Abstract
The eye has protective barriers (ie, the conjunctival and corneal membranes) and defense mechanisms (ie, reflex tearing, blinking, lacrimal drainage) which present challenges to topical drug delivery. Topical ocular corticosteroids are commonly used in the treatment of anterior segment diseases and inflammation associated with ocular surgery, and manufacturers continually strive to improve their characteristics. We describe the development of a novel ophthalmic gel formulation of loteprednol etabonate (LE), a C-20 ester-based corticosteroid with an established safety profile, in the treatment of ocular inflammatory conditions. The new LE gel formulation is non-settling, eliminating the need to shake the product to resuspend the drug, has a pH close to that of tears, and a low preservative concentration. The rheological characteristics of LE gel are such that the formulation is instilled as a drop and transitions to a fluid upon instillation in the eye, yet retains sufficient viscosity to prolong ocular surface retention. The new formulation provides consistent, uniform dosing as evidenced by dose extrusion studies, while pharmacokinetic studies in rabbits demonstrated rapid and sustained exposure to LE in ocular tissues following instillation of LE gel. Finally, results from two clinical studies of LE gel in the treatment of postoperative inflammation and pain following cataract surgery indicate that it was safe and effective. Most patients reported no unpleasant drop sensation upon instillation, and reports of blurred vision were rare.
Keywords: loteprednol etabonate, gel, drug delivery, clinical trial, ocular surface
Topical corticosteroids are commonly prescribed in ophthalmology for the treatment of anterior segment inflammation due to their broad anti-inflammatory effects. For instance, they inhibit prostaglandin and leukotriene synthesis through inhibition of phospholipase A2 by promoting the synthesis of lipocortins that block phospholipase A2; they suppress capillary dilation, inhibit macrophage and neutrophil migration, and reduce T cells and B cells responsible for the inflammatory response; and they also stabilize intracellular and extracellular membranes.1,2 Corticosteroids mediate their effects primarily by binding to and modifying the activity of cytosolic glucocorticoid receptor (GR) at the genomic level.1,3,4 Corticosteroids bind to the GR in the cytoplasm, after which the corticosteroid-GR complex migrates to the nucleus and inhibits the expression of pro-inflammatory proteins while inducing the expression of anti-inflammatory proteins. The corticosteroid-GR complex also elicits non-genomic effects, such as inhibition of vasodilation, vascular permeability, and migration of leukocytes.2,4 In addition, corticosteroids mediate non-genomic anti-inflammatory effects by binding to membrane-bound GRs and through direct nonspecific interactions with cellular membranes.2,5
Despite the availability of numerous topical corticosteroid preparations, there is a continued need to modify the formulations for improved ocular delivery into the anterior segment. We describe the development of loteprednol etabonate (LE) in a novel ophthalmic gel formulation for improved ocular delivery. The LE gel formulation has attributes intended to provide consistent dose uniformity, without the need to shake, and increased residence time of the drug on the ocular surface, as well as attributes intended to provide greater comfort for the patient. We begin with a brief review of the barriers to anterior segment delivery of a topically applied drug, before describing the development of LE in the novel gel-based formulation.
Barriers to topical ocular drug delivery
Topical ocular drug delivery is the preferred route for treating anterior segment diseases and inflammation associated with cataract and refractive surgery. Direct application of a small dose to the ocular surface typically provides a fast onset of action at the anterior segment with minimal systemic exposure. Tear fluid secretion, tear volume, and lacrimal drainage present the first barriers to topical ocular drug delivery6,7 The anterior surface of the eye is continuously rinsed by tear fluid secreted by the lacrimal glands and the goblet cells. The basal secretion rate for tear fluid is about 1.2–1.5 μL/min, while the normal tear volume on the surface of the eye is about 6–7 μL.7–9 At steady state, this means that the tear fluid has a mean residence time (τ = V/kdr) of 5–6 minutes on the surface of the eye. Instillation of a topical drop will increase the volume of liquid on the surface of the eye to a maximum capacity of about 25–30 μL as the formulation is mixed with the tear fluid.710 Excess fluid beyond the maximal capacity will overflow at the lacrimal lake or be splashed onto the eyelashes by reflex blinking, while nasolacrimal drainage will quickly return tear volume back to normal.6,11 Moreover, if the physical characteristics of the post-instillation tear fluid mixture cause any irritation or discomfort (eg, foreign body sensation, pH outside of the normal range, or osmolality outside of the 200–600 mOsm range), reflex tearing, reported to increase to as high as 300–400 μL/min, and reflex blinking will contribute further to lacrimal lake overflow and the dilution and loss of drug via nasolacrimal drainage.6,11,12 The approximate composition and properties of normal tear fluid are outlined in Table 1. The cornea is highly innervated providing immediate feedback to the lacrimal glands and the muscles controlling blinking in the event that tear fluid composition is altered. As a consequence, most aqueous formulations have extremely short residence times on the surface of the eye. Common formulation approaches to prolong ocular residence time and enhance drug delivery include the use of viscous aqueous formulations (ie, gels), which thicken the tear fluid and reduce the tear fluid drainage rate from the surface of the eye, and ointment formulations, which are not miscible with the tear fluid and therefore also slow the removal of the formulation from the surface of the eye.
Table 1.
| Characteristic | Typical range | Mean value | |
|---|---|---|---|
| Physical properties | pH | 7.3–7.5 | 7.4 |
| Osmolality (mOsm) | 300–350 | 320 | |
| viscosity (cps) | 1.3–5.9 | 2.9 | |
| Surface tension (dynes/cm2) | 40–50 | 45 | |
| Composition | Sodium (mmol/L) | 146 ± 10 | |
| Potassium (mmol/L) | 16 ± 5 | ||
| Chloride (mmol/L) | 128 ± 5 | ||
| Bicarbonate (mmol/L) | 20–40 | 26 | |
| Calcium (mmol/L) | 0.57 | ||
| Total nitrogen (mmol/L) | 113 | ||
| Meibomian oils (g/L) | 1.3 | ||
| Total protein (g/L) | 4.3–12.2 | 8 | |
| Proteins | Albumin (g/L) | 3.94 | |
| and enzymes | Lysozyme (g/L) | 1.7 | |
| Lactoferrin (g/L) | 2.75 |
The site of action for most topically applied drugs includes the tissues of the anterior segment – the cornea, conjunctiva, sclera, and iris-ciliary body. Drug penetration into the iris-ciliary body, of importance in the treatment of postoperative inflammation, is dependent on prior drug penetration through the cornea into the aqueous humor and/or penetration through the sclera followed by absorption into the iris-ciliary body. The cornea offers the major pathway for drug diffusion into the anterior chamber of the eye, especially for small molecules. Small lipophilic compounds generally penetrate through the epithelium via the intracellular route, while small hydrophilic compounds are limited to the paracellular route. The fraction of a lipophilic compound penetrating through the cornea is 20 times greater than a hydrophilic molecule of similar molecular size.17 A logD (log distribution coefficient at pH 7.65) of 2–3 for beta blockers was reported to provide optimal corneal permeation.18 Permeation through the conjunctival epithelium is limited by the tight junctions. The high conjunctival surface area of diffusion when compared with the cornea (17:1) contributes to the importance of this route especially for hydrophilic compounds and large molecules.19 However, unlike the cornea, the sclera and conjunctiva are vascularized tissues. The presence of blood vessels in the conjunctiva can limit drug penetration to the sclera, carrying drug instead to the systemic circulation.7,10 Compounds penetrating through the conjunctiva can continue passage into the eye through the sclera. Scleral permeation does not depend on the compound lipophilicity, but has been reported to be inversely proportional to the molecular radius.20 Drug molecules can be cleared directly from the aqueous humor through the aqueous humor drainage as well as through the blood vessels penetrating the vascularized tissues.
For topical ocular drugs that are neither charged nor are weak acids or bases (eg, corticosteroids), absorption follows Fick’s first law of diffusion – namely, the rate of drug penetration across or into ocular anterior segment tissues is proportional to the drug concentration in the tear fluid and the permeability of the drug through the ocular tissues.
Given that drug delivery to the ocular surface is limited by tear fluid secretion and turnover, it is not surprising that only 5% or less of a topically applied drug is estimated to penetrate the cornea and reach intraocular tissues.6 It follows that ocular therapy could be improved if the ocular surface residence time of a drug were increased without causing discomfort to the patient’s eye.
Properties of loteprednol etabonate
LE is an ester-based corticosteroid commonly prescribed for the topical treatment of various ocular inflammatory diseases as well as inflammation occurring after cataract surgery. Approved by the US Food and Drug Administration in 1998 in an aqueous suspension formulation (Lotemax®; Bausch and Lomb Incorporated, Rochester, NY, USA), LE differs from traditional corticosteroids in that it contains an ester group at the carbon 20 (C-20) position of the corticosteroid core structure in lieu of a ketone. Specifically, LE is a 17ß-chloromethylester derivative of Δ1-cortienic acid an inactive metabolite of prednisolone; LE also has a 17α-etabonate moiety (Figure 1). The ester substitution was the result of retrometabolic drug design, in which novel drugs are designed such that they are converted into inactive metabolites after exerting their therapeutic effects, thereby avoiding unwanted adverse effects.21,22 For ophthalmic use of corticosteroids, the primary side effect of concern is increased intraocular pressure (IOP),23 which is thought to result from structural and biochemical changes on the trabecular meshwork induced by corticosteroids, leading to increased resistance to aqueous outflow.24,25 As a function of its retrometabolic design, any LE not bound to GRs is rapidly metabolized to inactive carboxylic acid metabolites by tissue esterases (Figure 1), resulting in a decreased effect on IOP relative to traditional C-20 ketone corticosteroids.
Figure 1.
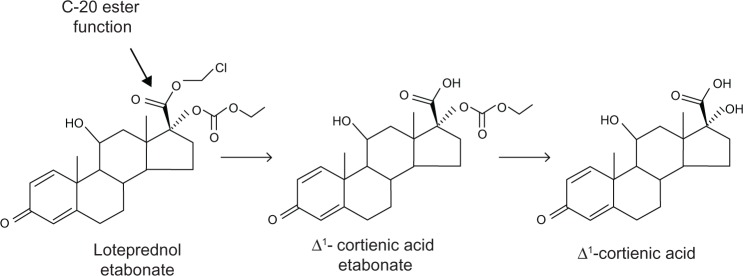
Molecular structure of loteprednol etabonate and metabolism of loteprednol etabonate to inactive metabolites.
Notes: Loteprednol etabonate has an ester function at C-20 position and is metabolized by esterases to Δ1-cortienic acid etabonate and then to Δ1-cortienic acid. Both metabolites lack glucocorticoid activity.
In addition to increased IOP, corticosteroids are also associated with the potential for cataract formation.23 The presence of an ester function at the C-20 position in the LE molecule has the added advantage of decreased potential for cataractogenicity. Manabe et al demonstrated that topical corticosteroids such as prednisolone form Schiff base intermediates with lens proteins, a first step in cataractogenesis, through the ketone moiety at the C-20 position.26 However, non-ketolic analogs are not able to form such adducts. LE is a non-ketolic C-20 ester corticosteroid and presumably is also unable to form such adducts, although other mechanisms of cataractogenesis cannot be ruled out.
LE has been shown to have the right balance of lipophilicity to aqueous solubility required for effective ocular tissue penetration. The lipophilicity of LE and other steroids was calculated according to their retention characteristics by reversed phase high pressure liquid chromatography (HPLC). The lipophilicity of LE (log K = 3.04) was found to be seven times greater than that of dexamethasone (log K = 2.19).27 While its water solubility is relatively low (0.5 μg/mL), early studies demonstrated that its solubility could be increased by inclusion of solubilizing agents (eg, propylene glycol or cyclodextrin) and permeability enhancers in the formulation.27 Druzgala et al were the first to demonstrate that LE readily penetrates ocular tissues.28 Instillation of 14C-labelled LE (three 50-μL drops of 0.5% LE in a 1% hydroxypropyl methylcellulose test formulation at 5-minute intervals) in the eyes of rabbits led to high concentrations in the conjunctiva and cornea, followed by the iris–ciliary body and aqueous humor, at 30 minutes following instillation. The authors attributed the relatively low aqueous humor concentration to the rapid metabolism of unbound LE to inactive metabolites by esterases. Inactive metabolites of LE were found in all three tissues, with the highest ratio of metabolites to unchanged drug in the cornea, suggesting that the cornea is the primary site for metabolism. More recently, Glogowski and Proksch studied the ocular tissue penetration of LE following instillation of a single 50-μL drop of either the 0.5% marketed suspension formulation (povidone-based) or ointment formulation in rabbits with corneal inflammation and likewise found high concentrations in the conjunctiva and cornea and low levels in the aqueous humor.29 Maximal concentrations (Cmax) in the conjunctiva, cornea, and aqueous humor were 7.77, 3.00, and 0.06 nmoles/g, respectively, following instillation of the suspension formulation and 4.41, 2.48, and 0.16 nmoles/g, respectively, following instillation of the ointment formulation.29 In both studies, the concentration–time profile of LE in the aqueous humor closely paralleled that in the cornea, suggesting that LE in the aqueous humor originates primarily from the cornea.
Preclinical research showed that LE has a high affinity for the cytosolic GR, an affinity 4.3 times stronger than that of dexamethasone, when tested in rat lung type II GR binding studies.30 Additional preclinical studies by Bodor and colleagues, reviewed in 2000 by Bodor and Buchwald, showed that the therapeutic index of LE was more than 20-fold better than that of other corticosteroids including hydrocortisone 17α-butyrate, betamethasone 17α-valerate, and clobetasone 17α-propionate, based on the cotton pellet granuloma test and thymolysis potency.22 Thus, LE was predicted to have potent anti-inflammatory activity along with decreased side-effect potential.
Clinical studies demonstrated the efficacy and safety of LE in a suspension formulation for the treatment of numerous ocular inflammatory conditions including, but not limited to, anterior uveitis, giant papillary conjunctivitis, and seasonal allergic conjunctivitis,31–35 and in both suspension and ointment formulations in the treatment of inflammation and pain following cataract surgery.36–38 Consistent with its retrometabolic design, the incidence of clinically significant elevations in IOP (≥10 mmHg) was similar between LE-treated patients and vehicle-treated patients in most studies and less than that observed in prednisolone-treated patients.39,40 Additional safety studies, including studies in steroid responders, demonstrated a significant difference in the incidence of IOP elevation in favor of LE compared with the C-20 ketone steroids prednisolone, dexamethasone, and fuorometholone.40–45
A non-settling gel formulation of loteprednol etabonate 0.5%
Current marketed formulations of LE 0.5% include a suspension (Lotemax®) and an ointment (Lotemax® ointment). The LE suspension formulation is a low viscosity suspension containing povidone and glycerin, both sometimes used as active ingredients for the relief of dry eye symptoms in over-the-counter products.46 It is preserved with 0.01% benzalkonium chloride (BAK), a quaternary ammonium preservative widely used in ophthalmic formulations for its potent antimicrobial efficacy and chemical stability. Despite its established efficacy, a drawback of the LE suspension formulation, or any other steroid suspension formulation, is the need to shake vigorously prior to dosing to assure a consistent dose of medication. In a study of patient compliance, Apt et al showed that less than one-third of patients shake their topical ophthalmic medication prior to instillation,47 underscoring the benefit of developing non-settling formulations. While ointment formulations do not need to be shaken, the LE ointment formulation has disadvantages common to all ophthalmic ointments – namely, blurred vision, due to refractive index difference between the tears and the ointment, and the potential for inaccurate dosing due to difficulty instilling a precise ribbon of ointment.48
The newly developed ophthalmic gel formulation of LE 0.5% provides sufficient structure such that LE stays in suspension and the formulation does not need to be shaken prior to dosing. LE 0.5% gel behaves as a semisolid gel in the bottle, shear-thins to a liquid when being dispensed from the dropper bottle, and converts to a liquid when mixed with tear fluid on the surface of the eye, while maintaining sufficient viscosity for ocular surface retention. All gels have a measurable yield stress (in Pascal [Pa]), or minimum stress that must be applied to initiate flow. Until a force greater than the yield stress is applied to the gel, it will not flow, and hence, particles in suspension will not settle. In the case of LE gel, when the bottle is tipped and squeezed, the force applied exceeds the yield stress and the gel is able to flow, like a liquid, and be expressed as a drop. LE gel has also been formulated in such a way as to result in minimal visual distortion compared with other ophthalmic gels, due to the use of a gelation polymer that becomes significantly less viscous, transitioning to a fluid after mixing with tears in the eye. This stands in contrast to so-called in situ gel-forming solutions which are designed to transition from a solution to a gel upon instillation in the eye and mixing with tears (eg, Timoptic-XE; [Merck and Co, Inc, Whitehouse Station, NJ, USA] and the generic Timolol Gel Forming Solution [Timolol GFS]). The composition of the new LE ophthalmic gel is compared with the LE suspension in Table 2. The gel formulation replaces the povidone suspending agent with polycarbophil. It is the polycarbophil polymer that provides the gel structure to the formulation to prevent sedimentation of LE. Polycarbophil also functions as a mucoadhesive and viscoelastic suspending agent. The specific level of polycarbophil in the formulation provides a sufficient yield stress to prevent settling of the LE in the dropper bottle but also provides viscosity low enough to allow delivery as a drop when the bottle is squeezed. From a clinical perspective, the new non-settling LE gel formulation is expected to deliver consistent, full doses of LE to the ocular surface for reliable drug delivery and subsequent clinical effect. For patients, these attributes translate into a formulation that does not need to be shaken prior to instillation, not unlike an ointment, yet having a delivery ease and simplicity similar to that of an aqueous drop formulation, and without expected blurred vision.
Table 2.
Comparison of 0.5% loteprednol etabonate suspension and gel formulations
| Ingredients | Function | LE suspension 0.5% | LE gel 0.5% |
|---|---|---|---|
| Active substance | |||
| Loteprednol etabonate | Steroid, anti-inflammatory | 5.00 mg/mL | 5.00 mg/g |
| Excipients | |||
| Povidone | Suspending and/or viscosity-increasing agent | + | |
| Polycarbophil | Suspending and/or viscosity-increasing agent | + | |
| Glycerin | Tonicity agent/humectant | + | + |
| Propylene glycol | Tonicity agent/humectant | + | |
| Sodium chloride | Tonicity agent | + | |
| Boric acid | Buffer/antimicrobial enhancer | + | |
| Tyloxapol | Surfactant and/or wetting agent | + | + |
| Edetate disodium dihydrate | Chelant/antimicrobial enhancer | + | + |
| Benzalkonium chloride | Antimicrobial preservative | 100 ppm | 30 ppm |
| Sodium hydroxide and/or HCl | pH adjuster | qs to pH 5.5 | qs to pH 6.5 |
| Water for injection | Vehicle | qs to 1 mL | qs to 1 g |
Note: The + sign indicates the presence of excipient in the formulation.
Abbreviations: LE, loteprednol etabonate; ppm, parts per million; qs, sufficient quantity.
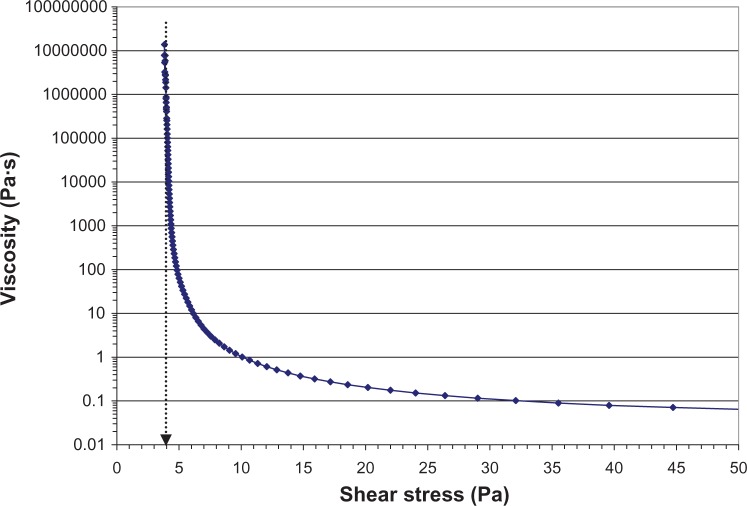
Rheology studies demonstrated that LE gel behaves as a semisolid gel at low shear and a fluid-like suspension at high shear.49Figure 2 shows the viscosity of the LE gel as a function of applied shear stress. Below a yield stress of 4 Pa, the gel does not flow and, hence, allows no settling or sedimentation of drug particles. At higher shear stress (ie, >30 Pa), the viscosity is less than 0.1 Pa·s or 100 centipoise (cps) and the product can be easily expressed as a liquid drop through a dropper tip. The conversion from a gel at rest to a liquid under high shear and back to a gel at low shear was found to occur in less than one second.49 Because the gel adapts to different shear forces almost instantaneously, LE particle sedimentation will not occur during use of the formulation. Figure 3 illustrates how, after delivery from the dropper bottle, LE gel immediately regains a gel structure, remaining at the site of delivery. In contrast, the lower viscosity of LE suspension causes it to flow away from the administration point.
Figure 2.
Viscosity of loteprednol etabonate 0.5% gel as a function of applied shear stress.
Notes: The viscosity is much higher at low shear, as indicated by the logarithmic y-axis. The yield stress for the gel is about 4 Pa. Below the yield stress, the gel does not flow, and hence, viscosity cannot be measured. At high shear, the formulation has sufficiently low viscosity (<100 cps) that it can be delivered as a drop. Data was obtained using a controlled-stress rheometer (TA Instruments AR2000 with firmware version 7.20; TA Instruments, New Castle, DE, USA) with a vaned-rotor and cup. A steady-state flow experiment was performed by scanning the shear rate from 1000 s−1 to 1 × 10−5 s−1 (log scale, 10 points/decade) at 25°C. Steady state equilibrium was defined as three consecutive measurements within the tolerance window of 2%. The sample period was 10 seconds, and the maximum time point was set to 5 minutes. Data were collected using Rheology Advantage software V5.7.13 (TA Instruments). The yield value for the formulation was determined by fitting the steady-state flow data to the Herschel-Bulkley equation.
Figure 3.
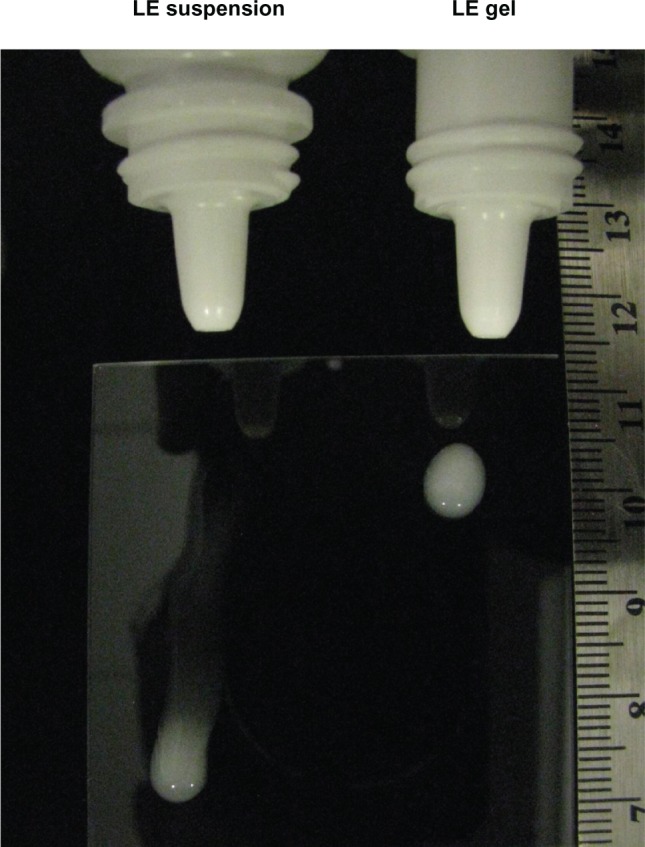
Delivery of LE 0.5% suspension and LE 0.5% gel from the dropper bottle.
Notes: Both formulations were delivered as an eyedrop to a glass plate (held at a 45 degree angle). After delivery, the gel formulation immediately regained its gel structure and remained where it was delivered. In contrast, the suspension formulation flowed away from the administration point. Photograph taken immediately after administering the drop of LE 0.5% suspension and approximately 1 minute after administering the drop of LE 0.5% gel.
Abbreviation: LE, loteprednol etabonate.
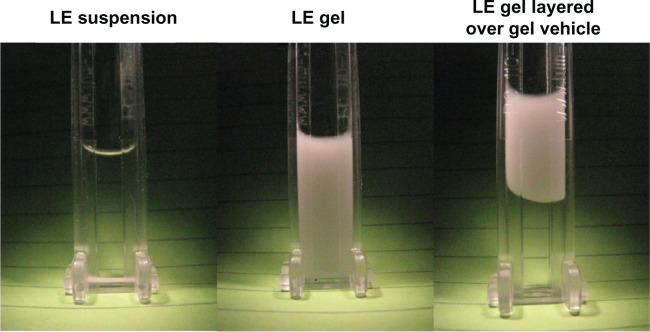
LE gel rheological characteristics were reflected in sedimentation studies. The concentration of LE suspended in the unshaken gel following 16 months of stability testing (upright storage at 25°C and 40°C) ranged from 100.4%–107.6% of label and did not differ whether sampled near the top or near the bottom of the bottled test samples.49 Furthermore, LE did not sediment out of the gel formulation under accelerated conditions. Figure 4 presents the visual sedimentation results following exposure of LE suspension and LE gel to 1000 rpm for 24 hours (116–145 times gravity) using a photocentrifuge. With this instrument, any sedimentation of drug particles out of the formulation is documented by a change in the clarity of the sample over time. The LE suspension formulation settled in less than 20 minutes while the LE gel did not show any settling over the 24-hour testing period. Moreover, the LE particles suspended in the gel formulation did not migrate when LE gel was layered on top of gel vehicle. These visual results were further confirmed by HPLC analysis (Bausch and Lomb, Inc., data on file, 2012).
Figure 4.
Sedimentation of LE 0.5% suspension and LE 0.5% gel formulations under 120× g at 1000 rpm (116–145× g) for 24 hours using a LUMiSizer dispersion analyzer (LUM GmbH, Berlin, Germany).
Notes: LE particles settled out of suspension within 20 minutes from the suspension formulation while remaining suspended in the gel formulation. Even when the LE 0.5% gel formulation was placed above additional placebo vehicle (clear), LE particles did not migrate under centrifugation.
Abbreviation: LE, loteprednol etabonate.
Drop/dose uniformity studies also confirmed the non-settling characteristic of the gel formulation. Drop uniformity (weight per drop), dose uniformity (amount of LE per drop), and drop potency (amount of LE/weight of drop) were evaluated following expression of LE gel from shaken and unshaken 10-mL dropper bottles, and the results are presented in Figure 5 (Bausch and Lomb, Inc., data on file, 2012). Drop weight and amount of LE per drop were highly consistent whether LE gel was delivered from shaken or unshaken dropper bottles, with an overall variation in drop weight and amount of LE per drop of less than 10% relative standard deviation (RSD). The resulting %RSD for drop potency was less than 5%, indicating that small variations in the delivered dose were directly attributable to the small variations in drop weight. These data, taken together with data from sedimentation studies, demonstrate that LE gel does not settle, does not require shaking prior to expressing drops from the dropper bottle, and provides consistent, uniform dosing.
Figure 5.
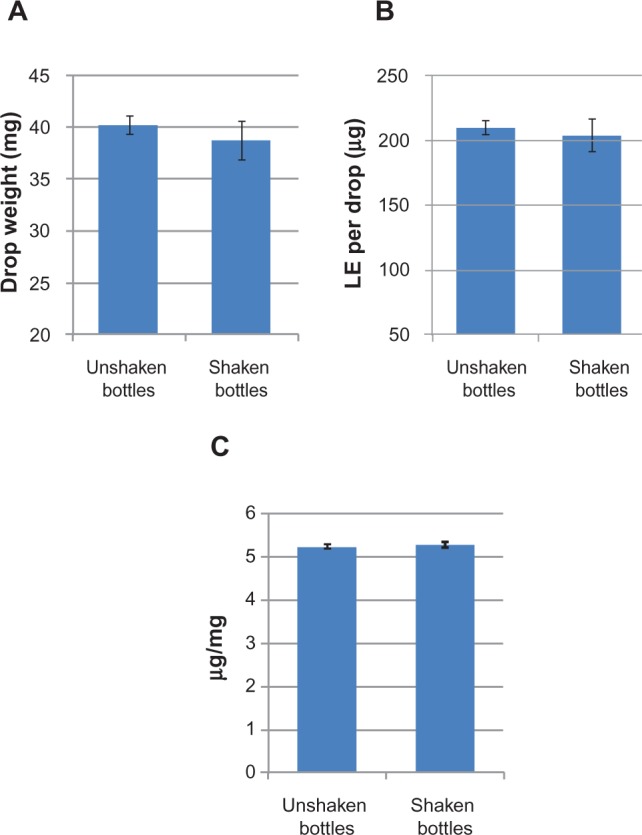
Drop weight (A), amount of dose delivered per drop (B), and resulting drop potency (C) of a representative lot of LE 0.5% gel.
Notes: Data represent the mean ± standard deviation for three test bottles, with six individual drops expressed per bottle. Bottles that were shaken were inverted ten times in rapid succession immediately prior to drop expression.
Abbreviation: LE, loteprednol etabonate.
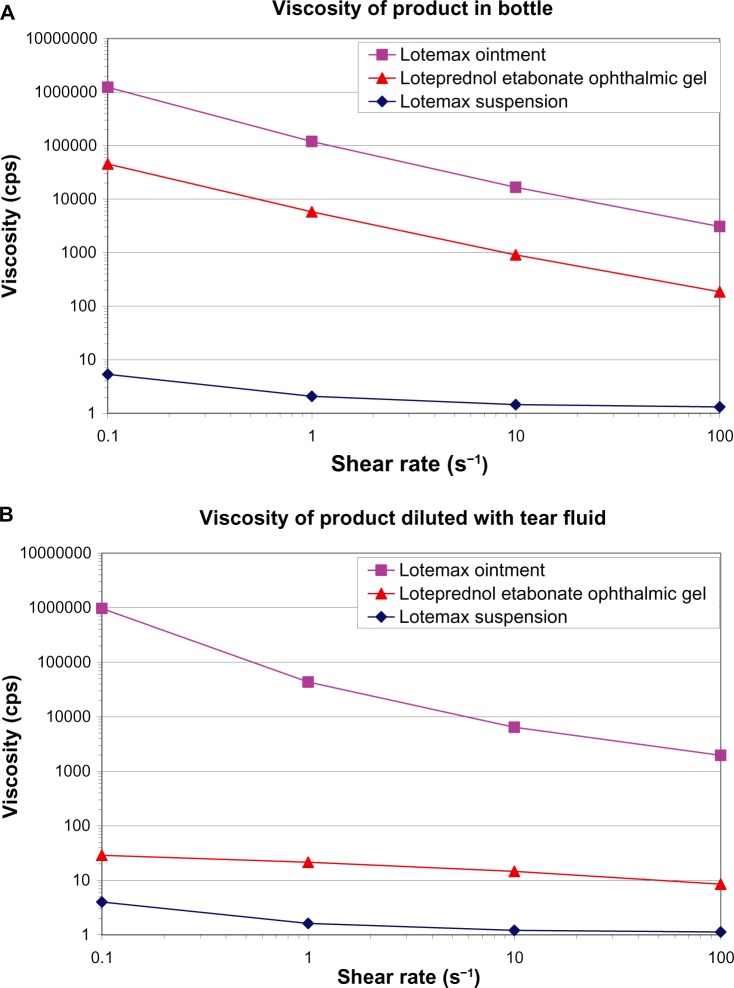
Once instilled in the eye, the LE gel formulation mixes with the tear fluid and loses its gel structure due to the interaction of the polycarbophil polymer with the electrolytes in tears and converts from a gel to a shear-thinning, moderately viscous, mucoadhesive suspension. In the bottle, the anionic carboxylate groups on the polymer cause the polymer to swell, through mutual charge repulsion, giving the gel its structure and stabilizing the formulation.50 In the eye, electrolytes in the tear fluid decrease the swelling of the polymer by partial screening of the charge repulsion, converting the gel into a viscous liquid. The shear-thinning suspension that results from mixing the LE gel with the tear fluid can spread easily over the surface of the eye during blinking due to the relatively low viscosity of the tear fluid mixture under high shear force, maximizing the potential for ocular absorption. Between blinks, or under low shear force, the relatively higher viscosity of the tear fluid mixture, although no longer a solid-like gel due to mixing and dilution with tears, is expected to increase the ocular surface contact time of the formulation, potentially increasing bioavailability. Thus, even after mixing with tears, the polycarbophil in the tear fluid mixture continues to affect viscosity. Figure 6 compares the viscosity of LE gel formulation with that of the current LE suspension and ointment formulations at low shear rates before and after dilution with tear fluid. Undiluted LE gel has a viscosity much greater than that of the LE suspension formulation but lower than that of the LE ointment formulation (Figure 6A). Diluting LE gel with simulated tear fluid at a ratio of 3:1 (the ratio expected when a normal drop is instilled in the eye and mixes with tear fluid) resulted in the LE gel transitioning to a mucoadhesive fluid with viscosity higher than that of the LE suspension formulation (Figure 6B) but low enough to prevent any potential discomfort to the patient.
Figure 6.
Viscosity of loteprednol etabonate gel 0.5%, suspension 0.5%, and ointment 0.5% at low shear rates before (A) and after (B) dilution with simulated tear fluid (3:1 dilution with Hank’s Balanced Salt Solution).
Notes: For a fluid being sheared between two surfaces, moving relative to each other, the shear rate is the relative velocity over the distance between the two surfaces (velocity/distance) and, hence, has units of seconds−1.
The pH of the LE gel formulation has been adjusted to be closer to that of normal tears (pH 7.4), from 5.5 in the LE suspension formulation to 6.5 in the LE gel formulation. For many patients, the natural buffering capacity of tears, mainly due to dissolved carbon dioxide and bicarbonate, will readily accommodate this small difference in pH with little noticeable difference. The osmolality of LE gel, 280–300 mOsm, is consistent with that of normal tear fluid (302–350 mOsm)11,13 and has been reported by patients to be comfortable.
As a further improvement, the concentration of BAK required to provide antimicrobial preservation has been reduced by 70%, from 0.01% (100 ppm) in the LE suspension formulation to 0.003% (30 ppm) in the LE gel formulation. BAK has been reported to be toxic in static cell cultures.51,52 Although there is ongoing controversy about the relevance of these in vitro findings to the clinical setting, the amount of BAK added to the LE gel formulation has been reduced by inclusion of boric acid (buffering agent) and disodium edetate (EDTA, a chelant) in the formulation. While neither boric acid nor EDTA provides preservation on its own, both are able to enhance the preservation provided by the lower concentration BAK. Ophthalmic products sold in the United States and Japan are required to demonstrate antimicrobial activity against pathogenic bacterial test organisms and stasis against fungal organisms.53,54 The requirements in Europe are more stringent and require a measurable level of antifungal efficacy as well.55 The LE gel formulation was evaluated against the more stringent preservative efficacy requirements as outlined in Europe and found to pass European Pharmacopoeia A standards, with a concentration of 30 ppm of BAK. Specifically, LE gel containing 30 ppm BAK reduced all required bacterial challenge organisms with ≥2 log kills at 6 hours and ≥3 log kills by 24 hours, while also producing ≥2 log reductions in fungi at 7 days (Bausch and Lomb, Inc., data on file, 2012).
The LE gel formulation contains both glycerin and propylene glycol. Glycerin and propylene glycol are listed as over-the-counter ocular lubricants for treatment of dry eye.46 These ingredients also function as humectants to retain moisture on the surface of the eye53 and are expected to contribute to patient comfort. Formulated together, glycerin and propylene glycol have been found to perform better than either humectant agent alone.56 The glycerin and propylene glycol also act as tonicity agents, contributing to the final osmolality of 280–300 mOsm.
Glogowski and Jiang evaluated the ocular and systemic pharmacokinetics of LE gel following a single 35-μL topical ocular instillation in healthy rabbits.57 LE was rapidly absorbed and distributed within the eye, with measurable concentrations observed in ocular tissues within 5 minutes after dosing. Maximal concentrations (Cmax) of LE were achieved within 0.5 hours in ocular tissues following a single, topical ocular dose with minimal levels in the plasma. Maximum concentrations of LE were highest in tear fluid (3.34 μmol/g), followed by bulbar conjunctiva (8.63 nmol/g), cornea (4.67 nmol/g), iris/ciliary body (0.35 nmol/g), and aqueous humor (0.03 nmol/mL), with a mean residence time of more than 9 hours in both the bulbar conjunctiva and cornea. Thus, it appears that formulating LE in a polycarbophil-based gel led to an increase in the Cmax of LE in conjunctival and corneal tissues of approximately 10% and 50%, respectively, compared with the less viscous LE suspension formulation. Perhaps more importantly, the overall exposure, as measured by the area under the concentration time curve from 0 to 24 hours (AUC(0–24)) for conjunctival tissue and cornea was 39.0 nm · h/g and 11.6 nm h/g, respectively, with the new LE gel formulation compared with 13.1 nm · h/g and 7.07 nm · h/g for the LE suspension formulation, representing a 200% and 65% increase in penetration into these tissues, respectively. These data suggest that the increased viscosity of the LE gel–tear fluid mixture due to polycarbophil may indeed provide greater ocular surface contact time for increased bioavailability. Because the tear fluid turnover rate in rabbits is about half that reported in humans (0.53 μL/min versus 1.2 μL/min),58 it has been reported that rabbit models may underestimate the benefit of viscosity increases on retention and drug delivery of topical ophthalmic formulations.59,60 It follows that the improvement in ocular surface contact time with LE gel may be even greater in humans. While these results are promising, head to head pharmacokinetic studies comparing the LE gel formulation with the LE suspension formulation in the same rabbit model with equal dosing volumes, or better yet in humans, are needed to confirm this apparent increase in ocular bioavailability with the LE gel formulation.
Clinical trials of loteprednol etabonate ophthalmic gel 0.5%
The clinical safety and efficacy of LE gel were evaluated in two randomized, multicenter, double-masked, vehicle-controlled studies in the treatment of pain and inflammation following cataract surgery.61,62 In both studies, patients aged 18 years or older, with postoperative anterior chamber cells (ACC) Grade 2 (6–15 cells) or greater following cataract surgery, were randomized to either LE gel or vehicle, both instilled four times daily for 14 days in the study eye, and followed up over seven visits. Concurrent use of nonsteroidal anti-inflammatory drugs (NSAIDs) was disallowed. The co-primary efficacy measures were the proportion of patients with complete resolution of ACC and the proportion of patients with Grade 0 (no) pain at postoperative day 8 in study eyes. A total of 406 patients were randomized in the first study (n = 203 for each treatment group) and 407 patients in the second study (n = 206 for LE gel 0.5%, n = 201 for vehicle). Results for the co-primary outcomes are presented in Table 3. In each study, significantly more LE gel-treated patients than vehicle-treated patients had complete resolution of ACC and Grade 0 (no) pain at day 8 (P < 0.001 for both). Significant treatment differences for resolution of ACC and Grade 0 (no) pain favoring LE gel were also found at day 15 in both studies (P < 0.001 for all). In addition, as expected, fewer patients in the LE gel group than in the vehicle group required rescue medication before day 15, with 17.2% versus 51.7% of patients in one study61 and 10.7% versus 42.3% of patients in the second study62 requiring such intervention (P < 0.001 for both).
Table 3.
| Day 8 | Day 15 | |||
|---|---|---|---|---|
|
| ||||
| LE gel | Vehicle | LE gel | Vehicle | |
| Complete resolution of ACCa | ||||
| Study 1 | 30.5% (62/203) | 16.3% (33/203) | 50.2% (102/203) | 21.7% (44/203) |
| P-valueb | <0.001 | <0.001 | ||
| Study 2 | 31.1% (64/206) | 13.9% (28/201) | 65.0% (134/206) | 35.8% (72/201) |
| P-valueb | <0.001 | <0.001 | ||
| Grade 0 (no) paina | ||||
| Study 1 | 72.9% (148/203) | 41.9% (85/203) | 75.9% (154/203) | 37.9% (77/203) |
| P-valueb | <0.001 | <0.001 | ||
| Study 2 | 75.7% (156/206) | 45.8% (92/201) | 77.7% (160/206) | 44.3% (89/201) |
| P-valueb | <0.001 | <0.001 | ||
Notes:aSubjects who had missing data or took rescue medication prior to Day 8 were imputed as failures;
P-values from Pearson Chi-squared statistic.
Abbreviations: ACC, anterior chamber cells; ITT, intent to treat.
Rates of ocular adverse events were low, and most were consistent with the surgical procedure itself. The incidence of drug related ocular adverse events was low in both studies, and all were mild to moderate in severity. Table 4 presents drug related ocular adverse events occurring at an incidence ≥1%. Drug related blurred vision, of particular interest in the development of a gel formulation, was reported for only two patients, both using vehicle (one event in each study), consistent with the expectation that LE gel transitions to a shear-thinning suspension after instillation. Drug related increased IOP, a concern with any steroid formulation, was reported for one patient in the LE gel group in one study and one patient in the vehicle group in the second study. As reported in numerous previous studies with other LE formulations,40–45 LE gel had little effect on IOP. Mean IOP was lower than the mean baseline pressure at postoperative days 8, 15, and 18 in both treatment groups. Two patients in the LE gel group (one per study) and one vehicle-treated patient demonstrated a transient, but clinically significant, increase (≥10 mm Hg) in IOP.
Table 4.
Drug-related ocular treatment emergent adverse events occurring at an incidence of ≥1% (safety populations)60,61
| Study 1 | LE gel (n = 203) | Vehicle (n = 203) |
|---|---|---|
| Number of patients with ≥1 AE | 9 (4.4%) | 14 (6.9%) |
| Total drug-related AEs | 16 | 25 |
| Anterior chamber inflammation | 3 (1.5%) | 3 (1.5%) |
| Eye pain | 2 (1.0%) | 3 (1.5%) |
| Photophobia | 2 (1.0%) | 2 (1.0%) |
| Foreign body sensation | 0 | 2 (1.0%) |
| Eye pruritus | 2 (1.0%) | 2 (1.0%) |
| Anterior chamber cells | 0 | 2 (1.0%) |
| Increased lacrimation | 2 (1.0%) | 0 |
| Study 2 | LE gel (n = 206) | Vehicle (n = 201) |
|
| ||
| Number of Subjects with ≥1 AE | 5 (2.4%) | 15 (7.5%) |
| Total drug-related AEs | 5 | 17 |
| Eye pain | 1 (0.5%) | 3 (1.5%) |
| Foreign body sensation | 1 (0.5%) | 3 (1.5%) |
| Eye irritation | 1 (0.5%) | 2 (1.0%) |
| Ocular hyperemia | 0 | 2 (1.0%) |
| Ocular discomfort | 0 | 2 (1.0%) |
Abbreviations: AE, adverse event; LE, loteprednol etabonate.
LE gel was well tolerated by patients. At most postoperative visits, patient reports for ocular pain, photophobia, and tearing due to the surgical procedure were significantly better in patients treated with LE gel; symptoms of ocular discharge, dryness, and itching, reported by few patients at baseline, either improved or did not change from baseline, with no significant differences between treatment groups. Drop comfort was assessed at postoperative visits as drop sensation (none, mild, moderate, or severe). In both studies, the majority (>85%) of patients in each group reported no drop sensation, while the few patients reporting drop sensation mostly characterized it as mild.
Implications for clinical practice
Cataract surgery has advanced dramatically in past decades leading not only to better visual outcomes but to less tissue damage and, consequently, less postoperative inflammation. Ophthalmic surgeons owe this to developments such as small-incision surgery, better ophthalmic viscosurgical materials, improved phacoemulsification equipment with enhanced fluidics, and more efficient delivery of ultrasound energy to the eye.63 Despite these advances, some degree of postoperative inflammation remains a predictable consequence of cataract surgery with intraocular lens (IOL) implantation. The sequela of inflammation may be the most serious concern during the post-surgical period. Clinically, iritis is the hallmark of intraocular inflammation, characterized by perilimbal injection and anterior chamber cell and fare.63 If not treated appropriately, this inflammation can result in significant discomfort for patients and impede the recovery of vision.64
Surgeons rely on corticosteroids to reduce postoperative ocular inflammation because of their potency and rapid onset of action; but, as indicated earlier, these drugs have the potential for adverse effects, most notably a risk of increasing IOP. Thus, choosing a corticosteroid to control inflammation following cataract surgery requires balancing the need for efficacy with concerns about safety. Historically, clinicians used topical steroids sparingly because of a fear of side effects. However, pharmacologic options have expanded greatly, with steroids now available in various strengths, combinations, and vehicles. Clinicians must not assume that all steroids are similar and should understand the safety, efficacy, and value of each formulation.
Today, most cataract surgeons in the US use a combination of a topical corticosteroid and NSAID in their postoperative regimen.65–67 NSAIDs inhibit miosis and, like steroids, reduce postoperative inflammation.63 This treatment paradigm has served patients well. However, while there are concerns about the use of topical ophthalmic corticosteroids and elevated IOP, surgeons must balance this concern against the risk of withholding effective treatment. In the case of cataract surgery, this could mean not using a steroid, stopping the steroid too soon (creating the risk of a rebound effect in which inflammation returns later in the healing process), or using a steroid that is too weak for the job. Herein lies the challenge: surgeons want a corticosteroid that is strong enough to speed the eradication of inflammation yet does not increase the risk of IOP elevation any more than is necessary. Based on studies over many years, it is clear that concerns regarding IOP spikes following cataract surgery are well founded. In particular, steroid responders are especially susceptible to elevated IOP with steroid use after cataract surgery.41 A breakthrough in drug design led to the development of LE, a steroid that provides the efficacy to control postoperative inflammation with a low risk of significant IOP elevation.39–41
With today’s less disruptive cataract surgery, it makes good clinical sense to select a corticosteroid with a pharmacokinetic/pharmacodynamic profile appropriate to the amount of inflammation expected. LE 0.5% fits these criteria well and is commonly used in cataract surgery patients to control postoperative inflammation. LE maximizes the safety profile while providing the necessary efficacy for the intended use. A recent prospective randomized study comparing the suspension formulation of LE 0.5% to prednisolone acetate (PA) 1% in patients undergoing routine cataract surgery resulted in good outcomes for both treatment groups with no significant difference observed throughout the 3 week follow-up period.68 However, there was less fluctuation in IOP for subjects treated with LE than with PA, particularly on days 1 and 3 following surgery.68
Although the currently marketed LE suspension is effective, the development of an improved formulation of this well characterized drug provides eye care practitioners with a useful alternative. Notwithstanding the apparent increase in pre-ocular contact time through the use of a polycarbophil-based gel formulation and subsequent increase in bioavailability of LE, the availability of a formulation that provides consistent dose uniformity without the need to shake is perhaps the greatest benefit of this new formulation. Topical ocular drug effectiveness starts with proper drug administration. While patients are typically instructed on proper administration of their postoperative medications in the clinic before going home, noncompliance with dosing instructions is problematic, especially with aqueous suspensions that require vigorous shaking to resuspend the drug particles. By eliminating the need to shake the product, it is anticipated that inconsistent patient compliance with shaking instructions will not affect the delivered dose of LE. This means that patients will receive consistently uniform doses of LE, maximizing the clinical benefit of the drug. In addition, the change in pH to more closely mimic the tear film, and the decrease in BAK concentration should improve tolerability and decrease any administration noncompliance that may be due to discomfort in sensitive patients.
Patient expectations following ophthalmic surgical procedures, especially cataract surgery, have never been higher. With the introduction of presbyopia-correcting IOLs and new high technology surgical procedures, patients and their physicians demand excellent results. As a consequence, the surgeon’s treatment of postoperative inflammation is critical to optimize patient outcomes. Topical ocular corticosteroids are one of the surgeon’s greatest allies, as they are the most effective and fastest acting anti-inflammatory compounds. In pursuit of the twin goals of reducing inflammation and limiting the risk of IOP elevation, while at the same time assuring consistent dose uniformity in a comfortable lubricating formulation, LE gel 0.5% strikes the balance that ophthalmic surgeons can use with confidence.
Conclusion
The new LE gel formulation utilizes polycarbophil to produce a formulation with adaptive viscosity – a formulation that behaves as a gel in the bottle, instills as a liquid drop under shear, transitions to a fluid upon mixing with the tear fluid, and yet retains sufficient viscosity for ocular surface retention. The new formulation has a pH close to that of tears, a low concentration of BAK, and contains known demulcents. These formulation attributes result in consistent, uniform dosing, as evidenced by drop extrusion studies, and improved patient comfort. Data from topical ocular administration of LE gel in rabbits suggests that the formulation also provides improved ocular bioavailability of LE, although confirmatory studies are still needed. Results from clinical studies of LE gel in the treatment of postoperative inflammation following cataract surgery indicate that it is safe and effective, with the majority of patients reporting no drop sensation upon instillation and rare reports of vision distortion. Finally, the formulation is non-settling, eliminating the need to shake the product to resuspend the drug – thus patient noncompliance with shaking instructions is not expected to affect the delivered dose of LE.
Disclosure
Drs Coffey and DeCory are employees of Bausch + Lomb, while Dr Lane is a consultant for Bausch + Lomb.
References
- 1.Gessi S, Merighi S, Borea PA. Glucocorticoid’s pharmacology: past, present and future. Curr Pharm Des. 2010;16:3540–3553. doi: 10.2174/138161210793797915. [DOI] [PubMed] [Google Scholar]
- 2.Stahn CB, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 3.Cidlowski JA. Glucocorticoids and their actions in cells. Retina. 2009;29(6):S21–S23. doi: 10.1097/IAE.0b013e3181ad2636. [DOI] [PubMed] [Google Scholar]
- 4.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 5.Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275(1–2):71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Ananthula HK, Vaishya RD, Barot M, Mitra AK. Bioavailability. In: Tasman W, Jaeger EA, editors. Duane’s Ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2011. [Google Scholar]
- 7.Rupenthal ID, Alany RG.Ocular drug delivery Gad SC.Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development and Manufacturing John Wiley and Sons, Inc; 20101–39.Published online March152010 10.1002/9780470571224Accessed October 19, 2012 [DOI] [Google Scholar]
- 8.Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 1966;5(3):264–276. [PubMed] [Google Scholar]
- 9.Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005;3(2):81–95. doi: 10.1016/s1542-0124(12)70157-x. [DOI] [PubMed] [Google Scholar]
- 10.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Ooteghem MM.Factors influencing the retention of ophthalmic solutions on the eye surface Saettone MS, Bucci M, Speiser P.Ophthalmic Drug Delivery: Biopharmaceutical, Technological and Clinical AspectsFidia Research Series.Padova, Italy: Liviana Press; 1987117–17. [Google Scholar]
- 12.Conrad JM, Reay WA, Polcyn RE, Robinson JR. Influence of tonicity and pH on lacrimation and ocular drug bioavailability. J Parenteral Drug Assoc. 1978;32(4):149–161. [PubMed] [Google Scholar]
- 13.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Del Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Bron AJ, Tiffany JM. The meibomian glands and tear film lipids: structure, function, and control. In: Sullivan DA, Dartt DA, Meneray MM, editors. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2: Basic Science and Clinical Relevance. New York: Plenum Press; 1998. pp. 281–295. [DOI] [PubMed] [Google Scholar]
- 15.Gilbard JP. Human tear film electrolyte concentrations in health and dry-eye disease; Proceedings of the Annual Meeting of the Association for Research and Vision in Ophthalmology; May 3–8, 1992; Sarasota, FL. [DOI] [PubMed] [Google Scholar]
- 16.Lentner C, editor. Geigy Scientific Tables: Units of Measurement, Body Fluids, Composition of the Body, and Nutrition. Basel: Ciba Pharmaceutical Co; 1981. pp. 178–184. [Google Scholar]
- 17.Zhang W, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99(2):241–258. doi: 10.1016/j.jconrel.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Huang H-S, Schoenwald RD, Lach JL. Corneal penetration of beta-blocking agents II: assessment of barrier contribution. J Pharm Sci. 1983;72:1272–1279. doi: 10.1002/jps.2600721109. [DOI] [PubMed] [Google Scholar]
- 19.Watsky MA, Jablonski MM, Edelhauser H F. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988;7(5):483–486. doi: 10.3109/02713688809031801. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39(5):244–254. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 21.Bodor N, Loftsson T, Wu W-M. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9(10):1275–1278. doi: 10.1023/a:1015849132396. [DOI] [PubMed] [Google Scholar]
- 22.Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000;20(1):58–101. doi: 10.1002/(sici)1098-1128(200001)20:1<58::aid-med3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Clark A F, Steely H T, Dickerson JE, Jr, et al. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001;42(8):1769–1780. [PubMed] [Google Scholar]
- 25.Clark A F, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Manabe S, Bucala R, Cerami A. Nonenzymatic addition of glucocorticoids to lens proteins in steroid-induced cataracts. J Clin Invest. 1984;74(5):1803–1810. doi: 10.1172/JCI111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberth M, Wu W-M, Winwood D, Bodor N. Lipophilicity, solubility, and permeability of loteprednol etabonate: a novel, soft, anti-inflammatory steroid. J Biopharm Sci. 1991;2(2):115–125. [Google Scholar]
- 28.Druzgala P, Wu WM, Bodor N. Ocular absorption and distribution of loteprendol etabonate, a soft steroid, in rabbit eyes. Curr Eye Res. 1991;10(10):933–937. doi: 10.3109/02713689109020329. [DOI] [PubMed] [Google Scholar]
- 29.Glogowski S, Proksch JW. Ocular pharmacokinetics of loteprednol etabonate following ocular administration of a novel ointment formulation or a suspension (Lotemax®) in rabbits with corneal inflammation; Proceedings of the Annual Meeting of the Association for Research and Vision in Ophthalmology; May 2–6, 2010; Fort Lauderdale, FL. [Google Scholar]
- 30.Druzgala P, Hochhaus G, Bodor N. Soft drugs – 10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991;38(2):149–154. doi: 10.1016/0960-0760(91)90120-t. [DOI] [PubMed] [Google Scholar]
- 31.Dell SJ, Lowry GM, Northcutt JA, Howes J, Novack GD, Hart K. A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol. 1998;102(2):251–255. doi: 10.1016/s0091-6749(98)70094-6. [DOI] [PubMed] [Google Scholar]
- 32.Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999;127(5):537–544. doi: 10.1016/s0002-9394(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 33.Shulman DG, Lothringer LL, Rubin JM, et al. A randomized, double-masked, placebo-controlled parallel study of loteprednol etabonate 0.2% in patients with seasonal allergic conjunctivitis. Ophthalmology. 1999;106(2):362–369. doi: 10.1016/S0161-6420(99)90077-5. [DOI] [PubMed] [Google Scholar]
- 34.Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30(1):10–13. doi: 10.1097/01.ICL.0000092071.82938.46. [DOI] [PubMed] [Google Scholar]
- 35.Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123(4):455–464. doi: 10.1016/s0002-9394(14)70171-0. [DOI] [PubMed] [Google Scholar]
- 36.Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Infammation Study Group 1. J Cataract Refract Surg. 1998;24(11):1480–1489. doi: 10.1016/s0886-3350(98)80170-3. [DOI] [PubMed] [Google Scholar]
- 37.A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. The Loteprednol Etabonate Postoperative Infammation Study Group 2. Ophthalmology. 1998;105(9):1780–1786. doi: 10.1016/s0161-6420(98)99054-6. [DOI] [PubMed] [Google Scholar]
- 38.Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–186. doi: 10.2147/OPTH.S16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novack GD, Howes J, Crockett RS, Sherwood MB. Change in intraocular pressure during long-term use of loteprednol etabonate. J Glaucoma. 1998;7(4):266–269. [PubMed] [Google Scholar]
- 40.Comstock TL, DeCory HH. Advances in corticosteroid therapy for ocular inflammation: loteprendol etabonate. Int J Inflam. 2012;2012:789623. doi: 10.1155/2012/789623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–165. doi: 10.1089/jop.1993.9.157. [DOI] [PubMed] [Google Scholar]
- 42.Holland EJ, Bartlett JD, Paterno MR, Usner DW, Comstock TL. Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. Cornea. 2008;27(1):50–55. doi: 10.1097/ICO.0b013e31815873c7. [DOI] [PubMed] [Google Scholar]
- 43.Holland EJ, Djalilian AR, Sanderson JP. Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. Cornea. 2009;28(10):1139–1143. doi: 10.1097/ICO.0b013e3181a3c52f. [DOI] [PubMed] [Google Scholar]
- 44.Chen M, Gong L, Sun X, et al. A multicenter, randomized, parallel-group clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunctivitis. Curr Med Res Opin. 2012;28(3):1–10. doi: 10.1185/03007995.2012.659723. [DOI] [PubMed] [Google Scholar]
- 45.Amon M, Busin M. Loteprednol etabonate ophthalmic suspension 0.5%: efficacy and safety for postoperative anti-inflammatory use. Int Ophthalmol. 2012;32(5):507–517. doi: 10.1007/s10792-012-9589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CFR – Code of Federal Regulations Title 21 Part 349.12 Ophthalmic demulcents, and Part 349.60 Labeling of ophthalmic demulcent drug productsRevised April 1, 2012. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cficfr/CFRSearch.cfm?CFRPart=349Accessed November 12, 2012
- 47.Apt L, Henrick A, Silverman LM. Patient compliance with use of topical ophthalmic corticosteroid suspensions. Am J Ophthalmol. 1979;87(2):210–214. doi: 10.1016/0002-9394(79)90145-4. [DOI] [PubMed] [Google Scholar]
- 48.Fiscella RG. Ophthalmic drug formulations. In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. 5th ed. St Louis, MO: Butterworth-Heinemann; 2008. [Google Scholar]
- 49.Coffey MJ, Davio SR. Viscoelastic and sedimentation characterization of loteprednol etabonate ophthalmic gel, 0.5%; Proceedings of the Annual Meeting of the Association for Research and Vision in Ophthalmology; May 10, 2012; Fort Lauderdale, FL. [Google Scholar]
- 50.Piau JM. Carbopol gels: elastoviscoplastic and slippery glasses made of individual swollen sponges. Meso- and macroscopic properties, constitutive equations and scaling laws. J Nonnewton Fluid Mech. 2007;144:1–29. [Google Scholar]
- 51.Kaur IP, Lal S, Rana C, Kakkar S, Singh H. Ocular perservatives: associated risks and newer options. Cutan Ocul Toxicol. 2009;28(3):93–103. doi: 10.1080/15569520902995834. [DOI] [PubMed] [Google Scholar]
- 52.Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(2):113–119. doi: 10.1089/jop.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.United States Pharmacopoeia (USP 35 – NF 30) The United States Pharmacopeia Convention: United States Pharmacopeia/National Formulary. Rockville, MD: US Pharmacopeia; 2012. [Google Scholar]
- 54.Ykuji Nippo Ltd: Japanese Pharmacopoeia. 15th ed. Tokyo: Maruzen Co, Ltd; 2006. [Google Scholar]
- 55.European Pharmacopoeia 7th ed2012(7.5). European Directorate for the Quality of Medicines and Health Care: efficacy of antimicrobial preservation Strasbourg: European Directorate for the Quality of Medicines; 20065129–5130. [Google Scholar]
- 56.Hu Z, Denick J. Bausch and Lomb Incorporated, assignee. Ophthalmic compositions including glycerin and propylene glycol. United States patent US 5800807. 1998 Sep 1;
- 57.Glogowski S, Jiang S. Ocular and systemic pharmacokinetics of loteprednol etabonate gel (0.5%) following topical ocular administration to rabbits; Paper presented at: Annual Meeting of the Association for Research and Vision in Ophthalmology; May 6–10, 2012; Fort Lauderdale, FL. [Google Scholar]
- 58.Chrai SS, Patton TF, Mehta A, Robinson JR. Lacrimal and instilled fluid dynamics in rabbit eyes. J Pharm Sci. 1973;62(7):1112–1121. doi: 10.1002/jps.2600620712. [DOI] [PubMed] [Google Scholar]
- 59.Saettone M F, Giannaccini B, Barattini F, Tellini N. The validity of rabbits for investigations on ophthalmic vehicles: a comparison of four different vehicles containing tropicamide in humans and rabbits. Pharm Acta Helv. 1982;57(2):47–55. [PubMed] [Google Scholar]
- 60.Zaki I, Fitzgerald P, Hardy JG, Wilson CG. A comparison of the effect of viscosity on the precorneal residence of solutions in rabbit and man. J Pharm Pharmacol. 1986;38(6):463–466. doi: 10.1111/j.2042-7158.1986.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 61.Rajpal RK, Roel L, Siou-Mermet R, Erb T. Evaluation of the efficacy and safety of loteprednol etabonate gel 0.5% in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2012 doi: 10.1016/j.jcrs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Fong R, Leitritz M, Siou-Mermet R, Erb T. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. Clin Ophthalmol. 2012;6:1113–1124. doi: 10.2147/OPTH.S32643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8. doi: 10.1097/00055735-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17(1):20–28. doi: 10.1177/112067210701700104. [DOI] [PubMed] [Google Scholar]
- 65.Wittpenn JR, Silverstein S, Heier J, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146(4):554–560. doi: 10.1016/j.ajo.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 66.Parekh JG, Newsom TH, Nielsen S. Safety of besifoxacin ophthalmic suspension 0.6% in cataract surgery patients. J Cataract Refract Surg. 2012;38(10):1864–1867. doi: 10.1016/j.jcrs.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 67.Raizman MB, Donnenfeld ED, Weinstein AJ. Clinical comparison of two topical prednisolone acetate 1% formulations in reducing inflammation after cataract surgery. Curr Med Res Opin. 2007;23(10):2325–2331. doi: 10.1185/030079907X226195. [DOI] [PubMed] [Google Scholar]
- 68.Lane SS, Holland EJ. A randomized, multicenter, masked evaluation of 0.5% loteprednol etabonate versus 1.0% prednisolone acetate for the treatment of inflammation following cataract surgery. J Cataract Refract Surg. 2012 doi: 10.1016/j.jcrs.2012.10.039. [DOI] [PubMed] [Google Scholar]