Abstract
Objective
Using the collagen-induced arthritis (CIA) model, we explored the characteristics of the T cell population which responds to an analog peptide (A9) of type II collagen (CII) and regulates autoimmunity.
Methods
A9 is a 26 amino acid peptide analogous to the sequence of a segment of CII (CII 245-270) but with substitutions made at amino acid positions 260 (alanine for isoleucine), 261 (hydroxyproline for alanine), and 263 (asparagine for phenylalanine). We have previously shown that A9 profoundly suppresses immunity to CII and CIA. In order to determine the mechanism of suppression, we used a transgenic mouse whose T cells express a CII specific receptor (TCR) and performed passive cell transfer experiments.
Results
The results demonstrate that suppression of CIA by the A9 is dependent upon T cells. Using multiparameter flow cytometry, we determined that the cells responsible for suppression were CD4+ and expressed high levels of FcεRIγ(FcRγ). To establish the significance of this finding, we obtained mice genetically deficient in FcRγ to perform passive transfer experiments. The resulting FcRγ-/- CD4+ T cells when primed by culture with A9 could not transfer the suppression of arthritis nor secrete cytokines in response to A9.
Conclusion
Taken together, these data suggest that the suppression of arthritis and the Th2 cytokine profile elicited by A9 is dependent upon the presence of FcRγ in the T cells. These findings are novel and may have therapeutic potential for patients with autoimmune arthritis.
Introduction
The collagen-induced arthritis (CIA) model of inflammatory arthritis is caused by immunizing susceptible animals with type II collagen (CII), the major structural component of cartilage (1). We have used this model to develop a highly specific immunotherapy capable of down regulating the response to CII and autoimmune arthritis in this model. The immunotherapy was based on devising an analog peptide representing the immunodominant epitope of CII but with several critical modifications. This peptide (A9) is analogous to CII 245-270 but with substitutions made for the amino acids at positions 260 (alanine for isoleucine), 261 (hydroxyproline for alanine), and 263 (asparagine for phenylalanine). When used to treat CIA, A9 can profoundly suppress immunity to CII and arthritis. Other analog peptides were either less effective or completely ineffective (2).
In order to have sufficient numbers of CII-specific T cells with which to study the mechanism of suppression, we used a CII-specific T cell receptor transgenic mouse (qCII24). These mice are transgenic for TCR that recognize the immunodominant CII epitope contained within the CII 245-270 region of the CII molecule. When immunized with intact CII, they develop a severe arthritis beginning 18 days after immunization (3). Arthritis in the transgenic mice is efficiently suppressed by A9. In this report we demonstrate that T cells activated by the A9 peptide can passively transfer suppression.
Functionally distinct subsets of CD4+ T cells are essential to orchestrate efficient immune responses and regulate immune-mediated inflammatory diseases. Although these subsets were initially defined on the basis of the secretion of specific cytokines (i.e. Th1, Th2, Th17), recent experiments have identified nuclear regulators of T cell differentiation and an array of molecular markers that allow a more precise characterization of T cells that perform regulatory functions in autoimmune diseases. Using flow cytometry and specific antibodies, we identified CII-specific CD4+ cells that were capable of suppressing arthritis in transgenic mice and established that these cells had upregulated FcεRIγ (FcRγ), a molecule known to associate with the TCR complex, but did not express Foxp3 that is characteristic of regulatory T cells (Treg). Using mice genetically deficient in FcRγ, we demonstrate that FcRγ is required both for A9-induced cytokine secretion and for transferring the suppression of arthritis. We believe that the A9 analog peptide functions by stimulating CD4+ T cells to increase both the expression of FcRγ and the secretion of Th2-type cytokines.
Methods
Preparation of Tissue Derived Type II Collagen
Native CII was solubilized from fetal calf articular cartilage by limited pepsin-digestion and purified as described earlier (4). The purified collagen was dissolved in cold 0.01M acetic acid at 4 mg/ml and stored frozen at -70°C until used.
Animals
DBA/1 mice were obtained from the Jackson Laboratories and raised in our animal facility. The transgenic mouse that expresses a CII-reactive TCR specific for the immunodominant determinant on CII has been developed and bred in our facility (3). Briefly, the Va11.1-Ja17 and Vb8.3-Db1-Jb1.4 gene segments derived from an I-Aq restricted, CII-specific, T-cell hybridoma were cloned into T cell expression vectors, co-injected into B6 fertilized eggs, and the transgenic mice backcrossed with DBA/1 for 12 generations to establish the recombinant TCR gene on the DBA/1 background. These mice were then interbred to establish a. strain, designated “qCII24”, which is indistinguishable from WT DBA/1 mice and reproduces without difficulty. Another strain of mice, genetically deficient in the FcRγ-chain has been bred onto the DBA/1 background for 12 generations (5-7). In some experiments these mice were intercrossed with qCII24 to produce a new strain expressing the TCR transgene, but deficient in the production of the FcRγ-chain.
All mice were fed standard rodent chow (Ralston Purina Co., St. Louis, Mo.) and water ad libitum. The environment was specific pathogen-free and sentinel mice were tested routinely for mouse hepatitis and Sendai viruses. All animals were kept until the age of 7-10 weeks before being used for experiments, which were conducted in accordance with approved IACUC protocols.
Immunization
CII was dissolved in 0.01 M acetic acid at a concentration of 4 mg/ml and emulsified with an equal volume of CFA containing 4 mg/ml of M. tuberculosis strain H37 Ra (Difco Microbiology Products, Becton Dickinson, NJ, USA) (8). Each mouse received 100μg of CII emulsified in CFA subcutaneously at the base of the tail in order to induce arthritis.
Measurement of the Incidence and Severity of Arthritis
The incidence and severity of arthritis were determined by visually examining each forepaw and hindpaw and scoring them on a scale of 0-4 as described previously (4). Scoring was conducted by two examiners, one of whom was unaware of the identity of the treatment groups. Each mouse was scored thrice weekly beginning three weeks post immunization and continuing for 8 weeks. The incidence of arthritis (number of animals with one or more arthritic limbs); percentage of arthritic limbs (total number of arthritic limbs per group, including both forelimbs and hindlimbs, expressed as a percent of the total number of limbs) and mean severity score (sum of the severity scores for the group on each day /total number of animals in the group) was recorded at each time point.
Passive Transfer Experiments
In transfer experiments, splenocytes or inguinal lymph node cells from qCII24 Tg mice previously immunized with the A9 analog peptide in CFA were collected 8 days after immunization and various cell subsets were fractionated (I-Aq positive cells, α/β TCR+ T cells, or γ/δ TCR+ T cells, CD8+ T cells) using ferromagnetic beads (Miltinyi Biotech, Gladbach, Germany) and subset specific antibodies for positive selection according to the manufacturers protocol. CD4+ cells were collected by negative selection, according to the manufacturer's protocol (Miltinyi Biotech, Gladbach, Germany). (In preliminary experiments CD4+ T cells were selected by either positive or negative selection, and the experimental results were similar.) The purity of each cell population was confirmed by flow cytometry to have > 95% purity. In each experiment 10 or 20 mice were used for collection of cells. An equal number of recipient mice were given cells intravenously using two protocols a.) 2.5 × 107 either I-Aq positive cells, α/β+ T cells, γ/δ+ T cells, or b.) 5×105 of CD4+ or CD8+ T cells. The cell number (2.5 × 107) was chosen for the first set of experiments to approximate the number of cells readily obtained from a single mouse spleen. The cell numbers were reduced in the second set of experiments when dose response experiments revealed that 5×105 purified CD4+ cells were sufficient to suppress arthritis. Controls were given equal numbers of undepleted spleen cells from CFA immunized controls. All recipient mice were immunized with CII either on the day of the cell transfer (prevention protocol) or on day 23 after immunization (therapy protocol) and observed for arthritis.
Measurement of Serum Antibody Titers
Mice were bled at four or six weeks after immunization and sera were analyzed for antibodies reactive with native CII using a modification of an enzyme-linked immunoassay (ELISA) previously described (4). Results are reported as units of activity, derived by comparison of test sera with the curve derived from the standard serum which was arbitrarily defined as having 50 units of activity. Reactivity to CII was not detected in sera obtained from normal mice.
Characterization of the cells
Splenocytes from qCII24 tg mice were isolated and cultured with various peptides for 24 hours. The phenotype induced by A9 was determined by multiparameter flow cytometry using a LSRII flow cytometer (BD Biosciences, San Jose, CA) and specific gating on the CD4+ population. Cells were cultured with fluorochrome-labeled antibodies specific for CD4, TCR-Vβ8, CD25, CD71, CD44, and CD62L (BD Biosciences, San Jose, CA). In some experiments intracellular labeling was performed using antibodies specific for FcRγ (cat # 06-727, Upstate Cell Signaling Solutions, Lake Placid, NY) and FoxP3 (FJK-16s, eBioscience, San Diego, Ca).
Measurement of Cytokines
To measure cytokines, several cell populations were used: a.) CD4+ T cells were isolated from inguinal nodes of DBA/1 FcRγ-/- mice immunized 14 days previously with CII/ CFA, b.) CD4+ T cells were isolated from spleens of either qCII24 mice or qCII24 FcRγ-/- mice. The CD4+ T cells were cultured (5 × 105 CD4+ T cells/ml) with wild type APC's (I-Aq-positive splenocytes) (1:2 ratio) which had been prepulsed with 100 μg/ml of the various peptides (A2 or A9). (The purity of each cell population was confirmed by flow cytometry to have > 95% purity). In some experiments 100μg of A2 was added to APCs together with increasing molar amounts of the A9 peptide to establish a dose response. Supernatants were collected 72 hours later and analyzed for the presence of multiple cytokines (IL-4, IL-10, IL-2, INFγ, and IL-17) by a Bio-plex mouse cytokine assay (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. Values are expressed as picograms per ml and represent the mean values for each group.
Statistical Analysis
The incidence of arthritis in various groups of mice was compared using Fisher's Exact Test. Mean severity scores were compared using the Mann and Whitney test and antibody and cytokine levels were compared using the Student's T test.
Results
Identification of the cell responsible for the A9 effect in vivo
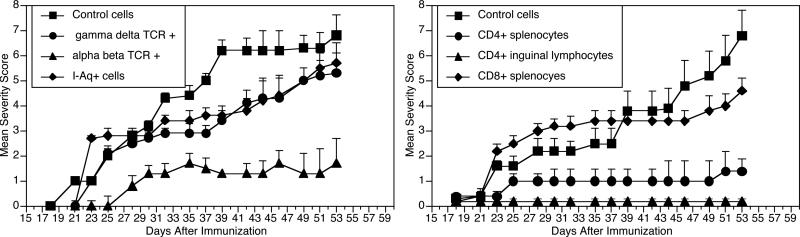
We have previously reported that A9-stimulated splenocytes are capable of transferring the suppression of arthritis in the CIA model (4). In order to identify the cell subset responsible for this effect, we used a transgenic mouse, qCII24tg, which expresses a transgenic TCR that is specific for the immunodominant CII epitope (3) to perform passive cell transfer experiments. These mice were necessary to provide adequate numbers of CII-reactive T cells for studies. Mice were immunized with A9/CFA and splenocytes collected for isolating various cell populations, using specific antibodies and ferromagnetic beads. As shown in Figure 1 (upper panel), mice receiving either I-Aq positive cells (APCs) or γ/δ+ TCR-expressing cells from spleens of A9 immunized donors contracted arthritis with an incidence and severity no different from controls (given splenocytes from mice injected with CFA alone). On the other hand, arthritis in mice receiving the α/β+TCR, A9-immune splenocytes was significantly attenuated compared to controls (incidence of 14% v 80%, p<0.01 using Fischer's Exact test). IgG antibodies to CII were also significantly suppressed in the mice receiving the α/β+ TCR A9-immune splenocytes (17.1 ± 8 v 69.5 ± 21 units, p <0.005). These data show that the suppression of CIA by the A9 analog peptide is dependent upon a population of α/β+ TCR cells rather than APCs or γ/δ TCR+ T cells.
Figure 1. Identification of suppressive T cells.
Left. Splenocytes from qCII24Tg mice immunized with A9/CFA were collected and cell subsets fractionated: I-Aq+ cells (□), α/β TCR+ T cells (▲), or γ/δ TCR+ T cells (●). Recipient mice (n = 10 per group) were given 2.5 × 107 either I-Aq+ cells, α/β+ T cells, γ/δ+ T cells, or control undepleted splenocytes from CFA injected controls (■). Recipients were immunized with CII and observed for arthritis. Only the α/β TCR+ T cells suppressed CIA (final severity scores 1.7 ± 2.7 vs 6.8 ± 3.3, p = 0.01).
Right. Splenocytes and inguinal lymph node cells from qCII24tg mice were collected as above and CD4+ and CD8+ T cells were isolated. Each mouse (n=10 per group) was given 5×105 cells of either A9-primed CD4+ T cells from spleen (●), A9-primed CD4+ T cells from lymph nodes (▲), A9-primed CD8+ T cells from spleens (□), or undepleted CFA-primed splenocytes (■) and immunized with CII and observed for arthritis. Final severity scores for mice given CD4+ draining lymph node cells 0.2 ± 0.8 vs 6.8 ± 3.5, p = 0.01 and for mice given CD4+ splenocytes 1.4 ± 1 vs 6.8 ± 3.5, p = 0.01).
The α/βTCR+ cells consist of two major subpopulations, characterized by expression of either CD4 or CD8 cell surface molecules. To distinguish which subpopulation is critical for suppression, splenocytes and draining lymph node cells from qCII24 tg mice were collected after immunization with either A9/ CFA or CFA alone and the CD4+ and CD8+ T cells isolated and infused intravenously into DBA/1 mice prior to immunization with CII. When observed for arthritis, (Figure 1, lower panel), CD4+ A9-immune T cells from either source suppressed arthritis, while CD8+ T cells had no effect. Taken together, these data confirm that CD4+ α/β+ TCR cells are responsible for the suppression of arthritis induced by the A9 peptide.
Characterization of the inhibitory T cell
Activation of naive CD4+T cells after interactions with antigen-bearing APCs typically leads to the generation of cells with well defined effector and memory phenotypes. Naive T cells activated by antigen/APC interaction are expected to expand and differentiate into effector T cells, forming a subpopulation which have a long-lived memory phenotype.
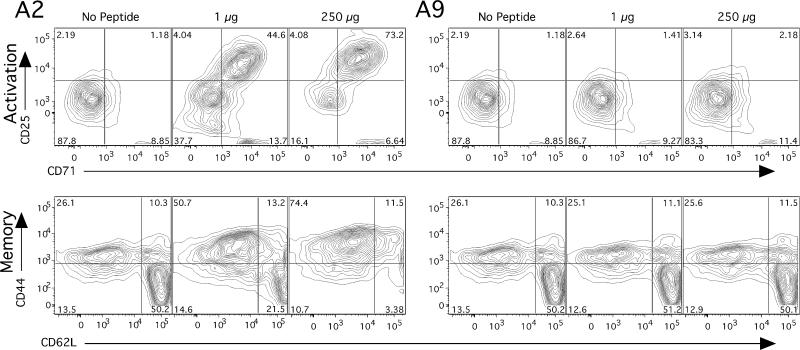
Our approach was to use multiparameter flow cytometry to characterize the phenotype of the CD4+ T cell subset generated by interaction with either A2 or A9. The qCII24tg splenocytes were cultured with either A2 or A9 in the presence of APCs. These cells were then tested for the presence of the activation markers CD44, CD62L CD25, and CD71. We found that A2 induced the emergence of a population of large blast-like cells with an activated phenotype (CD25hi, CD71hi), simultaneous with a rapid expansion of the memory phenotype (CD44hi and CD62Llow cells). In contrast, splenocytes cultured with A9 peptide and APCs did not diverge in phenotype from unstimulated control cells (Figure 2).
Figure 2. A2 induces both an activation and memory phenotype in transgenic mice; A9 does not.
When splenocytes from the CII-specific TcR transgenic mouse qCII24 were cultured for 48 hours in the presence of CII (245-271) peptide (A2), CD4+ T cells were found to upregulate both CD25 and CD71, indicating an activated phenotype. In addition, they increased expression of CD44 and changed their L-selectin (CD62L) expression from CD62Lhi to CD62Llo, indicating a memory phenotype. In contrast, the same cells (qCII24tg splenocytes), cultured with A9 peptide, developed no change in expression of either activation or memory markers. The analysis was performed three separate times using flow cytometry with specific gating on the CD4+ population.
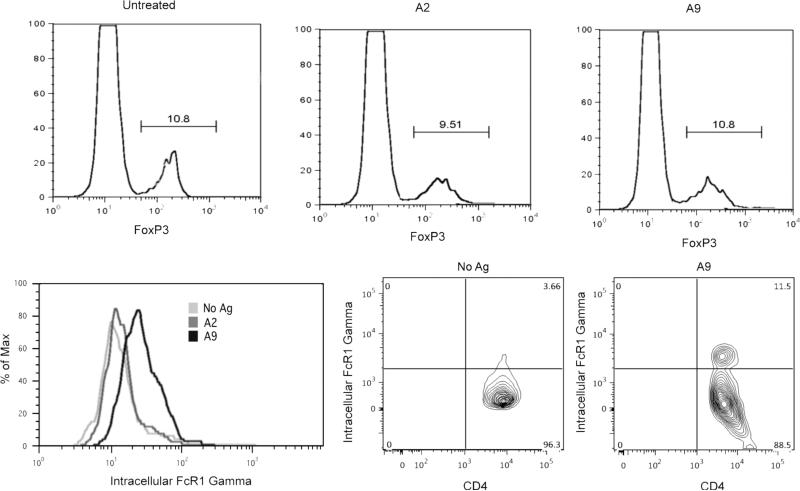
Regulatory T cells (Tregs) are a well defined subpopulation of CD4+ cells, characterized by expression of the transcription factor Foxp3 and are known to have immunomodulatory functions in autoimmunity (9). Therefore, quantification of the Treg cell subpopulation was performed on CD4+ qCII24tg splenocytes cultured with A2, A9, or media alone. As shown in figure 3 (upper panel), a small number of CD4+ cells expressing Foxp3, were identified using flow cytometry in each of the three culture conditions. However, there were no clear variations between the numbers of Tregs induced by either A2, A9, or control cells cultured with media alone. The experiments were repeated using CD4+ T cells taken from spleens 48 hours after intravenous administration of the peptides and the results again showed no differences between the two treatments (A2 = 10.1% Foxp3+ cells, A9 = 9.81% Foxp3+ cells, PBS = 9.78% Foxp3+ cells with gating specific for the CD4+ cells).
Figure 3. Upper: A9 does not induce Foxp3.
Splenocytes were isolated from qCII24 tg mice and cultured with A2, A9, or media. 48 hours later cells were collected and stained using an antibody for Foxp3 and analyzed by flow cytometry. Numbers represent the % Foxp3+ cells with gating on CD4+ T cells. The data are representative of results from three separate experiments.
Lower: A9 but not A2 treatment increases FcRγ expression.
Left: Splenocytes from qCII24 TCR transgenic mice were cultured for 48 hours in the presence of A2 (dark grey line), A9 (black line) or media alone (light grey line). Using an antibody specific for the FcRγ chain and specific gating on CD4+ cells, we demonstrate that T cells activated by A9 upregulate the expression of FcRγ. The cells cultured with A2 peptide are no different than unstimulated controls. (Mean fluorescence of three experiments: no antigen = 92 ± 22, A2 = 103 ± 32, A9 = 220 ± 35).
Right: Culture with A9 causes CD4+ T cells to expand and significantly upregulate the intra-cellular expression of FcRγ, creating an FcRγ-high-activated phenotype. The data shown are representative of data obtained from five separate experiments.
As none of the expected markers of activation and regulation were detectable in A9-stimulated CD4+ cells, we examined the contribution of the structure of the TCR itself in mediating these suppressive effects. T cells in disease states can undergo extensive down-regulation of the TCR-ζ chain with a reciprocal up-regulation of FcRγ and association of the FcRγ -chain with the TCR (10). Using an antibody specific for the FcRγ, we stained the qCII24tg splenocytes previously cultured with A2 or A9 peptide and found that CD4+ T cells stimulated with A9 and APCs, developed a significant upregulation in the expression of FcRγ (Figure 3, middle and lower panel), compared to stimulation with A2 or media alone. Taken together, these data suggest that activation with A9 induces a t cell that has a phenotype characterized by increased expression of FcRγ. The FcRγ induced by A9 most likely associates with the TCR, although other surface receptors might be involved.
FcRγ is required for the suppression of arthritis
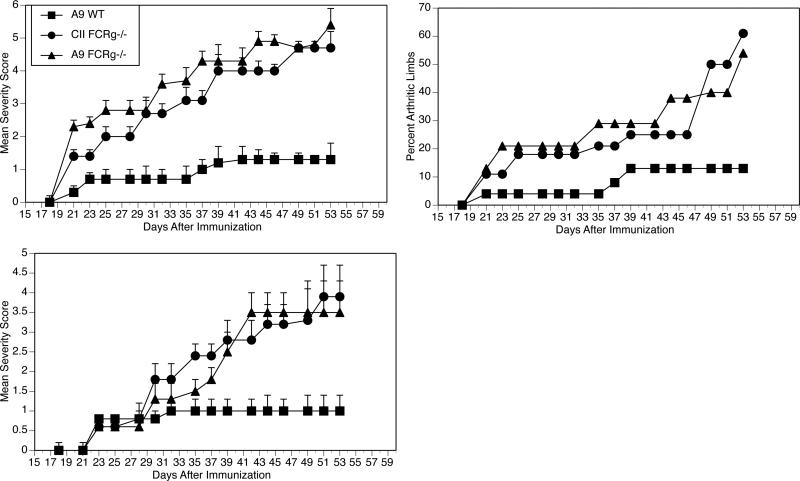
To investigate the possibility that upregulation of FcRγ might be a secondary epiphenomenon with no pathophysiological significance and to determine its importance, we obtained DBA/1 mice genetically deficient in FcRγ (6). To confirm that FcRγ-/- cells could successfully engraft into DBA/1 mice, we isolated CD4+ T cells from FcRγ-/- mice that had been previously immunized with CII/CFA and transferred them into DBA/1 mice which were subsequently immunized with collagen. Sera were obtained from the recipient animals four weeks later to test for the presence of autoantibodies to collagen. The mice which had received the CII-specific FcRγ-/- cells developed significantly greater antibody titers than control mice which did not receive cells (180 ± 35 vs 65 ± 15, p < 0.05). Similar results were obtained in two separate experiments and confirm that the CII-specific FcRγ-/- engrafted cells were viable and capable of secreting cytokines which enhance the production of autoantibodes. Using a similar approach, FcRγ-/- mice were immunized withA9/CFA and the CD4+ T cells were isolated from the draining lymph nodes and passive transfer experiments were performed as described above so that naïve wild type DBA/1 mice were given A9-primed CD4+ T cells prior to immunization with CII (prevention protocol) to observe for arthritis. As demonstrated in Figure 4, upper and middle panel, mice genetically deficient in the FcRγ did not transfer suppression while wild type A9-immune CD4+ T cells significantly down-regulated the severity of the arthritis. Similar results were obtained when the cells were given to mice after arthritis was firmly established (therapeutic protocol) (Figure 4, lower panel). Mean antibody titers to CII taken eight weeks after immunization were 17 ± 7 (p ≤ 0.05) in mice given A9-immune CD4+ wild type T cells, 50 ± 12 in mice given A9-immune CD4+ T cells from FcRγ-/- mice, and 53 ± 13 in mice given CII-immune CD4+ T cells from FcRγ-/- mice. When the A9-immune CD4+ T cells were infused at an even later time point, specifically day 28 after immunization, the wild type A9-immune CD4+ T cells significantly suppressed arthritis (final severity scores of 3.5 ± 1.2 compared to 6.2 ± 1.1 in controls) while the FcRγ -/- CD4+ T cells were ineffective (final severity scores of 5.9 ± 2.1 compared to 6.2 ± 1.1 in controls).
Figure 4. FcRγ is required for the A9 suppression of arthritis.
(Upper) Prevention. CD4+ inguinal lymph nodes cells were collected from qCII24 mice either wild type or FcRγ-/- immunized with either A9/CFA or CII/CFA. DBA/1 mice (n = 20) were infused with 5×105 cells of either A9-primed CD4+ T cells from FcRγ -/- mice (▲), A9-primed CD4+ T cells from wild type mice (■), or CII-primed CD4+ T cells from FcRγ -/- mice (●). Recipients were immunized with CII and observed for arthritis. Only the wild type A9-immune cells prevented CIA (final severity scores comparing ■ vs▲ of 1.3 ± 2.5 vs 5.4 ± 3.2, p = 0.01. Final percentage of arthritic limbs was 10% vs 60% comparing ■ vs ▲, p ≤ 0.001). (Lower) Suppression. CD4+ lymph node cells were collected as above and on day 23 after immunization, 5×105 cells of either A9-primed CD4+ T cells from FcRγ -/-mice (▲), A9-primed CD4+ T cells from wild type mice (■), or CII-primed CD4+ T cells from FcRγ -/- mice (●) were infused iv. The wild type A9-immune cells suppressed CIA (final severity scores comparing ■ vs ▲ of 1.0 ± 0.6 vs 3.9 ± 1.6, p = 0.02).
Mechanism of suppression
One of the functions of T cells is to secrete cytokines. Depending on the specific cytokines secreted, different effects are noted in the host. We have previously reported that the A9 analog peptide induces T cells to secrete cytokines that are characteristic of Th2-type T cells (11, 12). Therefore, it was of interest to quantify cytokine secretion of T cells genetically deficient in FcRγ in response to A2 and A9. The FcRγ-/- mice were immunized with type II collagen (CII) and the draining lymph nodes were collected for in vitro studies to determine the response to the A2 and A9 peptide in the presence of normal APCs. As shown in Table IA, the supernatants from cultures of CD4+ cells deficient in FcRγ that were cultured with A2 contained levels of Th1, Th17, and Th2 cytokines significantly greater than background. On the other hand supernatants from cells cultured with A9, elicited only background levels of Th1, Th2, and Th17 cytokines. These data also show that the FcRγ-/- T cells had higher background level of cytokines compared to wild type DBA/1 cells, although the reasons for this are unclear.
Table I.
Cytokine Responses
| A^ Cytokine responses in Fcry-/- Mice | |||||
|---|---|---|---|---|---|
| Antigen | Cytokines | (pg/ml) | |||
| IL-2 | IFN-γ | IL-17 | IL-4 | IL-10 | |
| FcR-/- | |||||
| No Ag | 116 ± 15 | 378 ± 55 | 1,422±185 | 15±5 | 443±52 |
| A2 | 215±25* | 1,041±92** | 10,934±500** | 38±12* | 1,053±88** |
| A9 | 117 ± 13 | 427± 42 | 1,646±420 | 17±10 | 481±41 |
| Wild Type | |||||
| No Ag | 66 ± 5 | 346 ± 290 | 269 ± 25 | 12 ± 2 | 108 ± 11 |
| A2 | 347 ± 25* | 6,390±400** | 14,891± 220* | 76 ± 8* | 3,331 ± 31* |
| A9 | 17 ± 2 | 647 ± 200 | 380 ± 25 | 52 ± 4* | 2,640 ± 22* |
| B^^. Cytokine responses in mice treated with A9-immune CD4+ T cells | ||||
|---|---|---|---|---|
| Treatment | Cytokines | (pg/ml) | ||
| IL-2 | IFN-γ | IL-17 | IL-4 | |
| FcRy-/- CD4+ cells | 1,302 ± 122 | 545 ± 22 | 6,482 ± 252 | 6 ± 4 |
| WT CD4+ cells | 147 ± 15 | 132 ± 13 | 278 ± 23 | 47 ± 8 |
| PBS | 1, 522 ± 132 | 655 ± 26 | 6,891 ± 280 | 8 ± 5 |
Groups of three DBA/1 mice either wild type (lower panel) or genetically deficient in FcRγ (upper panel) were immunized with CII/CFA. Draining inguinal lymph node cells were harvested 14 days after the immunization, and the CD4+ cells were isolated and cultured (5 × 105 cells/ml) with 1 × 106 I-Aq+ APCs (purified from spleens of wild type DBA/1 mice) which had been prepulsed in vitro with 100 μg/ml of either the immunodominant wild type peptide A2 or the analog A9 or media alone. Supernatants were collected 72 hours later and analyzed for the presence of the indicated cytokines. Values are expressed as picograms per ml and represent the mean values for each group.
(p ≤ 0.05)
(p < 0.001)
DBA/1 mice (n = 3 per group) were immunized with CII/CFA and observed for the development of arthritis. On day 23 after immunization, when arthritis was established, each mouse was infused intravenously with either 5×105 cells of A9-primed CD4+ T cells from FcRγ -/- mice, or A9-primed CD4+ T cells from wild type mice or PBS as a control. Inguinal lymph node cells were harvested 14 days after the cell infusion and cultured (5 × 106 cells/ml) with 100 μg/ml of the mouse collagen immunodominant wild type peptide. Supernatants were collected 72 hours later and analyzed for the presence of the indicated cytokines. Values are expressed as picograms per ml and represent the mean values for each group. Cytokines IL-2, IFN-γ, and IL-17 were all significantly lower in response to the mouse collagen immunodominant peptide in supernatants from the mice treated with WT CD4+ cells, compared with supernatants from mice treated with FcRg-/- CD4+ T cells or PBS (P≤0.05). On the other hand, IL-4 was significantly greater in the mice treated with WT CD4+ T cells (P≤0.05).
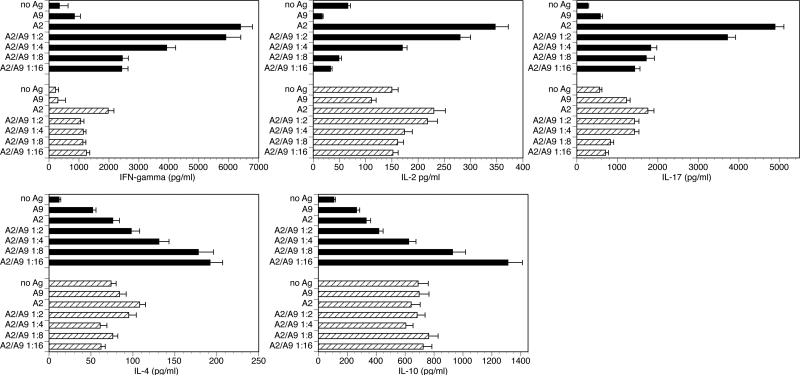
In a second set of experiments the mice genetically deficient in FcRγ were intercrossed with the qCII24 mice to obtain T cells expressing the collagen-specific TCR together with a genetic deficiency of FcRγ. The resulting FcRγ-/- CD4+ T cells from spleens were used to further establish the T cell responses to A9. A set of competitive inhibition studies was performed in which the A9 analogue peptide was cultured at various concentrations relative to A2 using T cells from qCII24 mice that are either deficient or sufficient in FcRγ. When the supernatants were collected and tested (Figure 5, panels A and B), A9 cultured with qCII24 tg cells successfully competitively inhibited the Th1 and Th17 cytokine responses to A2 while it enhanced the Th2 cytokine response in a dose-dependent manner. On the other hand, the T cells genetically deficient in FcRγ neither competitively inhibited nor enhanced the cytokine responses induced by A2.
Figure 5. Cytokine responses to A2 and A9.
CD4+ T cells were isolated from spleens of either qCII24 mice (black bars) or qCII24 mice deficient inFcRγ (hatched bars) and were cultured (5 × 105 CD4+ T cells/ml) with wild type APC (I-Aq-positive splenocytes) (1:2 ratio) prepulsed in vitro with either 100 μg/ml of A2 or 100μg of A2 together with increasing molar ratios of A9) for 6 days. Supernatants were tested for cytokines using a multiplexed ELISA. Results shown represent three separate experiments and are expressed in pg/ml. When supernatants were tested for inflammatory cytokines (IL-2, IL-17, IFN-γ, upper panel) A9/I-Aq inhibited inflammatory T cells responses in a dose-dependent manner (black bars). On the other hand, the FcRγ-/- CD4+ qCII24 tg cells gave a very poor response to A9/ I-Aq and did not decrease the Th1, and Th17 responses (hatched bars). When supernatants were tested for Th2 cytokines, (lower panel), A9/I-Aq enhanced qCII24 tg CD4+T cell responses to IL-4 and IL-10 in a dose-dependent manner (black bars). On the other hand, the FcRγ-/- CD4+ qCII24 tg cells gave a very poor response to A9/ I-Aq and did not enhance the Th2 responses (hatched bars).
Discussion
We have identified a unique mechanism by which an analog peptide prevents the development of inflammatory arthritis and inhibits the immune response to CII. This mechanism differs significantly from previously described immunomodulatory mechanisms in its dependence upon the presence of FcRγ and the propensity to stimulate the secretion of Th2 cytokines relative to Th-1 or Th17 cytokines. Further experiments will be necessary to determine if the T cells responsible for this effect express specific markers other than enhanced expression of FcRγ.
The concept of “suppressor” T cells, which are able to suppress antigen-specific responses and transfer tolerance in animal models, was first put forward 30 years ago (13). Since that report, an increasing number of immune cell populations have been described which play a role in regulation of autoimmunity (14), each characterized by specific markers, different mechanisms of action, and interference with different stages of the immune response. By using cell-specific antibodies and flow cytometry, we have excluded from consideration, immune cells previously reported to have regulatory functions, including monocytes, CD8+ T cells, γδ T cells, NK cells, double negative CD3+CD4-CD8- T cells, NKT cells, or Tregs expressing the transcription factor Foxp3. We report that the A9-induced inhibitory cells have an αβ TCR and express CD4.
There are three known functionally distinct subsets of CD4+ T cells, defined on the basis of the secretion of specific sets of cytokines (i.e. Th1, Th2, Th17), and a fourth subset (Tfh) present in germinal centers, which are essential to orchestrate efficient immune responses and regulate immune-mediated inflammatory diseases (15). The A9-induced cytokine profile of predominately, IL-4 and IL10, is similar to the well described Th2 CD4+ subset. Yet it differs from that subset in that it does not develop an activated or memory phenotype following exposure to A9 (16). Moreover, the proposed A9-induced inhibitory T cell does not express Foxp3, the characteristic phenotype of regulatory T cells. Neither is it an NKT cell, which is an innate-type T cell which responds prior to antigen challenge (17). Recently Thomson and coworkers described a population of double negative murine T cells, characterized by intracellular phosphorylation of both FcRγ and Syk, which suppressed immune responses in vitro (18). Our cell bears functional similarities to these cells although the phenotypes are not identical.
We are cognizant of the possibility that the TCR expressed by qCII24 Tg mice may not be representative of the T-cell repertoire found in wild type mice. In these studies, we have been careful to confirm that T cells from TCR transgenic mice react like cells from non-transgenic mice in their ability to transfer suppression of arthritis and to secrete predominantly Th2 cytokines (especially IL-4) after stimulation with A9. An animal model becomes most useful when it allows study of a mechanism that would take years to delineate in humans. In that regard, the T-cell-transfer model is unique in its ability to allow precise definition of the cell subpopulation with suppressive ability. The greater significance of this work however, lies in its similarity to T cells reported to play a critical role in human autoimmune disease. Some Lupus patients have effector CD4+ T cells with reduced expression of CD3-ζ so that they phosphorylate Syk rather than Zap-70 (10, 19, 20). Syk is also expressed at high levels in some human CD4+ effector T cells, induced by antibodies directed against CD4 (21). Recently Chauhan et. al. have reported that human T cells which express the receptor FcγRIII (i.e. γδ, NKT, and CD4+ T cells) can be induced by circulating immune complexes to become activated, differentiate, and proliferate via a Syk-mediated T cell pathway, in which FcRγ binds to the FcγRIII. These data bear striking similarities to our system, although the FcRγ interacts with the TCR in our A9-induced signaling pathway, (Park, et. al., manuscript in preparation). More work must be done clarify which surface receptors and antigenic triggers are required to induce this alternate pathway (22).
In this study and in our previous work, we demonstrate that the A9-immune CD4+ T cells secrete both IL-4 and IL10 (Table I). These data suggest that A9 induces and expands a population of inhibitory T cells which secrete predominately Th2 cytokines. We believe the induction and expansion of these inhibitory cells explains the profound suppression of arthritis observed following treatment with analog A9. The shift to a Th2 cytokine profile is quite significant, because Th2 cytokines are known to have inhibitory effects on autoimmune arthritis (23-25). Both IL-10 and IL-4 suppress CIA when they are administered to mice (26). Our previous work is consistent with the concept that IL-4 has a unique role in the suppression of arthritis that is only partially duplicated by other Th2-type cytokines in the absence of IL-4 (27).
Despite this observation, the molecular mechanism by which FcRγ mediates the suppressor capacity is unknown. We hypothesize that the structure of TCR is altered in response to A9 such that the TCR ζ is replaced by FcRγ. Since FcRγ is known to bind to Syk and not ZAP-70, this implies that binding between Syk and FcRγ rewires the cells to produce a different outcome. We have previously provided several lines of evidence that support this hypothesis: 1) expression of Syk but not ZAP-70 is enhanced in response to A9, 2) inhibition of Syk suppressed downstream events such as TCR-induced cytokine secretion and the induction of the Th2 associated nuclear factor GATA-3 (28). The differential expression of TCR ζ and FcRγ in T cell subsets might explain differential functional outcomes of TCR signaling. Therefore, we propose that FcRγ can be upregulated in T cells following exposure to certain carefully designed peptides and could serve as an important molecular target for controlling autoimmune arthritis.
In summary, we describe an inhibitory CD4+ cell which is induced by exposure to analog peptide/MHC complexes. Following activation, the T cells upregulate FcRγ and intracellular signaling leads to the secretion of predominately Th2-type cytokines rather than Th-1 or Th17 cytokines. This T inhibitory cell develops a phenotype characterized by the intracellular upregulation of FcRγ, without increasing typical activation or memory markers, nor Fox-P3. Our data suggest that the induction and expansion of these inhibitory cells leads to a profound suppression of arthritis, dependent upon the intracellular presence of FcRγ to suppress arthritis and secrete cytokines.
One of the fundamental, and most challenging, goals of immunological research is to devise a treatment that suppresses immunity to a particular antigen, thus halting an injurious autoimmune process, without disrupting the beneficial functions of the immune system such as surveillance for opportunistic infections and tumors. To this end, analog (or altered) peptide ligands are a particularly desirable type of antigen-specific immunotherapy and are well suited for treating autoimmune diseases (29, 30). Although the use of peptides as therapies for human illness is still in its infancy (29, 31, 32), recent reports together with our new data suggest that peptide ligands can modulate autoimmune arthritis by inducing regulatory or inhibitory T cells (33-35).
Acknowledgments
This work was supported, in part, by USPHS Grants AR-55661, AR-55266, and program-directed funds from the Department of Veterans Affairs and the Arthritis Foundation.
Abbreviations
- CII
Type II collagen
- RA
rheumatoid arthritis
- MHC
major histocompatibility complex
- APC
antigen presenting cell
- CIA
collagen induced arthritis
- TCR
t cell receptor
- FcRγ
the gamma chain of the FcεRI Receptor
- A2
a peptide representing the immunodominant determinant on CII in the region of amino acids 245-270
- qCII24tg
transgenic mice that express a TCR specific for A2
- A9
a synthetic peptide representing the sequence CII 245-270 but with substitutions at amino acids 260, 261 and 263
- Foxp3
transcription factor forkhead box P3 protein
References
- 1.Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61(19):1861–78. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- 2.Myers LK, Tang B, Rosioniec EF, Stuart JM, Kang AH. An altered peptide ligand of type II collagen suppresses autoimmune arthritis. Crit Rev Immunol. 2007;27(4):345–56. doi: 10.1615/critrevimmunol.v27.i4.40. [DOI] [PubMed] [Google Scholar]
- 3.Brand DD, Myers LK, Whittington KB, Latham KA, Stuart JM, Kang AH, et al. Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J Immunol. 2002;168(1):490–8. doi: 10.4049/jimmunol.168.1.490. [DOI] [PubMed] [Google Scholar]
- 4.Rosloniec EF, Cremer M, Kang AH, Myers LK, Brand DD. Collagen-induced arthritis. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im1505s89. Chapter 15:Unit 15 5 1-25. [DOI] [PubMed] [Google Scholar]
- 5.Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000;191(9):1611–6. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinau S. The impact of Fc receptors on the development of autoimmune diseases. Curr Pharm Des. 2003;9(23):1861–70. doi: 10.2174/1381612033454414. [DOI] [PubMed] [Google Scholar]
- 7.Nandakumar KS, Andren M, Martinsson P, Bajtner E, Hellstrom S, Holmdahl R, et al. Induction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIB. Eur J Immunol. 2003;33(8):2269–77. doi: 10.1002/eji.200323810. [DOI] [PubMed] [Google Scholar]
- 8.Rosloniec EF, Kang AH, Myers LK, Cremer MA. Collagen-induced arthritis. In: Coico R, Shevach E, editors. Current Protocols in Immunology. Wiley & Sons; New York, NY: 1997. pp. 15.5.1–24. [Google Scholar]
- 9.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan S, Juang YT, Chowdhury B, Magilavy A, Fisher CU, Nguyen H, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181(11):8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers L, Rosloniec E, Seyer J, Stuart J, Kang A. A synthetic peptide analogue of a determinant of type II collagen prevents the onset of collagen-induced arthritis. J Immunol. 1993;150:4652–4658. [PubMed] [Google Scholar]
- 12.Myers LK, Tang B, Rosloniec EF, Stuart JM, Chiang TM, Kang AH. Characterization of a peptide analog of a determinant of type II collagen that suppresses collagen-induced arthritis. J Immunol. 1998;161(7):3589–95. [PubMed] [Google Scholar]
- 13.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108(3):586–90. [PubMed] [Google Scholar]
- 14.Lehner T. Special regulatory T cell review: The resurgence of the concept of contrasuppression in immunoregulation. Immunology. 2008;123(1):40–4. doi: 10.1111/j.1365-2567.2007.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annu Rev Immunol. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 18.Thomson CW, Teft WA, Chen W, Lee BP, Madrenas J, Zhang L. FcR gamma presence in TCR complex of double-negative T cells is critical for their regulatory function. J Immunol. 2006;177(4):2250–7. doi: 10.4049/jimmunol.177.4.2250. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171(7):3325–31. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172(12):7821–31. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 21.Chentouf M, Ghannam S, Bes C, Troadec S, Cerutti M, Chardes T. Recombinant anti-CD4 antibody 13B8.2 blocks membrane-proximal events by excluding the Zap70 molecule and downstream targets SLP-76, PLC gamma 1, and Vav-1 from the CD4-segregated Brij 98 detergent-resistant raft domains. J Immunol. 2007;179(1):409–20. doi: 10.4049/jimmunol.179.1.409. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan A, Atkinson J, Moore TL. Altered T Cell Signaling in Systemic Lupus Erythematosus (SLE): A Role for Fcgamma RIII and Immune Complexes (ICs). Arthritis Rheum. 2010;62(10S):S352–353. [Google Scholar]
- 23.Joosten LA, Lubberts E, Helsen MM, Saxne T, Coenen-de Roo CJ, Heinegard D, et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1(1):81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166(5):3499–505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- 25.Nishibori T, Tanabe Y, Su L, David M. Impaired Development of CD4+ CD25+ Regulatory T Cells in the Absence of STAT1: Increased Susceptibility to Autoimmune Disease. J Exp Med. 2004;199(1):25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20. J Immunol. 2001;167(7):3545–9. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 27.Myers LK, Tang B, Stuart JM, Kang AH. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin Immunol. 2002;102(2):185–91. doi: 10.1006/clim.2001.5162. [DOI] [PubMed] [Google Scholar]
- 28.Tang B, Zhou J, Park JE, Cullins D, Yi AK, Kang AH, et al. T cell receptor signaling induced by an analog peptide of type II collagen requires activation of Syk. Clin Immunol. 2009;133(1):145–53. doi: 10.1016/j.clim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6(10):1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 30.Bielekova B, Martin R. Antigen-specific immunomodulation via altered peptide ligands. J Mol Med. 2001;79(10):552–65. doi: 10.1007/s001090100259. [DOI] [PubMed] [Google Scholar]
- 31.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6(10):1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Antel JP, Duquette P, Alleva DG, Conlon PJ, Bar-Or A. Persistence of immune responses to altered and native myelin antigens in patients with multiple sclerosis treated with altered peptide ligand. Clin Immunol. 2002;104(2):105–14. doi: 10.1006/clim.2002.5258. [DOI] [PubMed] [Google Scholar]
- 33.Leipe J, Skapenko A, Lipsky PE, Schulze-Koops H. Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):93. doi: 10.1186/ar1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101(12):4228–33. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL, et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60(11):3207–16. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]