Abstract
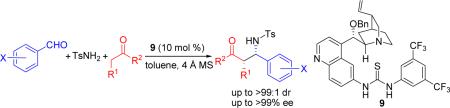
A highly stereoselective three-component direct Mannich reaction between aromatic aldehydes, p-toluenesulfonamide, and unfunctionalized ketones was achieved through an enolate mechanism for the first time with a bifunctional quinidine thiourea catalyst. The corresponding N-tosylated β-aminoketones were obtained in high yields and excellent diastereo- and enantioselectivities (up to >99:1 dr and >99% ee).
The Mannich reaction is a very powerful tool for constructing carbon-carbon bond in organic chemistry.1 The reaction is especially useful for the synthesis of β-amino carbonyl derivatives.1 Due to the importance of the Mannich products in organic synthesis, various methods for conducting highly diastereoselective and/or enantioselective Mannich reactions have been developed in the past.1
Since List reported the first example of a proline catalyzed direct Mannich reaction in 2000,2 organocatalyzed Mannich reactions have been undergoing vigorous development in the past decade.1b,3 Amino acid derivatives, mainly those derived from proline,4 chiral Brønsted acids,5 chiral amine thioureas,6 and cinchona alkaloids7 have been used as the catalysts in Mannich reactions, and high diastereoselectivities and/or enantioselectivities have been achieved in many cases.1b,3 Nonetheless, among those reported organocatalyzed direct Mannich reactions, catalysts that can perform the three-component direct Mannich reactions of unfunctionalized ketones or aldehydes are still very limited because many of the reported catalysts and/or reaction conditions are not compatible with the in situ generation of the imines.8,9, To our knowledge, with the exception of a chiral phosphoric acid reported by Gong and coworkers,9a all the other catalysts are mainly amine derivatives4,9b that catalyze the reaction through the enamine mechanism.9
Recently we demonstrated that Brønsted base catalysts are capable of inducing aldol reactions of unfunctionalized ketones.10a The reaction works through the enolate mechanism11 and is complementary to the amine-catalyzed aldol reactions in terms of the substrate scope.10 Because the reaction mechanisms of the Mannich and aldol reactions are very similar, we envisioned that Brønsted bases should be also good catalysts for the Mannich reaction of unfunctionalized ketones via the enolate mechanism. With this in mind, we recently realized a TMG-catalyzed high diastereoselective three-component direct Mannich reaction of unfunctionalized ketones.12 Herein we wish to report a bifunctional Brønsted base-catalyzed highly enantioselective and diastereoselective three-component direct Mannich reaction of unfunctionalized ketones. To our knowledge, although enolate-mediated organocatalyzed enantioselective direct Mannich reactions of active methylene compounds are known,6,7 such a three-component direct Mannich reaction of unfunctionalized ketones has not been reported.9,11
Using benzaldehyde (1a), p-toluenesulfonamide, and 1,2-diphenylethanone (3a) as the model substrates, we initially screened several chiral Brønsted bases (5–12, Figure 1) for their ability to effect the desired enolate mediated three-component Mannich reactions. The results are summarized in Table 1.
Figure 1.
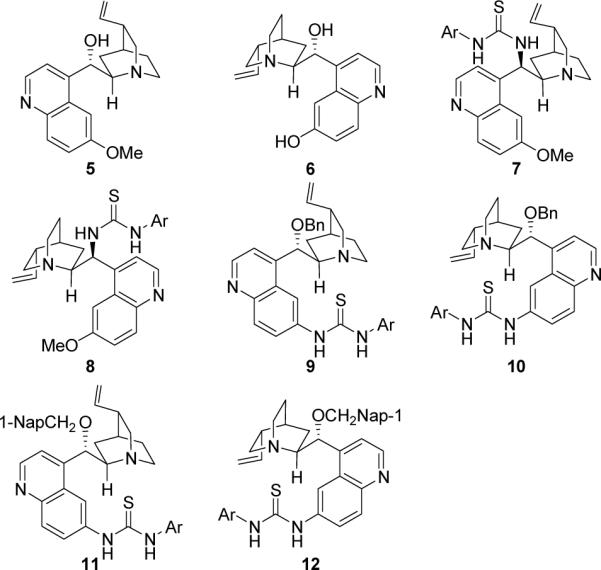
Catalyst Screened for the Mannich Reaction (Ar = 3,5-(CF3)2C6H3-; 1-Nap= 1-naphthyl)
Table 1.
Catalyst screening and condition optimizations for the base-catalyzed three-component direct Mannich reactiona
| entry | catalyst | solvent | yield (%)b | drc | ee (%)d |
|---|---|---|---|---|---|
| 1 | 5 | toluene | 10 | >99:1 | 45 |
| 2 | 6 | toluene | trace | --- | --- |
| 3 | 7 | toluene | 44 | >99:1 | 60 |
| 4 | 8 | toluene | 49 | >99:1 | 78e |
| 5 | 9 | toluene | 96 | >99:1 | 94 |
| 6 | 10 | toluene | 96 | >99:1 | 90e |
| 7 | 11 | toluene | 78 | >99:1 | 94 |
| 8 | 12 | toluene | 68 | >99:1 | 92e |
| 9 | 9 | benzene | 96 | >99:1 | 92 |
| 10 | 9 | o-xylene | 95 | >99:1 | 92 |
| 11 | 9 | CH2Cl2 | 95 | >99:1 | 86 |
| 12 | 9 | Et2O | 87 | >99:1 | 72 |
| 13 | 9 | THF | 60 | >99:1 | 50 |
| 14f | 9 | toluene | 96 | >99:1 | 96 |
Unless otherwise specified, all reactions were carried out at rt with benzaldehyde (1a, 0.20 mmol), toluenesulfonamide (2, 0.40 mmol), ketone 3a (0.40 mmol), and the Lewis base catalyst (0.020 mmol, 10 mol %) in the presence of 4 Å MS (50.0 mg) in the indicated solvent (2 mL) for 24 h.
Yield of the isolated product after column chromatography.
Determined by 1H NMR analysis of the crude reaction product.
Values of ee were determined by chiral HPLC analysis on a ChiralCel AD-H column.
The opposite enantiomer was obtained as the major product in this case.
The reaction was conducted at 0 °C for 48 h.
With toluene as the solvent, the reaction catalyzed by quinidine (5) gave a poor yield (10%) and a low ee value (45%) of the desired 4a (Table 1, entry 1). Similarly, poor results were obtained when cupreine (6) was used (Table 1, entry 2). In contrast, quinidine- and quinine-derived thioureas 7 and 8 led to much improved yields and moderate ee values (Table 1, entries 3–4). To our pleasure, when quinine or quinidine-derived thioureas 9, 10, 11, and 12 were applied,13 good yields and high diastereoselectivities as well as excellent enantioselectivities were obtained (Table 1, entries 5–8). Catalyst 9 was adopted for further optimizations (Table 1, entries 9–14) since it yields the highest product yield and ee value. Toluene (entry 5) was identified as the best solvent for this reaction since the other tested solvents all led to less satisfactory results. However, it was found that the ee value of 4a may be improved to 96% when the reaction was carried out at 0 °C (entry 14).
Once the reaction conditions were optimized, the scope of this reaction was evaluated, and the results are collected in Table 2.
Table 2.
Three-component direct Mannich reaction catalyzed by bifunctional catalysts 9 or 10a
| entry | X/ R1 / R2 | 1/3/4 | time (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | H/Ph/Ph | a/a/a | 48 | 96 | 96 |
| 2 | 4-Me/Ph/Ph | b/a/b | 40 | 95 | 96 |
| 3 | 4-MeO/Ph/Ph | c/a/c | 48 | 88 | 96 |
| 4 | 4-F/Ph/Ph | d/a/d | 36 | 96 | 95 |
| 5 | 4-Cl/Ph/Ph | e/a/e | 24 | 95 | 94 |
| 6 | 4-Br/Ph/Ph | f/a/f | 24 | 97 | 94 |
| 7 | 4-CN/Ph/Ph | g/a/g | 40 | 97 | 97 |
| 8 | 4-NO2/Ph/Ph | h/a/h | 24 | 93 | 93 |
| 9 | 2-Br/Ph/Ph | i/a/i | 48 | 96 | 95 |
| 10 | 3-Br/Ph/Ph | j/a/j | 24 | 96 | 95 |
| 11 | H/4-BrC6H4/Ph | a/b/k | 30 | 95 | 98 |
| 12 | H/4-ClC6H4/Ph | a/c/l | 35 | 95 | 96 |
| 13 | H/4-MeOC6H4/Ph | a/d/m | 72 | 75 | 96 |
| 14 | H/Et/Ph | a/e/n | 96 | 75 | >99 |
| 15 | H/PhCH2/Ph | a/f/o | 24 | 95 | 96 |
| 16 | H/Ph/OBoc | a/g/p | 24 | 96d | >99 |
| 17 | 4-Br/Ph/OBoc | f/g/q | 40 | 96e | >99 |
| 18f | H/4-MeOC6H4/OBoc | a/h/r | 48 | 95g | >99 |
Unless otherwise indicated, all reactions were carried out at 0 °C with aldehyde 1 (0.20 mmol), p-toluenesulfonamide (2, 0.40 mmol), ketone 3 (0.40 mmol), catalyst 9 (0.020 mmol, 10 mol %), and 4 Å MS (50. 0 mg) in toluene (2.0 mL).
Yield of the isolated product after column chromatography. Only a single diastereomer was detected by the 1H NMR analysis of the crude reaction product in all cases (dr >99:1) except for entries 16–18.
Determined by HPLC analysis.
The dr of this reaction was 93:7.
The dr of this reaction was 92:8.
Catalyst 10 (10 mol %) was used, and the opposite enantiomer was obtained as the major product.
The dr of this reaction was 90:10.
Firstly, the aldehyde substrates were evaluated using 1,2-diphenylethanone (3a) and p-toluenesulfonamide (2) as the substrates. As the data in Table 2 show, benzaldehyde (1a) and benzaldehyde derivatives that bear either an electron-withdrawing or an electron-donating group (1b–j) all produce the desired Mannich products 4 in high yields (≥ 88%) and excellent ee values (93–97% ee) as a single diastereomer (>99:1 dr, entries 1–10). Next the ketone substrates were studied using benzaldehyde (1a) and p-toluenesulfonamide (2) as the imine precursors. Again, excellent results (>99:1 dr, 96–98% ee) were obtained for 1,2-diphenylethanone derivatives 3b, 3c, and 3d (entries 11–13). The aliphatic 1-phenylbutan-2-one (3e) is slightly less reactive, and a lower yield (75%) of 4n was obtained, but this product was obtained in over 99% ee (entry 14). Excellent results were also obtained for the aliphatic 1,3-diphenylpropan-2-one (4o, >99:1 dr, 96% ee, entry 15). Phenacyl tert-butyl carbonate (3g) was also evaluated as substrate in this reaction and the corresponding Mannich product 4p was obtained in 96% yield and >99% ee with a dr of 93:7 (entry 16). Similarly, when 4-bromobenzaldehyde (1f) was used with 3g, the desired product 4q was obtained in 96% yield and >99% ee with a dr of 92:8 (entry 17). The stereochemistry of the major enantiomer generated with catalyst 9 was determined to be (2S,3S) according to the X-ray crystallographic analysis of the reaction product 4k.14
When an aldehyde was applied under these optimized conditions, no desired Mannich reaction product was obtained, probably due to the interference of the self-aldol reaction of the aldehyde. However, when phenylacetaldehyde was used with the pre-prepared N-tosylimine, the desired Mannich product was obtained in a high yield. To facilitate the purification and ee value determination, the primary Mannich product was in-situ reduced to the corresponding alcohol 13, which was obtained in excellent yield, dr, and ee value (Scheme 1).
Scheme 1.
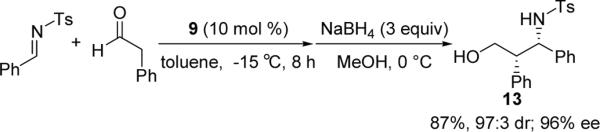
Mannich Reaction between N-tosylimine and phenylacetaldehyde
The N-tosyl β-aminoketone products obtained in this reaction are very useful in organic synthesis. For example, when catalyst 10 was used under these optimized conditions, the reaction of 1a, 2, and 3h led to the formation of (2R,3R)-4r with an ee value of >99% and a dr of 90:10 (Table 2, entry 18). This product may be oxidized by using mCPBA to produce the corresponding N-tosyl-β-amino ester 14 (Scheme 2), which may be readily benzoylated to give compound 15. After removing the N-tosyl group, the O-Boc-protected syn-β-amino ester 16 was obtained in high yield (78% over three steps). Since the Boc15 and PMP16 groups are readily removable during synthesis, compound 16 may be regarded as a protected Paclitaxel side chain and should be useful in the synthesis of Paclitaxel (Scheme 2).
Scheme 2.
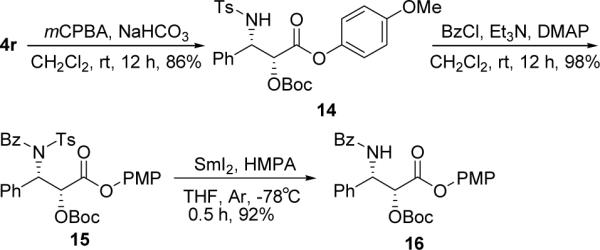
Synthesis of protected Paclitaxel side chain.
In summary, we have realized the first enantioselective enolate-mediated three-component direct Mannich reaction of unmodified ketones using bifunctional cinchona alkaloid thioureas as the base catalyst. The corresponding N-tosylated β-aminoketones were obtained in high yields, high enantioselectivities, and excellent syn diastereoselectivities. We also demonstrated that, with a preformed imine, aldehyde may also be used as the substrate in this reaction.
Supplementary Material
Acknowledgment
The generous financial support of this research from the National Science Foundation (Grant No. CHE 0909954), the NIH-NIGMS (Grant-No. SC1GM0828718), and the Welch Foundation (Grant No. AX-1593) is gratefully acknowledged. The authors also thank Dr. Hadi Arman (UTSA) for his assistance in the X-ray crystallographic analyses of the reaction products.
Footnotes
Supporting Information Available Full experimental procedures, compound characterization data, ORTEP drawings and cif file of compound 4k, and copy of NMR spectra and HPLC chromatograms. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For reviews on Mannich reactions, see: Kobayashi S, Ishitani H. Chem. Rev. 1999;99:1069. doi: 10.1021/cr980414z.. Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem. Rev. 2011;111:2626. doi: 10.1021/cr100204f.. Arend M, Westermann B, Risch N. Angew. Chem., Int. Ed. 1998;37:1044. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E.; Hart DJ, Ha D-C. Chem. Rev. 1989;89:1447..
- (2).List B. J. Am. Chem. Soc. 2000;122:9336. [Google Scholar]
- (3).For reviews, see: Dalko PI, Moisan L. Angew. Chem., Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650.. Marques MMB. Angew. Chem., Int. Ed. 2006;45:348. doi: 10.1002/anie.200502630.. Verkade JMM, van Hemert LJC, Quaedflieg P, Rutjes F. Chem. Soc. Rev. 2008;37:29. doi: 10.1039/b713885g.. Xu L-W, Lu Y. Org. Biomol. Chem. 2008;6:2047. doi: 10.1039/b803116a.. Bhadury PS, Song B-A. Curr. Org. Chem. 2010;14:1989.; Benohoud M, Hayashi Y. In: Science of Synthesis, Asymmetric Organocatalysis. List B, Maruoka K, editors. Vol. 1. Georg Thieme Verlag; Stuttgart: 2012. pp. 73–134.; Mukherjee S, Yang JW, Hoffmann S, List B. Chem. Rev. 2007;107:5471. doi: 10.1021/cr0684016..
- (4).For some leading examples, see: List B, Pojarliev P, Biller WT, Martin HJ. J. Am. Chem. Soc. 2002;124:827. doi: 10.1021/ja0174231.. Córdova A, Notz W, Zhong G, Betancort JM, Barbas CF., III J. Am. Chem. Soc. 2002;124:1842. doi: 10.1021/ja017270h.. Córdova A, Watanabe SI, Tanaka F, Notz W, Barbas CF., III J. Am. Chem. Soc. 2002;124:1866. doi: 10.1021/ja017833p.. Carlone A, Cabrera S, Marigo M, Jørgensen KA. Angew. Chem., Int. Ed. 2007;46:1101. doi: 10.1002/anie.200604479.. Frisch K, Landa A, Saaby S, Jørgensen KA. Angew. Chem., Int. Ed. 2005;44:6058. doi: 10.1002/anie.200501900.. Ibrahem I, Casas J, Córdova A. Angew. Chem., Int. Ed. 2004;43:6528. doi: 10.1002/anie.200460678.. Sunden H, Ibrahem I, Eriksson L, Córdova A. Angew. Chem., Int. Ed. 2005;44:4877. doi: 10.1002/anie.200500811.. Hayashi Y, Tsuboi W, Ashimine I, Urushima T, Shoji M, Sakai K. Angew. Chem., Int. Ed. 2003;42:3677. doi: 10.1002/anie.200351813.. Hayashi Y, Tsuboi W, Shoji M, Suzuki N. J. Am. Chem. Soc. 2003;125:11208. doi: 10.1021/ja0372513.. Yang JW, Stadler M, List B. Angew. Chem., Int. Ed. 2007;46:609. doi: 10.1002/anie.200603188..
- (5).For some leading examples, see: Uraguchi M, Terada M. J. Am. Chem. Soc. 2004;126:5356. doi: 10.1021/ja0491533.. Terada M, Machioka K, Sorimachi K. J. Am. Chem. Soc. 2007;129:10336. doi: 10.1021/ja0739584.. Matsubara M, Kawai N, Kobayashi S. Angew. Chem., Int. Ed. 2006;45:3814. doi: 10.1002/anie.200600471.. Seayad J, Seayad AM, List B. J. Am. Chem. Soc. 2006;128:1086. doi: 10.1021/ja057444l.. Yamanaka M, Itoh J, Fuchibe K, Akiyama T. J. Am. Chem. Soc. 2007;129:6756. doi: 10.1021/ja0684803.. Sickert M, Schneider C. Angew. Chem., Int. Ed. 2008;47:3631. doi: 10.1002/anie.200800103.
- (6).For some leading examples, see: Uraguchi M, Terada M. J. Am. Chem. Soc. 2004;126:5356. doi: 10.1021/ja0491533.. Terada M, Machioka K, Sorimachi K. J. Am. Chem. Soc. 2007;129:10336. doi: 10.1021/ja0739584.. Matsubara M, Kawai N, Kobayashi S. Angew. Chem., Int. Ed. 2006;45:3814. doi: 10.1002/anie.200600471.. Seayad J, Seayad AM, List B. J. Am. Chem. Soc. 2006;128:1086. doi: 10.1021/ja057444l.. Yamanaka M, Itoh J, Fuchibe K, Akiyama T. J. Am. Chem. Soc. 2007;129:6756. doi: 10.1021/ja0684803.; Sickert M, Schneider C. Angew. Chem., Int. Ed. 2008;47:3631. doi: 10.1002/anie.200800103..
- (7).For some leading examples, see: Robak MT, Trincado M, Ellman JA. J. Am. Chem. Soc. 2007;129:15110. doi: 10.1021/ja075653v.. Wang C-J, Dong X-Q, Zhang Z-H, Xue Z-Y, Teng H-L. J. Am. Chem. Soc. 2008;130:8606. doi: 10.1021/ja803538x.. Liu T-Y, Cui H-L, Long J, Li BJ, Wu Y, Ding L-S, Chen Y-C. J. Am. Chem. Soc. 2007;129:1878. doi: 10.1021/ja068703p.. Song J, Wang Y, Deng L. J. Am. Chem. Soc. 2006;128:6048. doi: 10.1021/ja060716f.. McCooey SH, Connon SJ. Angew. Chem., Int. Ed. 2005;44:6367. doi: 10.1002/anie.200501721.. Zhang H, Chuan Y-M, Li Z-Y, Peng Y-G. Adv. Synth. Catal. 2009;351:2288.. Han X, Kwiatkowski J, Xue F, Huang K-W, Lu Y. Angew. Chem., Int. Ed. 2011;50:2664..
- (8).For examples of Lewis acid-catalyzed three-component direct Mannich reactions of unmodified ketones and aldehydes, see: Xu L-W, Xia C-G, Li L. J. Org. Chem. 2004;69:8482. doi: 10.1021/jo048778g.. Salter MM, Kobayashi J, Shimizu Y, Kobayashi S. Org. Lett. 2006;8:3533. doi: 10.1021/ol0613012.. Das B, Majhi A, Reddy KR,, Suneel K. J. Mol. Catal. A: Chem. 2007;274:83.. Phukan P, Kataki D, Chakraborty P. Tetrahedron Lett. 2006;47:5523.. Rafiee E, Eavani S, Nejad FK, Joshaghani M. Tetrahedron. 2010;66:6858.. Kureshy RI, Agrawal S, Saravanan S, Khan NH, Shah AK, Abdi SHR, Bajaj HC, Suresh E. Tetrahedron Lett. 2010;51:489.
- (9).For examples of enol-mediated Mannich reactions of unmodified ketones, see: Guo Q-X, Liu H, Guo C, Luo S-W, Gu Y, Gong L-Z. J. Am. Chem. Soc. 2007;129:3790. doi: 10.1021/ja068236b.. Yalalov DA, Tsogoeva SB, Shubina TE, Martynova IM, Clark T. Angew. Chem., Int. Ed. 2008;47:6624. doi: 10.1002/anie.200800849..
- (10).(a) Guo Q, Bhanushali M, Zhao C-G. Angew. Chem., Int. Ed. 2010;49:9460. doi: 10.1002/anie.201004161. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guang J, Guo Q, Zhao JC-G. Org. Lett. 2012;14:3174. doi: 10.1021/ol301270w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For examples of enolate-mediated Mannich reactions of thioesters, see: Kohler MC, Yost JM, Garnsey MR, Coltart DM. Org. Lett. 2010;12:3376. doi: 10.1021/ol101152b.. Utsumi N, Kitagaki S, Barbas CF., III Org. Lett. 2008;10:3405. doi: 10.1021/ol801207x.. For examples of enolate-mediated Mannich reactions of sulfonylimidates, see: Van Nguyen H, Matsubara R, Kobayashi S. Angew. Chem., Int. Ed. 2009;48:5927. doi: 10.1002/anie.200900309.. Matsubara R, Berthiol F, Kobayashi S. J. Am. Chem. Soc. 2008;130:1804. doi: 10.1021/ja077054u..
- (12).Guo Q, Zhao JC-G, Arman H. Tetrahedron Lett. 2012;53:4866. [Google Scholar]
- (13).Catalysts 9–12 were prepared according to the procedures reported in Marcelli T, van der Haas R, van Maarseveen JH, Hiemstra H. Angew. Chem. Int. Ed. 2006;45:929. doi: 10.1002/anie.200503724.. Liu Y, Sun B, Wang B, Wakem M, Deng L. J. Am. Chem. Soc. 2009;131:418. doi: 10.1021/ja8085092..
- (14).For details, please see the Supporting Information
- (15).Ke B, Qin Y, Zhao F, Qu Y. Bioorg. Med. Chem. Lett. 2008;18:4783. doi: 10.1016/j.bmcl.2008.07.101. [DOI] [PubMed] [Google Scholar]
- (16).Watson DA, Fan X, Buchwald SL. J. Org. Chem. 2008;73:7096. doi: 10.1021/jo800907e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


