Casal et al., 2006; Strutt and Strutt, 2007). Complementary effects are seen when cells are manipulated to express higher Fz or Vang levels (Adler et al., 2000a; Adler et al., 1997).
5.1 The basis for directional cell non-autonomy
Early evidence suggested that fz had both cell autonomous and cell nonautonomous functions (Vinson and Adler, 1987). Several fz alleles behaved cell autononmously (Vinson and Adler, 1987) and that the cell nonautonomous function of fz was independent of dsh and preceded and was temporally separate from the cell autonomous function (Lee and Adler, 2002; Strutt and Strutt, 2002). It is worth noting that the PCP system does not require patterned expression of any of the fz pathways genes as even expression of these genes provides complete rescue of null alleles (Axelrod, 2001; Bastock et al., 2003; Collier and Gubb, 1997; Collier et al., 2005; Feiguin et al., 2001; Krasnow and Adler, 1994; Park et al., 1996; Strutt, 2001; Strutt and Warrington, 2008; Usui et al., 1999; Yan et al., 2008). The basis for the domineering nonautonomy has been studied at length. Early studies established that it was a property of abnormal local signaling between cells (Adler et al., 2000a). The current consensus model is that the intercellular signaling between neighboring cells due to the asymmetric accumulation of PCP proteins is propagated on a cell by cell basis and this is the basis for the domineering nonautonomy (Amonlirdviman et al., 2005; Goodrich and Strutt, 2011; Maung and Jenny, 2011; Tree et al., 2002; Wu and Mlodzik, 2008). Thus a cell with no Fz cannot recruit Vang to the juxtaposed membrane (Wu and Mlodzik, 2008). This leads to a high level of Vang on the side of the cell distal to the mutant membrane. This would be propagated for a number of cells and such a system could be at the heart of the intercellular signaling required for PCP. One drawback to such a model is that the function of a gene such as dsh that acts cell autonomously is required for the antisymmetric accumulation of other PCP proteins (Axelrod, 2001; Klingensmith et al., 1994; Strutt, 2001; Theisen et al., 1994; Tree et al., 2002; Usui et al., 1999). At first glance this result would seem to contradict the asymmetry propagation model. However, computer simulations indicate that the model can explain the observations (Amonlirdviman et al., 2005; Schamberg et al., 2010) as only modest levels of asymmetry may be required for signaling and this may be difficult to detect in immunostaining experiments. There remains however, a need for an explanation for the results that indicate the cell nonautonomous function precedes the cell autonomous function (Strutt and Strutt, 2002).
It is worth noting that this mechanism works well to explain both normal PCP development as well as domineering non-autonomy in a tissue where all cells express the fz/stan pathway proteins and show the asymmetric accumulation. In the eye only the R3 and R4 cells need to express fz pathway genes for the development of normal PCP (Zheng et al., 1995). In these cells fz/stan pathway proteins accumulate asymmetrically with proteins that accumulate on the distal side of wing cells accumulating in the R3 cell membrane that is juxtaposed to the R4 cell (Strutt et al., 2002a). Similarly, proteins that accumulate on the proximal side of wing cells accumulate on the R4 cell membrane that is juxtaposed to R3 (Strutt et al., 2002a). Thus, heterotypic interactions across cell membranes seems likely to be a mechanism used in the generation of the two domains (for example see (Wu and Mlodzik, 2008.
6. Downstream Effectors
6.1 Planar Polarity Effector (PPE) Genes
The 3 planar polarity effector proteins, In, Fy and Frtz are recruited to the proximal side of wing cells in a core group dependent manner (Fig 2A) (Adler et al., 2004; Strutt and Warrington, 2008). For example, in a fz mutant the asymmetric accumulation of In and Frtz is lost and indeed these proteins are found at much lower levels in such a mutant. It seems likely that at least one of these proteins is recruited by binding (either directly or indirectly) to either Pk or Vang, the two proximal specific proteins. As is the case for the upstream genes, each of the PPE genes need to be functional for the proteins to preferentially accumulate on the proximal side (Adler et al., 2004; Strutt and Warrington, 2008). In contrast to the situation with the upstream genes the over expression of PPE genes has not been reported to produce a gain of function PCP phenotype (Park et al., 1996). This seems somewhat surprising as over expression often leads to a loss in the spatially restricted accumulation of a protein, which results in altered function. One possibility is that the over expression does not saturate the system that localizes the proteins to the proximal side. This seems unlikely. A more attractive possibility is that protein that is not properly localized is not active due to a lack of one or more binding partners.
6.2 mwh
The mwh gene, which by epistasis tests is the most downstream member of the fz/stan pathway also accumulates on the proximal side of wing cells and this is dependent on both the core and PPE proteins and genes (Fig 2A) (Strutt and Warrington, 2008; Yan et al., 2008). Two papers have suggested possible mechanisms. In one, Lu and colleagues found that Mwh and In directly interacted and they suggested that In on the proximal side of wing cells recruited Mwh (Lu et al., 2010). In the other, Strutt and colleagues found that Mwh was phosphorylated in a PPE dependent manner and suggested this was important in the action of Mwh in ensuring a single distally pointing hair is formed (Strutt and Warrington, 2008). These two mechanisms are of course, not mutually exclusive. A variety of results argue that Mwh serves as an inhibitor of the actin cytoskeleton (Fig 6). How this is accomplished is unclear but it is worth noting that the amino terminal half of the large Mwh protein contains and G protein binding domain (GBD) and has sequence similarity to diaphanous family formins. These formins, are known to function as dimers, be activated by Rho:GTP and to promote the formation of long actin filaments (Campellone and Welch, 2011; Chesarone et al., 2011; Goode and Eck, 2007). Consistent with the sequence similarity between Mwh and Dia both genetic interactions and co-immunoprecipiation experiments argue that Rho1 activates Mwh (Yan et al., 2009). This raises the possibility that Mwh might inhibit the actin cytoskeleton by acting as a dominant negative formin.
6.3 A distal target of Dsh?
The cytoskeleton is activated to form a hair in the vicinity of the distal most vertex of pupal wing cells (Wong and Adler, 1993). The level of activity is important for insuring a single hair is formed and that it is the proper size and shape. For example, if the actin cytoskeleton is inhibited by drug treatment or by mutations in actin cytoskeleton components such as myosin II, multiple shorter than normal hairs are formed (Franke et al., 2010; Turner and Adler, 1998). Inhibition at the proximal side of wing cells from the recruitment of Mwh seems insufficient to specify the distal vertex. Several lines of data indicate that distal edge proteins can stimulate the cytoskeleton. Although mutations in in, fy, frtz and mwh are epistatic to loss of function mutations in distal proteins such as dsh and fz that is not the case for all gain of functions in those genes. The late over expression of dsh leads to the formation of multiple hair cells and an increase is seen even when cells also lack in or mwh function (Lee and Adler, 2002). Indeed, the effects of these two genetic changes are additive. Thus, at least when over expressed Dsh is able to affect cytoskeleton function and it must be doing so by interacting with an unidentified cellular constituent and not known fz/stan downstream components such as in or mwh. Independent evidence supporting the distal edge proteins stimulating the cytoskeleton came from Strutt and colleagues who found that Fz acted to promote hair formation (Strutt and Warrington, 2008). Several experimental results supported this idea including a delay in hair initiation in fz mutant cells compared to wild type neighbors. This was seen even in when comparing fz- and fz+ cells in a Vang mutant background where Fz was not properly localized.
The identity of the putative distal target(s) is currently unknown. In principle the target could be a cytoskeletal activator that is activated by the distal proteins or alternatively a cytoskeletal inhibitor that is inactivated by the distal proteins (Fig 6). If the distal target is an activator than we can predict that a loss of function mutation would result in delayed hair initiation and perhaps small or short hairs. Several such genes have been reported including shavenoid which has a strong hair delay/loss phenotype (Ren et al., 2006). However, the phenotype of null alleles of sha is strongly enhanced by cells simultaneously being mutant for fz/stan pathway genes. Thus, if Sha is a target it cannot be the only one. Other possible activator targets are proteins that modulate the activity of the actin cytoskeleton such as formins (Campellone and Welch, 2011; Chesarone et al., 2011; Goode and Eck, 2007) or the Arp23 complex (Campellone and Welch, 2011; Zigmond, 2004). No evidence suggesting this is the case has been published. It is also possible that the target is an inhibitor, and if so we can predict that loss of function mutations would result in premature hair initiation. No possible targets with this property have been reported.
ACKNOWLEDGMENT
The author is supported by a grant from the NIGMS (GM-37136). I thank Simon Collier for generously providing the images of wing ridges.
References
- Adler P, Taylor J, Charlton J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000a;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev. Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Adler PN, Liu J, Charlton J. Cell size and the morphogenesis of epidermal hairs. Genesis. 2000b;28:82–91. doi: 10.1002/1526-968x(200010)28:2<82::aid-gene60>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Adler PN, Zhu C, Stone D. Inturned Localizes to the Proximal Side of Wing Cells under the Instruction of Upstream Planar Polarity Proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2011;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–1327. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nusslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. Embo J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Blair A, Tomlinson A, Pham H, Gunsalus KC, Goldberg ML, Laski FA. Twinstar, the Drosophila homolog of cofilin/ADF, is required for planar cell polarity patterning. Development. 2006;133:1789–1797. doi: 10.1242/dev.02320. [DOI] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2011;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–1268. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2011;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2011;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- Cho B, Fischer JA. Ral GTPase promotes asymmetric Notch activation in the Drosophila eye in response to Frizzled/PCP signaling by repressing ligand-independent receptor activation. Development. 2011;138:1349–1359. doi: 10.1242/dev.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- Davis I, Ish-Horowicz D. Apical localization of pair-rule transcripts requires 3' sequences and limits protein diffusion in the Drosophila blastoderm embryo. Cell. 1991;67:927–940. doi: 10.1016/0092-8674(91)90366-7. [DOI] [PubMed] [Google Scholar]
- del Alamo D, Mlodzik M. Frizzled/PCP-Dependent Asymmetric Neuralized Expression Determines R3/R4 Fates in the Drosophila Eye. Developmental Cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Donoughe S, DiNardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–2759. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle K, Hogan J, Lester M, Collier S. The Frizzled Planar Cell Polarity signaling pathway controls Drosophila wing topography. Dev Biol. 2008;317:354–367. doi: 10.1016/j.ydbio.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature. 1999;397:523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled - dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Elphick L, Nusslein-Volhard C, St Johnston D. Staufen protein associates with the 3'UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994;79:1221–1232. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II is required for cell proliferation, cell sheet adhesion and wing hair morphology during wing morphogenesis. Dev Biol. 2010;345:117–132. doi: 10.1016/j.ydbio.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within drosophila ovarian follicles. Development. 2001;128:3209–3220. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- Gault WJ, Olguin P, Weber U, Mlodzik M. Drosophila CK1-gamma, gilgamesh, controls PCP-mediated morphogenesis through regulation of vesicle trafficking. J. Cell Biol. 2012;196:605–621. doi: 10.1083/jcb.201107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M, Schweisguth F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature. 1998;393:178–181. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EE, Odde DJ, Seydoux G. Regulation of the MEX-5 gradient by a spatially segregated kinase/phosphatase cycle. Cell. 2011;146:955–968. doi: 10.1016/j.cell.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B' regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Adler PN. The frizzled pathway regulates the development of arista laterals. BMC Developmental Biology. 2002;2:7. doi: 10.1186/1471-213X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held LI., Jr Bristles induce bracts via the EGFR pathway on Drosophila legs. Mech Dev. 2002;117:225–234. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Held LI, Jr, Duarte CM, Derakhshanian K. Extra joints and misoriented bristles on Drosophila legs. Prog Clin Biol Res. 1986;217A:293–296. [PubMed] [Google Scholar]
- Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–1276. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Hogan J, Valentine M, Cox C, Doyle K, Collier S. Two frizzled planar cell polarity signals in the Drosophila wing are differentially organized by the Fat/Dachsous pathway. PLoS Genet. 2011;7:e1001305. doi: 10.1371/journal.pgen.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Krasnow RE, Adler PN. A single frizzled protein has a dual function in tissue polarity. Development. 1994;120:1883–1893. doi: 10.1242/dev.120.7.1883. [DOI] [PubMed] [Google Scholar]
- Kumar JP. Building an ommatidium one cell at a time. Dev Dyn. 2012;241:136–149. doi: 10.1002/dvdy.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. Gradients in the insect segment: the orientation of hairs in the milkweed bug Oncopeltus fasciatus. J. Exp Biol. 1966;44:452–458. doi: 10.1242/jeb.44.3.507. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Adler PN. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Lu B, Usui T, Uemura T, Jan L, Jan YN. Flamingo controls the planar polarity of sensory 0bristles and asymmetric division of sensory organ precursors in drosophila [In Process Citation] Curr Biol. 1999;9:1247–1250. doi: 10.1016/s0960-9822(99)80505-3. [DOI] [PubMed] [Google Scholar]
- Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins Inturned and Multiple Wing Hairs interact physically and function together. Genetics. 2010;185 doi: 10.1534/genetics.110.114066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Amonlirdviman K, Raffard RL, Abate A, Tomlin CJ, Axelrod JD. Cell packing influences planar cell polarity signaling. Proc Natl Acad Sci U S A. 2008;105:18800–18805. doi: 10.1073/pnas.0808868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeil H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988;336:595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 2011;25:131–136. doi: 10.1101/gad.610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Current biology : CB. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara D, Horiuchi SY, Shimono K, Usui T, Uemura T. The seven-pass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons. Genes Dev. 2011;25:1982–1996. doi: 10.1101/gad.16531611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung SM, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7:165–179. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola G, Classen AK, Gonzalez-Gaitan M, Eaton S, Zerial M. A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development. 2010;137:2353–2364. doi: 10.1242/dev.048413. [DOI] [PubMed] [Google Scholar]
- Moyer KE, Jacobs JR. Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev Biol. 2008;8:99. doi: 10.1186/1471-213X-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkusich EM, Flanagan DJ, Whitington PM. The core planar cell polarity gene prickle interacts with flamingo to promote sensory axon advance in the Drosophila embryo. Dev Biol. 2011;358:224–230. doi: 10.1016/j.ydbio.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Mummery-Widmer JL, Yamazaki M, Stoeger T, Novatchkova M, Bhalerao S, Chen D, Dietzl G, Dickson BJ, Knoblich JA. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458:987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Adler PN. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development. 2012;139:906–916. doi: 10.1242/dev.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Olguin P, Glavic A, Mlodzik M. Intertissue mechanical stress affects Frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr Biol. 2011;21:236–242. doi: 10.1016/j.cub.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M. The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. Embo J. 1999;18:4669–4678. doi: 10.1093/emboj/18.17.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- Pataki C, Matusek T, Kurucz E, Ando I, Jenny A, Mihaly J. Drosophila Rab23 is involved in the regulation of the number and planar polarization of the adult cuticular hairs. Genetics. 2010;184:1051–1065. doi: 10.1534/genetics.109.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MH, Roberts DM, McCartney BM, Jezuit E, Peifer M. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J Cell Sci. 2006;119:403–415. doi: 10.1242/jcs.02761. [DOI] [PubMed] [Google Scholar]
- Purvanov V, Koval A, Katanaev VL. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci Signal. 2010;3:ra65. doi: 10.1126/scisignal.2000877. [DOI] [PubMed] [Google Scholar]
- Ren N, Charlton J, Adler PN. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics. 2007;176:2223–2234. doi: 10.1534/genetics.107.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N, He B, Stone D, Kirakodu S, Adler PN. The shavenoid gene of Drosophila encodes a novel actin cytoskeleton interacting protein that promotes wing hair morphogenesis. Genetics. 2006;172:1643–1653. doi: 10.1534/genetics.105.051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repiso A, Saavedra P, Casal J, Lawrence PA. Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat. Development. 2010;137:3411–3415. doi: 10.1242/dev.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamberg S, Houston P, Monk NA, Owen MR. Modelling and analysis of planar cell polarity. Bull Math Biol. 2010;72:645–680. doi: 10.1007/s11538-009-9464-0. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of FAt:Dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010;24:2157–2168. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova NI, Shaw S, Saburi S, Matakatsu H, Blair S, McNeill H. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr Biol. 2009;19:1112–1117. doi: 10.1016/j.cub.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr Biol. 2002a;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Johnson R, Cooper K, Bray S. Asymmetric localisation of Frizzled and the determination of Notch-dependent cell fate in the Drosophila eye. Curr Biol. 2002b;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell. 2011;20:511–525. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Struhl G. Decoding vectorial information from a gradient: sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development. 1999;126:5725–5738. doi: 10.1242/dev.126.24.5725. [DOI] [PubMed] [Google Scholar]
- Tree DRP, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle Mediates Feedback Amplification to Generate Asymmetric Planar Cell Polarity Signaling. Cell. 2002;109:1–11. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–192. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Valentine M, Collier S. Planar cell polarity and tissue design: Shaping the Drosophila wing membrane. Fly (Austin) 2011;5 doi: 10.4161/fly.5.4.15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema DR, Zeev-Ben-Mordehai T, Auld VJ. Transient apical polarization of Gliotactin and Coracle is required for parallel alignment of wing hairs in Drosophila. Dev. Biol. 2004;275:301–314. doi: 10.1016/j.ydbio.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–3629. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-Associated Kinase (Drok) Links Frizzled-Mediated Planar Cell Polarity Signaling to the Actin Cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin G. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, Adler PN. The multiple-winghairs Gene Encodes a Novel GBD-FH3 Domain-Containing Protein That Functions Both Prior to and After Wing Hair Initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Lu Q, Fang X, Adler PN. Rho1 has multiple functions in Drosophila wing planar polarity. Dev Biol. 2009;333:186–199. doi: 10.1016/j.ydbio.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned Gene Expression Directs Bipolar Planar Polarity in Drosophila. Developmental Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–1372. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- Zigmond SH. Beginning and ending an actin filament: control at the barbed end. Curr Top Dev Biol. 2004;63:145–188. doi: 10.1016/S0070-2153(04)63005-5. [DOI] [PubMed] [Google Scholar]
NOTE ADDED IN PROOF
Several quite important papers on fly PCP were published recently and interested readers should take a look at them (see below).
- Ambegaonkar A, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-fat Planar Cell Polarity. Current Biology. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar Polarity Specification through Asymmetric Localization of Fat and Dachsous. Current Biology. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Han C, Axelrod JD. Planar Polarized Protrusions Break the Symmetry of EGFR Signaling during Drosophila Bract Cell Fate Induction. Developmental Cell. 2012;23:1–12. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Julicher F, Eaton S. Establishment of Global Patterns of Planar Polarity during Growth of the Drosophila Wing Epithelium. Current Biology. 2012;22:1296–1301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–3674. doi: 10.1242/dev.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]