Abstract
Increasingly, it is recognized that understanding and predicting nanoparticle behavior is often limited by the degree to which the particles can be reliably produced and adequately characterized. Two examples that demonstrate how sample preparation methods and processing history may significantly impact particle behavior are: 1) an examination of cerium oxide (ceria) particles reported in the literature in relation to the biological responses observed and 2) observations related that influence synthesis and aging of ceria nanoparticles. Examining data from the literature for ceria nanoparticles suggests that thermal history is one factor that has a strong influence on biological impact. Thermal processing may alter many physicochemical properties of the particles, including density, crystal structure, and the presence of surface contamination. However, these properties may not be sufficiently recorded or reported to determine the ultimate source of an observed impact. A second example shows the types of difficulties that can be encountered in efforts to apply a well-studied synthesis route to producing well-defined particles for biological studies. These examples and others further highlight the importance of characterizing particles thoroughly and recording details of particle processing and history that too often are underreported.
Keywords: nanomaterials, nanoparticles, ENM, cerium oxide, ceria, synthesis, nanocrystallite, biological endpoints
Introduction
Potential environmental and health impacts from the increasing use of various types, shapes, and sizes of engineered nanomaterials (ENMs) for applications in biomedicine; energy production and storage; sensors; and consumer products, such as cosmetics, have generated concern among members of the public and regulating authorities [1–4]. There also is growing awareness of the need to understand and characterize the properties of ENMs as they change from the time of synthesis to their final state during application or use and possible release in the environment [5–7]. Nanomaterials generally do not retain the same properties from their point of synthesis to their state of application, and both the particle processing and storage histories often are poorly documented.
Accurate characterization of ENMs often is compounded by the factors that make them interesting [8]. Their relative instability with respect to environmental and experimental probes [5], the impact of unintended surface contamination or deliberate coatings [9], the enrichment or depletion of elements on the particle surfaces, aging-based changes in the physical and chemical properties [10], and sample handling issues [11] all can lead to inaccurate or, at least, confusing nanoparticle characterizations [5, 9, 12]. We have sometimes observed that subtle changes in synthesis temperature (room temperature versus heating) and storage conditions (duration, light, and dark), as well as the nature of the synthesis or storage containers can lead to unpredictable or non-reproducible properties for some types of ENMs. Such subtle changes can, for example, shift the equilibrium of nanomaterials from relatively stable to unstable and reactive under what are thought to be “identical” conditions.
To formulate structure-property relationships of ENMs and evaluate their environmental and health impacts, knowledge of processing and sample history can be important. Insufficient surface characterization and under-emphasized processing history of ENMs often resulted in mixed and confusing reports about their biological impacts [9, 13–14]. A careful analysis of the nanoparticle literature points to the fact that nanomaterials are not necessarily created equal and may not behave in similar manners [15]. Even small differences in the handling and storage of nanomaterials synthesized using the same protocols may alter their properties. Although not a subtle process change, it has been shown that the chemical reactivity of nanoparticles formed in solution and tested without drying can be up to 10 times more reactive than particles from the same synthesis process dried and resuspended in solution before testing [16].
This short review highlights two examples demonstrating that sample preparation methods and processing history may significantly impact particle behavior: 1) an examination of cerium oxide (ceria) particles reported in the literature in relation to the biological responses observed and 2) our observations related to factors, known and unknown, which influence the synthesis and aging of ceria nanoparticles and impact our ability to deliver nearly identical particles for biological studies.
Variations in Biological properties of Cerium Oxide Nanoparticles Synthesized by Different Processes
Ceria nanoparticles form an important class of oxide nanomaterials popular for their use as polishing agents in chemical mechanical planarization (CMP), an electrolyte in solid oxide fuel cells (SOFC), and catalysts in automotive exhaust systems. Many of the applications derive from the redox ability of ceria nanoparticles, wherein the oxidation state of cerium can switch between Ce4+ and Ce3+ while maintaining the fluorite lattice structure [17–19]. The oxidation state of cerium can be controlled by synthesis, size, and addition of various dopants [20–21]. For example, the trivalent oxidation state can be increased by doping the ceria lattice with trivalent lanthanide cations, such as Sm, or by decreasing the size of the nanoparticles that creates surface oxygen vacancies resulting in a localized reduction of cerium cations [21].
Recently, some types of ceria nanoparticles have been found to possess biological antioxidant properties that can protect cells from damage caused by reactive oxygen species (ROS) in various in vitro and in vivo conditions, including radiotherapy and cancer therapy [17–19, 22–26]. In direct contrast, several investigations reported that the oxidative properties of ceria nanoparticles cause severe oxidative stress, resulting in cell death at physiologically relevant conditions [27–38]. Still, other studies have found ceria nanoparticles to be rather unreactive and benign to cells [39–44]. In an attempt to understand the apparent discrepancies in the literature, we examined the types of ceria particles, characterization levels, and the processing history of these ENMs in studies published between 2005 and June 2011. Many of the reports involved nanoparticles synthesized using entirely different synthesis protocols. Some of the ENMs were purchased directly from commercial vendors and used in toxicological studies without reporting any detailed characterization of the surface and bulk properties [34, 45]. In one study, researchers identified the presence of an unknown surfactant using Fourier transform infrared spectroscopic analysis of the “as-is” ENMs received from a commercial vendor. However, the possible importance of the surfactant in the ENMs’ reported toxicity did not appear to be considered [34]. At this time, there does not appear to be any published attempt to systematically compare the biological activity of similar-sized ceria nanoparticles prepared by entirely different procedures.
Classification of ENMs based on synthesis conditions
Among the reported studies on the biological properties of ceria nanoparticles, it was evident that at least three important characteristics of cerium oxide nanoparticles were not always adequately identified: surface composition, sample processing, and sample storage (duration and conditions). Although the data are not complete in many ways (e.g., the data were insufficient to allow direct comparison of the oxidation state of cerium, the surface composition, or processing history), it is well known that the temperatures used during sample synthesis and processing can have significant impact. Synthesis temperature affects many properties of ENMs, such as agglomeration, crystallite size, crystal structure, surface defects, and oxidation state. Therefore, the synthesis of ceria nanoparticles from the published studies were divided into following categories based on their exposure to specific ranges of temperature:
High Temperature: Nanomaterials heated or calcined at >300°C (e.g., sintering, calcination, high-temperature or flame pyrolysis, and thermal decomposition)—no surfactant or coatings are expected after pyrolysis though surfactants may be added after heat treatment to disperse nanoparticles
Heated in Solvent: Nanomaterials heated in solvents <100°C (e.g., thermal hydrolysis, solvothermal, and hydrothermal)—with or without surfactants/coatings
Room Temperature: Nanomaterials synthesized at room temperature (e.g., acid or base hydrolysis or microemulsion)—with or without surfactants/coatings.
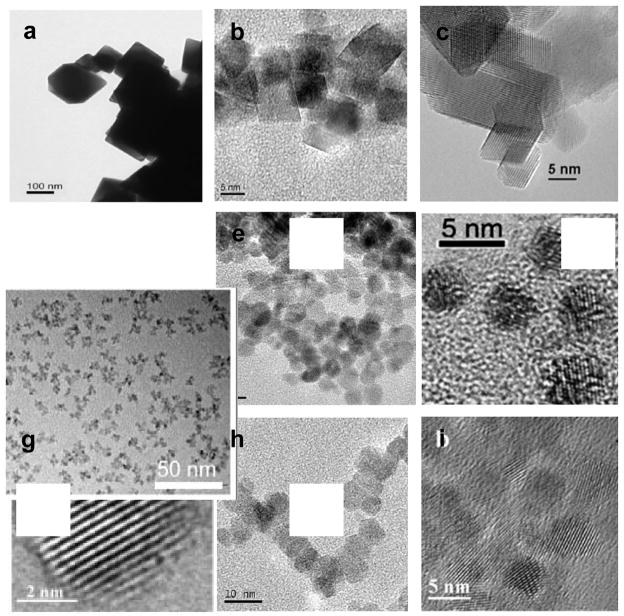
An illustration of particle sizes and morphologies from each of these categories is shown in Figure 1. The figure demonstrates that studies reporting different conclusions on the biological impact of ceria have used particles with significantly different sizes, shapes, and morphologies. High-temperature methods often involve techniques such as spray pyrolysis and sol-gel synthesis followed by high temperature calcination of nanoparticles to crystallize nanoparticles and remove unwanted impurities. Ceria nanoparticles prepared by high-temperature processing may be larger in size (>25 nm), crystalline, dense (fewer vacancies or other defects), and more likely to form hard agglomerates during the processing than particles formed or processed at lower temperatures. High-temperature processing also is more apt to create ceria nanoparticles with sharp facets or edges resembling the octahedron or truncated octahedron morphology of cerium oxide. In the catalysis community, it is well known that edges and facets frequently have different chemical reactivity than the usually spherical or non-faceted crystallites. In addition, high-temperature processing may make ceria nanoparticles more stable by annealing surface defects that may not be as prone to demonstrating the environmental and time-dependent shifts in the physical and chemical state of nanoparticles observed for smaller particles.
Figure 1.
Representative sizes, shapes, morphologies, and agglomeration states of cerium oxide nanoparticles synthesized by high-temperature processing (a–c) (28; 34; 35); indirect heating of precursors or nanoparticles in solution (d–f) (39; 42; 43); and room-temperature synthesis of nanoparticles (g–h) (25; 46; 47).
In contrast to high-temperature processing where the nanomaterials experience direct heating, ceria nanoparticles synthesized by heating the precursors or nanoparticles indirectly in solvent media may be smaller and have more uniform size distribution. These synthesis methods include techniques such as hydrothermal, solvothermal, and high-temperature hydrolysis of cerium salts. These nanoparticles are often less agglomerated, less crystalline and frequently appear to have uniform spherical morphology (though they may be faceted at the nanoscale) than particles formed or processed at higher temperatures. Indirect heating in the presence of solvents leads to weak agglomeration of nanoparticles and better dispersibility in aqueous media. Nanoparticles prepared by this method are more likely to have a layer of surfactant or solvent molecules as an intentional or unintentional coating on their surface. Thus, detailed surface characterization should be performed to characterize composition of the particle surfaces.
The third temperature category involves synthesis of ceria nanoparticles at room temperature without any direct or indirect heating of nanoparticles. These nanoparticles are weakly agglomerated even in absence of surfactants or coatings compared with the hard agglomerates formed during high-temperature calcinations. Room-temperature synthesis can sometimes create nanoparticles with relatively uniform size distribution—roughly spherical in morphology. Often, 10–20 nm particles are made up of smaller 3- to 6-nm crystalline grains. In addition, the room-temperature synthesis of ceria nanoparticles produces particles that retain more surface defects; have a higher Ce3+/Ce4+ ratio; appear to be less stable; and are more likely to undergo time-, temperature-, and environment-induced changes usually reflected in agglomerate sizes, surface charge, self-assembly, and changing oxidation states [46–49]. For example, ceria nanoparticles react with phosphate-buffered saline, resulting in the loss of the superoxide dismutase properties otherwise exhibited by cerium oxide [50]. Previously, we showed these nanoparticles to undergo time-dependent changes in the oxidation state during their synthesis with hydrogen peroxide [46, 48].
Biological responses of ceria nanoparticles as classified by synthesis
To evaluate the relationship between bioactivity and the method of particle synthesis, the results from studies on the biological properties of ceria nanoparticles were sorted by the three synthesis categories already described. In Table 1, biological effects from the exposure of ceria nanoparticles were grouped in three basic responses as: 1) pro-oxidative (red), 2) anti-oxidative (blue), or 3) no effect or ambiguous (green). For simplification, all reports indicating inflammatory response from ceria nanoparticles were grouped as pro-oxidative while all studies reporting beneficial effects were grouped as anti-oxidative. In addition studies that have mixed response or showed no effect from addition of ceria nanoparticles were grouped as no effect or ambiguous. As the table indicates, most of the pro-oxidative results reported on ceria nanoparticles involved the nanoparticles being synthesized using direct high-temperature exposure processes. Conversely, studies using ceria nanoparticles synthesized by low heat in solvents or at room temperature more often reported little or ambiguous response or anti-oxidative response. It must be noted that numerous results from Seal and coworkers [22–26, 48, 51–57] consistently describe anti-oxidative activity of ceria nanoparticles synthesized at room temperature. Per Table 1, it is clear that the synthesis method used to create nanoparticles may explain some of the discrepancies among the biological responses reported. However, the method of synthesis is not the only variable to be considered. In addition to the materials characteristics, the protocols for biological/toxicological processing, surface composition, and the specific biological system and endpoint being tested also are important variables in these studies that are not included in this review. Other processing parameters, such as sample storage and handling, surface composition, impurities, surface coatings, and aging, also are important ENM characteristics that may influence the diversity in biological responses to ceria nanoparticles. Furthermore, during processing, the high surface area of ENMs makes them particularly susceptible for adsorption of surface impurities.
Table 1.
Classification of Biological Responses of Cerium Oxide Nanoparticles based on Synthesis Protocols
| Synthesis | Study | Response | Ref |
|---|---|---|---|
| High temperature | Inflammation in lungs by metal oxide nanoparticles | Pro-oxidative | [36] |
| Oxidative stress of ceria nanoparticles in bronchial epithelial cells | Pro-oxidative | [28] | |
| Inflammatory response in mice treated with ceria nanoparticles by intratracheal instillation | Pro-oxidative | [37] | |
| Biodistribution and oxidative stress of commercial ceria nanomaterials | Pro-oxidative | [34] | |
| Toxicity of cerium oxide nanoparticles in human lung cancer cells | Pro-oxidative | [30] | |
| Ceria-nanoparticle-induced pulmonary inflammation in rats | Pro-oxidative | [31] | |
| Oxidative stress induced by ceria nanoparticles in BEAS-2B cells | Pro-oxidative | [32] | |
| Cerium oxide nanoparticles trigger neuronal survival | Anti-oxidative | [69] | |
| Cardioprotective effects of ceria nanoparticles | Anti-oxidative | [70] | |
| Comparison of toxicity of zinc oxide and ceria nanoparticles based on dissolution of metal ions | Anti-oxidative | [71] | |
| Screening nanoparticulate ceria as a diesel fuel additive | Neutral or Both | [72] | |
| Hazard and risk assessment of ceria nanoparticles | Neutral or Both | [73] | |
| Effect of ceria nanoparticles in vascular endothelial cells | Neutral or Both | [74] | |
| Heated in Solvent | Cytotoxicity of ceria nanoparticles for E coli | Neutral or Both | [44] |
| Nanoceria exhibits no detrimental effects on eye lens proteins | Neutral or Both | [41] | |
| Interaction between ceria nanoparticles and 3T3 fibroblasts | Neutral or Both | [43] | |
| DNA damage in human dermal fibroblasts by ceria nanoparticles | Pro-oxidative | [27] | |
| Brain distribution and toxicological evaluation of ceria nanoparticles | Neutral or Both | [39] | |
| Adverse effects of ceria nanoparticles at environmentally relevant concentrations | Pro-oxidative | [35] | |
| Room Temperature | Altered vascular reactivity and ischemia-reperfusion injury following ceria nanoparticle instillation | Pro-oxidative | [45] |
| pH-dependent antioxidant activity of ceria nanoparticles | Anti-oxidative | [75] | |
| Yttria and ceria nanoparticles are neuroprotective | Anti-oxidative | [76] | |
| Ceria nanoparticles inhibit oxidative stress in H9c2 cardiomyocytes exposed to cigarette smoke | Anti-oxidative | [77] | |
| Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor stroma–interactions | Anti-oxidative | [22] | |
| Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides | Anti-oxidative | [23] | |
| Protection from radiation-induced pneumonitis using cerium oxide nanoparticles | Anti-oxidative | [24] | |
| Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons | Anti-oxidative | [25] | |
| Vacancy-engineered ceria nanostructures for protection from radiation-induced cellular damage | Anti-oxidative | [26] | |
| PEGylated Nanoceria as Radical Scavenger with Tunable Redox Chemistry | Anti-oxidative | [48] | |
| The role of cerium redox state in the SOD mimetic activity of nanoceria | Anti-oxidative | [52] | |
| Anti-inflammatory properties of cerium oxide nanoparticles | Anti-oxidative | [53] | |
| Rare earth oxides as nanoadditives in 3-D nanocomposite scaffolds for bone regeneration | Anti-oxidative | [55] | |
| Superoxide dismutase mimetic properties exhibited by vacancy-engineered ceria nanoparticles | Anti-oxidative | [56] | |
| Nanoceria Inhibit the Development and Promote the Regression of Pathologic Retinal Neovascularization in the Vldlr Knockout Mouse | Anti-oxidative | [57] |
Although the surfaces of ENMs usually are coated with proteins or other biomolecules in biological environments, surface impurities can affect the biological responses by altering the particle surface or affecting the interaction of ENMs with proteins or biomolecules and modifying the overall surface charge and size distribution of ENMs. Elucidating the effect of each of the sample processing parameters is outside the scope of this current review. However, the possible effects of storage and handling on the overall stability of the ENMs and pH of the solvent medium as a function of time is described in the next section.
The observation that particles with different crystalline structures or a high number of defects may have differing biological endpoints is not new and results for silica nanoparticles appear consistent with the implied behaviors here. For instance, ceria nanoparticles synthesized by direct heating at high temperature show higher toxicity that might be explained, in part, by the formation of crystalline nanoparticles. Crystalline (silica) or quartz particles induce sustained lung inflammation and cytotoxicity whereas spherical amorphous silica particles induce reversible and transient inflammatory responses in the lung that resolve within a few months [58–61]. Long term exposures to quartz can lead to silicosis, an occupational lung disease, and pose a carcinogenic risk to human [59, 62–63]. However, reported cases of silicosis and lung cancer among workers in glass factories, where workers are exposed to vitreous silica particles that are amorphous [64–65], raise the possibility that crystallinity might not be a necessary prerequisite for toxicity. A recent study showed that unlike amorphous silica spheres, vitreous silica and quartz particles have irregular shapes with sharp edges, potential to release free radicals, and strong hydrophilic sites [66], all of which could account for the observed toxicity. Quartz toxicity has been thought to partially result from inability to clear the particles from the lung [67]. The dissolution rate of vitreous silica is higher than that of quartz but lower than any other amorphous silica forms, supporting the idea that the degree of dissolution and clearance might play a critical role in particle toxicity.
Variations in Ceria Nanoparticle Behavior During Aging and Storage
For many years, members of this research team and collaborators have synthesized ceria nanoparticles at room temperature by wet chemical processing and studied the effect of time, temperature, and aging on their chemistry, self-assembly, and agglomeration [46, 49]. Briefly, stoichiometric amounts of hydrogen peroxide are added to a homogenous solution of cerium nitrate hexahydrate at room temperature, and the solution is allowed to age at room temperature under ambient laboratory conditions. The advantage of using this synthesis process is the formation of 12–20 nm particles that are loose agglomerates constructed of 5–7 nm sized nanocrystallite grains. Hydrogen peroxide converts Ce3+ ions to Ce4+, which, in an aqueous environment, leads to immediate hydrolysis and formation of ceria nanoparticles. The pH of this solution changes (between 3.0–3.8) with the onset of nanoparticle aging (0–4wks) as the ceria nanoparticles nucleate and grow into individual nanocrystallites, depending upon the concentration of precursor cerium ions and hydrogen peroxide [46].
During aging, several things happen during that are affected by time, temperature, and nanoparticle concentrations. During initial growth, Ce3+ is converted to Ce4+ while simultaneously forming ceria nanoparticles. With aging, hydrogen peroxide decomposes and the oxidation potential of the solution decreases leading to the formation of ceria nanoparticles with a predominantly Ce3+ oxidation state. Changes in the ceria particles’ oxidation state has been observed in solution by optical adsorption via visible color changes and on particles removed from solution using X-ray photoelectron spectroscopy [5]. Aging of a low concentration (≈5mM) of ceria nanoparticles in aqueous solution leads to random agglomeration in the absence of any surfactants. A low pH helps avoid heavy precipitation or hard agglomeration of nanoparticles. Aging of these nanoparticles in aqueous solution at a concentration of ≈30mM leads to the self-assembly of the nanoparticles into sharp and faceted fractal superoctahedra (>50nm) while maintaining the individual 3–5nm ceria nanocrystallites [49, 68]. Refrigeration of nanoparticles inhibits this fractal self-assembly. However, freezing this solution of nanoparticles in standard laboratory refrigerators leads to their self-assembly as polycrystalline nanorods (oriented aggregation) [47]. Although we have synthesized and characterized these nanoparticles with great detail previously and they retained their stability beyond one year, we observed inconsistent behavior of these nanoparticles when only minimal changes were introduced in the synthesis protocol for purposes of biological studies.
As we prepared a set of nanoparticles for toxicological studies, we used sterile plastic PET (polyethylene terephthalate) containers (VWR International – Cat number 89132-064) rather than the previously used acid-washed and autoclaved glassware and pyrogen-free water in lieu of standard deionized water used previously. In addition, we used a new supply of the cerium nitrate hexahydrate precursor. With these changes we found that in some circumstances particles synthesized by a process for which the products had been previously stable for periods of up to a year would start to dissolve within 60 days. When a few differing characteristics were discovered, we began a systematic investigation. Particles were grown in both glass and plastic vials. Although the particles that nucleated and grew were initially similar in size, the nanoparticles in different types of vials aged differently as shown in Figure 2.
Figure 2.
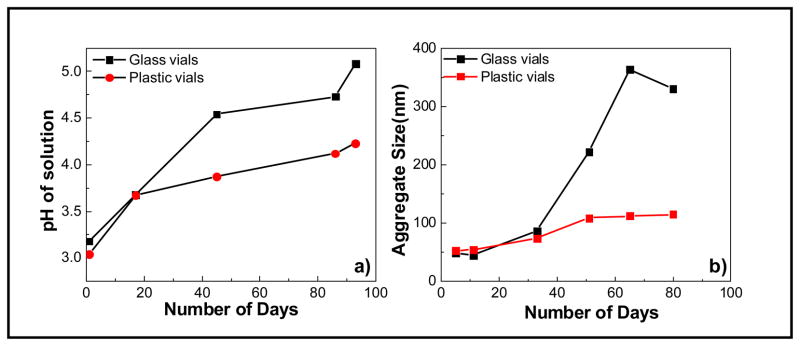
Time-dependent changes in a) pH and b) agglomeration of cerium oxide nanoparticles stored and aged in glass and plastic vials (color coded version appears online).
In contrast to our historical observations of a gradual increase in pH from around 3.0 to 3.8 within a span of 4–8 weeks, the pH values increased from 3.0 to greater than 4.5 within 6 weeks of aging. Thus, the changes in water, the new supply of the cerium nitrate hexahydrate, or the different storage containers and vials used for aging the nanoparticles are causing a change in the kinetics of the pH increase in comparison to the historic data. In addition, particles synthesized and stored in plastic showed different kinetics of change in pH compared to particles in glass. Figure 2a shows the change in the pH of these nanoparticles aged in glass and plastic vials. While the pH of the nanoparticles stored in glass vials changes from an initial value of 3.0 to 4.5 within 6 weeks of aging, the pH of the solution containing the nanoparticles stored in plastic vials remained below 4.0 during a similar aging time and stabilized at a final value of 4.25 after 13 weeks. The tendency of the nanoparticles to agglomerate is a direct consequence of the change in pH. It is well known that nanoparticles, especially oxides, form stable colloidal suspensions at high acidic or basic pH far away from their isoelectric point (IEP) due to the charged nanoparticle surfaces that repel each other. As the pH of the solution increases and approaches the IEP, the surface charge decreases, this, in turn, decreases the electrostatic stabilization resulting in nanoparticle agglomeration.
The same agglomeration patterns were observed for ceria nanoparticles aged in glass and plastic vials. The nanoparticles aged in glass vials showed higher agglomerate sizes as opposed to nanoparticles aged in PET vials. The agglomeration trend is correlated with the rate of change in pH and the final value of pH obtained for ceria nanoparticles. While we are evaluating the cause of the spontaneous pH increase of the solution compared to the typically observed behavior, the effect of aging and storage condition illustrates the importance of paying close attention to the handling, storage, and reactive and responsive nature of nanoparticles.
Summary
The observations summarized in this paper for ceria nanoparticles focus attention on the well-established but often ignored fact that the chemical and physical properties of many nanoparticles are highly dependent on the details surrounding their synthesis and subsequent processing, as well as the time and environment where they are stored or applied. Examination of the literature suggests that the biological impact of ceria nanoparticles can be influenced by the pervious thermal history with particles exposed to higher temperatures during synthesis (likely more crystalline, larger, and sometimes with well-developed surface facets) often more pro-oxidative than particles that have not been heated.
It is highly likely that the factors controlling the biological impact of particles also include other factors such as surface chemistry, residue from the synthesis process, or a variety of other factors—some of which are not reported in the publications examined in this paper. These uncertainties further emphasize the idea that many different factors can control particle behavior and one type of ceria nanoparticle does not necessarily behave in the same manner as other ceria nanoparticles, which may be the same size but have different histories. Such variability in particle behavior has both positive and negative implications for understanding behaviors, including environmental and biological impact or toxicity. The variability in properties may explain the diversity of behaviors reported in the literature, but it places increased importance on the need for accurate record keeping relative to particle history and the need for more thorough particle characterization.
While many parts of the synthesis process are well understood and reproducible in the hands of experienced researchers, our results show that small and seemingly inconsequential differences can significantly alter either the final product or the aging process. This also may explain some of the variability in results.
Still, it must be emphasized that our understanding of the behaviors of the numerous nanoparticle types being created essentially is in its infancy. It is highly probable that additional fundamental research on particle synthesis and properties, as well as additional experience in producing particles for technological applications, will help overcome what currently appears as a significant barrier to high-quality research addressing important questions. Previously, others have indicated the need for additional analysis tools [7] and more careful application of the tools already available [5–6]. At this point, it may be adequate to appropriately acknowledge nanoparticles are not necessarily created equal and thorough characterization (recognizing the various characterization challenges) along with careful records associated with synthesis, processing, storage, and handling can assist in developing the needed information and understanding to measure and ultimately predict the behaviors and impacts of specific nanoparticles in different applications or environments.
Acknowledgments
Aspects of the work have been supported by the U.S. Department of Energy’s (DOE) Offices of Basic Energy Sciences and Biological and Environmental Research (BER) and the National Institute of Environmental Health Sciences under Grant NIH U19 ES019544. Portions of this research were performed using EMSL, a national scientific user facility sponsored by DOE-BER and located at Pacific Northwest National Laboratory.
References
- 1.Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- 2.Oberdorster G, Ferin J, Lehnert BE. Environmental Health Perspectives. 1994;102:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberdorster G, Oberdorster E, Oberdorster J. Environmental Health Perspectives. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nel A, Xia T, Madler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 5.Baer DR, Amonette JE, Engelhard MH, Gaspar DJ, Karakoti AS, Kuchibhatla S, Nachimuthu P, Nurmi JT, Qiang Y, Sarathy V, Seal S, Sharma A, Tratnyek PG, Wang CM. Surface and Interface Analysis. 2008;40:529–537. [Google Scholar]
- 6.Grainger DW, Castner DG. Advanced Materials. 2008;20:867–877. [Google Scholar]
- 7.Grassian VH. Journal of Physical Chemistry C. 2008;112:18303–18313. [Google Scholar]
- 8.Baer DR. Journal of Surface Analysis. 2011;17:163–169. doi: 10.1384/jsa.17.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baer DR, Gaspar DJ, Nachimuthu P, Techane SD, Castner DG. Analytical and Bioanalytical Chemistry. 2010;396:983–1002. doi: 10.1007/s00216-009-3360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarathy V, Tratnyek PG, Nurmi JT, Baer DR, Amonette JE, Chun CL, Penn RL, Reardon EJ. Journal of Physical Chemistry C. 2008;112:2286–2293. [Google Scholar]
- 11.I. T. 14187:2011, ISO, 2011.
- 12.Baer DR, Engelhard MH. Journal of Electron Spectroscopy and Related Phenomena. 2010;178:415–432. [Google Scholar]
- 13.Cressey D. Nature. 2010;467:264–265. doi: 10.1038/467264b. [DOI] [PubMed] [Google Scholar]
- 14.Stuart C. Small Times. 2006. p. 6. [Google Scholar]
- 15.Moore K, Forsberg B, Baer DR, Arnold WA, Penn RL. Journal of Environmental Engineering. 2011 [Google Scholar]
- 16.Erbs JJ, Gilbert B, Penn RL. Journal of Physical Chemistry C. 2008;112:12127–12133. [Google Scholar]
- 17.Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Chemical Society Reviews. 2010;39:4422–4432. doi: 10.1039/b919677n. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov VK, Shcherbakov AB, Usatenko AV. Russian Chemical Reviews. 2009;78:855–871. [Google Scholar]
- 19.Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Nanoscale. 2011;3:1411–1420. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande S, Patil S, Kuchibhatla S, Seal S. Applied Physics Letters. 2005:87. [Google Scholar]
- 21.Patil S, Seal S, Guo Y, Schulte A, Norwood J. Applied Physics Letters. 2006:88. [Google Scholar]
- 22.Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S, Seal S, Brenneisen P. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Chen JP, Patil S, Seal S, McGinnis JF. Nature Nanotechnology. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 24.Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, Seal S, Jenkins DW, Baker CH. Nanomedicine-Nanotechnology Biology and Medicine. 2009;5:225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ. Biomaterials. 2007;28:1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarnuzzer RW, Colon J, Patil S, Seal S. Nano Letters. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 27.Auffan M, Rose J, Orsiere T, De Meo M, Thill A, Zeyons O, Proux O, Masion A, Chaurand P, Spalla O, Botta A, Wiesner MR, Bottero JY. Nanotoxicology. 2009;3:161–U115. [Google Scholar]
- 28.Eom HJ, Choi J. Toxicology Letters. 2009;187:77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Garcia A, Espinosa R, Delgado L, Casals E, Gonzalez E, Puntes V, Barata C, Font X, Sanchez A. Desalination. 2011;269:136–141. [Google Scholar]
- 30.Lin WS, Huang YW, Zhou XD, Ma YF. International Journal of Toxicology. 2006;25:451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 31.Ma JY, Zhao H, Mercer RR, Barger M, Rao M, Meighan T, Schwegler-Berry D, Castranova V, Ma JK. Nanotoxicology. 2011;5:312–325. doi: 10.3109/17435390.2010.519835. [DOI] [PubMed] [Google Scholar]
- 32.Park EJ, Choi J, Park YK, Park K. Toxicology. 2008;245:90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Srinivas A, Rao PJ, Selvam G, Murthy PB, Reddy PN. Toxicology Letters. 2011;205:105–115. doi: 10.1016/j.toxlet.2011.05.1027. [DOI] [PubMed] [Google Scholar]
- 34.Yokel RA, Florence RL, Unrine JM, Tseng MT, Graham UM, Wu P, Grulke EA, Sultana R, Hardas SS, Butterfield DA. Nanotoxicology. 2009;3:234–248. doi: 10.3109/17435390.2013.868059. [DOI] [PubMed] [Google Scholar]
- 35.Zhang HFZ, He XA, Zhang ZY, Zhang P, Li YY, Ma YH, Kuang YS, Zhao YL, Chai ZF. Environmental Science & Technology. 2011;45:3725–3730. doi: 10.1021/es103309n. [DOI] [PubMed] [Google Scholar]
- 36.Cho WS, Duffin R, Poland CA, Howie SEM, MacNee W, Bradley M, Megson IL, Donaldson K. Environmental Health Perspectives. 2010;118:1699–1706. doi: 10.1289/ehp.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park EJ, Cho WS, Jeong J, Yi JH, Choi K, Kim Y, Park K. Journal of Health Science. 2010;56:387–396. [Google Scholar]
- 38.Zeyons O, Thill A, Chauvat F, Menguy N, Cassier-Chauvat C, Orear C, Daraspe J, Auffan M, Rose J, Spalla O. Nanotoxicology. 2009;3:284–295. [Google Scholar]
- 39.Hardas SS, Butterfield DA, Sultana R, Tseng MT, Dan M, Florence RL, Unrine JM, Graham UM, Wu P, Grulke EA, Yokel RA. Toxicological Sciences. 2010;116:562–576. doi: 10.1093/toxsci/kfq137. [DOI] [PubMed] [Google Scholar]
- 40.He XA, Zhang HF, Ma YH, Bai W, Zhang ZY, Lu K, Ding YY, Zhao YL, Chai ZF. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/28/285103. [DOI] [PubMed] [Google Scholar]
- 41.Pierscionek BK, Keenan J, Yasseen A, Colhoun LM, Li YB, Schachar RA, Chen W. Current Analytical Chemistry. 2010;6:172–176. [Google Scholar]
- 42.Pierscionek BK, Li YB, Yasseen AA, Colhoun LM, Schachar RA, Chen W. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/3/035102. [DOI] [PubMed] [Google Scholar]
- 43.Safi M, Sarrouj H, Sandre O, Mignet N, Berret JF. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/14/145103. [DOI] [PubMed] [Google Scholar]
- 44.Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Environmental Science & Technology. 2006;40:6151–6156. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- 45.Wingard JC, Walters DM, Cathey BL, Hilderbrand SC, Katwa P, Lin S, Ke PC, Podila R, Rao A, Lust RM, Brown JM. Nanotoxicology. 2010:1–15. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karakoti AS, Kuchibhatla S, Babu KS, Seal S. Journal of Physical Chemistry C. 2007;111:17232–17240. [Google Scholar]
- 47.Karakoti AS, Kuchibhatla S, Baer DR, Thevuthasan S, Sayle DC, Seal S. Small. 2008;4:1210–1216. doi: 10.1002/smll.200800219. [DOI] [PubMed] [Google Scholar]
- 48.Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla S, Wozniak K, Self WT, Seal S. Journal of the American Chemical Society. 2009;131:14144. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuchibhatla S, Karakoti AS, Seal S. Nanotechnology. 2007:18. doi: 10.1088/0957-4484/18/7/075303. [DOI] [PubMed] [Google Scholar]
- 50.Singh S, Dosani T, Karakoti AS, Kumar A, Seal S, Self WT. Biomaterials. 2011;32:6745–6753. doi: 10.1016/j.biomaterials.2011.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowding JM, Lubitz S, Karakoti A, Kim A, Seal S, Ellisman M, Perkins G, Bossy-Wetzel E, Self W. Free Radical Biology and Medicine. 2010;49:S181–S181. [Google Scholar]
- 52.Heckert EG, Karakoti AS, Seal S, Self WT. Biomaterials. 2008;29:2705–2709. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 54.Karakoti AS, Monteiro-Riviere NA, Aggarwal R, Davis JP, Narayan RJ, Self WT, McGinnis J, Seal S. Jom. 2008;60:33–37. doi: 10.1007/s11837-008-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karakoti AS, Tsigkou O, Yue S, Lee PD, Stevens MM, Jones JR, Seal S. Journal of Materials Chemistry. 2010;20:8912–8919. [Google Scholar]
- 56.Korsvik C, Patil S, Seal S, Self WT. Chemical Communications. 2007:1056–1058. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 57.Zhou XH, Wong LL, Karakoti AS, Seal S, McGinnis JF. Plos One. 2011:6. doi: 10.1371/journal.pone.0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA. Food and Chemical Toxicology. 1991;29:341–354. doi: 10.1016/0278-6915(91)90205-l. [DOI] [PubMed] [Google Scholar]
- 59.Warheit DB. 2001;20:9. [Google Scholar]
- 60.Warheit DB, McHugh TA, Hartsky MA. Scand J Work Environ Health. 1995;2:19–21. [PubMed] [Google Scholar]
- 61.Sayes CM, Reed KL, Warheit DB. Toxicological Sciences. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 62.McLaughlin JK, Chow WH, Levy LS. Journal of Toxicology and Environmental Health. 1997;50:553–566. doi: 10.1080/15287399709532054. [DOI] [PubMed] [Google Scholar]
- 63.Markus R. Mutation Research/Reviews in Mutation Research. 727:72–85. [Google Scholar]
- 64.Munn NJ, Thomas SW, DeMesquita S. Chest. 1990;98:871–874. doi: 10.1378/chest.98.4.871. [DOI] [PubMed] [Google Scholar]
- 65.Sankila R, Karjalainen S, Pukkala E, Oksanen H, Hakulinen T, Teppo L, Hakama M. British Journal of Industrial Medicine. 1990;47:815–818. doi: 10.1136/oem.47.12.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghiazza M, Polimeni M, Fenoglio I, Gazzano E, Ghigo D, Fubini B. Chemical Research in Toxicology. 2010;23:620–629. doi: 10.1021/tx900369x. [DOI] [PubMed] [Google Scholar]
- 67.Arts JHE, Muijser H, Duistermaat E, Junker K, Kuper CF. Food and Chemical Toxicology. 2007;45:1856–1867. doi: 10.1016/j.fct.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Kuchibhatla S, Karakoti AS, Sayle DC, Heinrich H, Seal S. Crystal Growth & Design. 2009;9:1614–1620. [Google Scholar]
- 69.D’Angelo B, Santucci S, Benedetti E, Di Loreto S, Phani RA, Falone S, Amicarelli F, Ceru MP, Cimini A. Current Nanoscience. 2009;5:167–176. doi: 10.2174/156720509788486518. [DOI] [PubMed] [Google Scholar]
- 70.Niu JL, Azfer A, Rogers LM, Wang XH, Kolattukudy PE. Cardiovascular Research. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi HB, Yeh JI, Zink JI, Nel AE. Acs Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park B, Martin P, Harris C, Guest R, Whittingham A, Jenkinson P, Handley J. Particle and Fibre Toxicology. 2007;4:1–10. doi: 10.1186/1743-8977-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park B, Donaldson K, Duffin R, Tran L, Kelly F, Mudway I, Morin JP, Guest R, Jenkinson P, Samaras Z, Giannouli M, Kouridis H, Martin P. Inhalation Toxicology. 2008;20:547–566. doi: 10.1080/08958370801915309. [DOI] [PubMed] [Google Scholar]
- 74.Gojova A, Lee JT, Jung HS, Guo B, Barakat AI, Kennedy IM. Inhalation Toxicology. 2009;21:123–130. doi: 10.1080/08958370902942582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez JM, Asati A, Nath S, Kaittanis C. Small. 2008;4:552–556. doi: 10.1002/smll.200700824. [DOI] [PubMed] [Google Scholar]
- 76.Schubert D, Dargusch R, Raitano J, Chan SW. Biochemical and Biophysical Research Communications. 2006;342:86–91. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- 77.Niu JL, Wang KK, Kolattukudy PE. Journal of Pharmacology and Experimental Therapeutics. 2011;338:53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]