Abstract
4-vinylcyclohexene diepoxide (VCD) destroys ovarian primordial and small primary follicles via apoptosis. In mice, VCD exposure induces ovarian mRNA expression of glutathione S-transferase (GST) family members, including isoform mu (Gstm). Extra-ovarian GSTM negatively regulates pro-apoptotic apoptosis signal-related kinase 1 (ASK1) through protein complex formation, which dissociates during stress, thereby initiating ASK1-induced apoptosis. The present study investigated the ovarian response of Gstm mRNA and protein to VCD. Induction of Ask1 mRNA at VCD-induced follicle loss onset was determined. Ovarian GSTM:ASK1 protein complex formation was investigated and VCD exposure effects thereon evaluated. Phosphatidylinositol-3 kinase (PI3K) regulation of GSTM protein was also studied. Postnatal day (PND) 4 rat ovaries were cultured in control media ±: 1) VCD (30 μM) for 2–8d; 2) VCD (30 μM) for 2d, followed by incubation in control media for 4d (acute VCD exposure); or 3) LY294002 (20 μM) for 6d. VCD exposure did not alter Gstm mRNA expression, however, GSTM protein increased (P < 0.05) after 6d of both the acute and chronic treatments. Ask1 mRNA increased (0.33-fold; P < 0.05) relative to control after 6d of VCD exposure. Ovarian GSTM:ASK1 protein complex formation was confirmed and, relative to control, the amount of GSTM bound to ASK1 increased 33% (P < 0.05) by chronic but with no effect of acute VCD exposure. PI3K inhibition increased (P < 0.05) GSTM protein by 40% and 71% on d4 and d6, respectively. These findings support involvement of GSTM in the ovarian response to VCD exposure, through regulation of pro-apoptotic ASK1.
Keywords: 4-vinylcyclohexene diepoxide, ovotoxicity, glutathione S-transferase mu, apoptosis
Introduction
The ovary is the female gonad composed of oocyte-containing follicles at various stages of development. Females are born with a finite number of the most immature follicular stage, termed primordial follicles, which, once destroyed, cannot be regenerated (Hirshfield, 1991). Exposure to environmental factors that cause follicular damage and depletion can impair fertility and induce ovarian failure (menopause; Mattison et al., 1980). An occupational chemical known to selectively destroy primordial and small primary follicles in the ovaries of mice and rats is 4-vinylcyclohexene diepoxide (VCD, Kao et al., 1999; Smith et al., 1990). VCD is the active metabolite of 4-vinylcyclohexene (VCH), an industrial diluent for epoxides (IARC, 1976). Repeated daily dosing of rats with VCD (80 mg/kg/day, intraperitoneal) for 15 days results in approximately 50% depletion of primordial and small primary follicles relative to controls, with no significant effect on larger follicles or corpora lutea (Smith et al., 1990; Flaws et al., 1994; Springer et al., 1996a,b). VCD-induced primordial and small primary follicle loss begins on day 6 of in vitro exposure in postnatal day (PND) 4 cultured Fisher 344 (F344) rat ovaries (Keating et al., 2009). Mechanistic studies have revealed that VCD induces follicle loss by accelerating the natural process of atresia (apoptosis) in both a time- and dose-dependent manner (Springer et al., 1996a; Hu et al., 2001a; Devine et al., 2002).
VCH may be metabolized (bioactivated) in the ovary to VCD by the action of the cytochrome P450 (CYP) family member CYP 2E1 (Cannady et al., 2003; Rajapaksa et al., 2007). Cyp 2e1-null mice had less follicle loss compared to their wild type control littermates (Rajapaksa et al., 2007), suggesting the involvement of CYP 2E1 in VCH bioactivation. Conversely, VCD can be detoxified in the ovary to a less ovotoxic tetrol metabolite through the action of microsomal epoxide hydrolase (mEH) (Flaws et al., 1994; Cannady et al., 2002; Keating et al., 2008; Bhattacharya et al., 2012). Studies have shown that VCD induces mEH mRNA and protein expression both in vivo and in vitro prior to an observed follicle loss in mice and rats (Cannady et al., 2002; Keating et al., 2008; Bhattacharya et al., 2012). Additionally when mEH is inhibited in vitro using cyclohexene oxide (CHO) in the presence of VCD, more follicle loss results relative to those ovaries treated only with VCD, thereby supporting an ovarian detoxification role for mEH during VCD exposure (Bhattacharya et al., 2012).
Glutathione (GSH) is a ubiquitous antioxidant that protects cells against oxidative stress and electrophilic compounds (DeLeve and Kaplowitz, 1991). GSH functions in two major ways: 1) through conjugation to electrophilic compounds resulting in their more rapid excretion from the body; or 2) directly reducing and neutralizing reactive oxygen intermediates in a coupled reaction involving GSH peroxidase (DeLeve and Kaplowitz, 1991). The glutathione S-transferase (GST) enzyme family catalyze conjugation of GSH to xenobiotics (Jakoby et al., 1978), and the ovary is capable of synthesizing GSH (Luderer et al., 2001). The mRNA encoding the GST isoforms pi (Gstp) and mu (Gstm) are increased in response to VCD exposure in cultured PND4 mouse ovaries (Keating et al., 2008). Additionally, VCD increases Gstp mRNA and protein in cultured PND4 rat ovaries prior to (d4) and at times of (d6, d8) VCD-induced follicle loss (Keating et al., 2010). These observations support that the GST isoforms mu and pi are involved in the ovarian response to VCD exposure, and may potentially catalyze VCD:GSH conjugation.
In addition to GSH conjugation, GST enzymes are involved in cell signaling pathway regulation (Cho et al., 2001; Dorion et al., 2002; Keating et al., 2010). GSTP forms a protein complex with c-JUN N-terminal kinase (JNK; Adler et al., 1999) and is likely to negatively regulate its action in the rat ovary (Keating et al., 2010). The amount of JNK protein bound to GSTP increased after 6 days of VCD exposure (Keating et al., 2010) with a concomitant decrease in unbound JNK and phosphorylated c-JUN (p-c-JUN) protein levels. Furthermore, a decrease in p-c-JUN preceded the increase in JNK bound to GSTP, indicating that JNK activity was impaired through the GSTP interaction (Keating et al., 2010).
Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein kinase kinase kinase (MAPKKK) which is capable of activating the pro-apoptotic JNK and p38 MAPK signaling pathways (Ichijo et al., 1997). In extra-ovarian tissues, GSTM physically interacts with ASK1 and functions to repress ASK1-mediated apoptosis (Cho et al., 2001). The GSTM:ASK1 protein complex dissociates in response to stress and thereby triggers activation of apoptosis by ASK1 (Dorion et al., 2002). It is therefore known that GSTM modulates stress-activity signals by suppressing ASK1. Interestingly, VCD-induced apoptosis involves the action of JNK and p-c-JUN (Hu et al., 2002). Thus, ovarian GSTM is potentially involved in the ovarian response to VCD exposure.
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase involved in various cellular functions including cell growth and survival, proliferation, and differentiation (Vivanco and Sawyers, 2007). In the ovary, the PI3K signaling pathway plays an important role in primordial follicles by maintaining oocyte viability and directing their recruitment into the growing follicular pool (Castrillon et al., 2003; Reddy et al., 2005; Liu et al., 2006). Impairment of PI3K signaling occurs during VCD-induced ovotoxicity (Keating et al., 2011), while PI3K inhibition prevented VCD-induced primordial follicle loss in cultured PND4 rat ovaries (Keating et al., 2009). Recently, it has been shown that the xenobiotic biotransformation enzymes mEH and Gstp are regulated by PI3K signaling in cultured PND4 rat ovaries and Gstm mRNA was increased by PI3K inhibition (Bhattacharya et al., 2012; Bhattacharya and Keating, 2012). Taken together, these findings also point to regulation of Gstm by PI3K signaling.
To determine if GSTM is associated with the ovarian response to VCD as well as in regulation of pro-apoptotic ASK1, several approaches were taken: the first was to study the temporal pattern of Gstm mRNA and protein expression in response to VCD. The second was to determine any effect of VCD on Ask1 mRNA expression at the time of the onset of VCD-induced follicle loss. The third approach was to establish the presence of a GSTM:ASK1 protein complex in the rat ovary and to study the effect of VCD exposure (both acute and chronic) upon this complex. Finally, because Gstm mRNA expression is increased in cultured PND4 rat ovaries during inhibition of the PI3K pathway (Bhattacharya and Keating, 2012) regulation of GSTM by PI3K was evaluated.
Materials and Methods
Reagents
4-vinylcyclohexene diepoxide (VCD, mixture of isomers, >99% purity), ascorbic acid, transferrin, bovine serum albumin (BSA), 2-β-mercaptoethanol, 30% acrylamide/0.8% bis-acrylamide, ammonium persulphate, glycerol, N′N′N′N′-Tetramethyl-ethylenediamine (TEMED), Tris base, Tris HCL, sodium chloride, Tween-20 were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1x (DMEM/Ham’s F12), Albumax, penicillin (5000U/ml), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2 or MgSO4), Gstm and β-actin primers, and superscript III one-step RT-PCR system were obtained from Invitrogen Co. (Carlsbad, CA). Gapdh and Ask1 primers were obtained from the DNA facility of the Office of Biotechnology at Iowa State University. Millicell-CM filter inserts and anti-GSTM antibodies were purchased from Millipore (Bedford, MA). The anti-ASK1 primary antibody was obtained from Cell Signaling Technology (Danvers, MA). 48-well cell culture plates were purchased from Corning Inc. (Corning, NY). RNeasy Mini kit, QIA shredder kit, RNeasy Mini Elute kit, and Quantitect™ SYBR Green PCR kit were purchased from Qiagen Inc. (Valencia, CA). RNAlater was obtained from Ambion Inc. (Austin, TX). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002; CAS#154447-36-6) was purchased from A.G. Scientific, Inc. (San Diego, CA). Pierce BCA protein Assay Kit was purchased from Thermo Scientific (Rockford, IL). The goat anti-rabbit secondary antibody was obtained from Thermo Scientific, (Lafayette, CO). Ponceau S was from Fisher Scientific (Asheville, NC). ECL plus chemical luminescence detection kit was obtained from GE Healthcare, Amersham (Buckinghamshire, UK).
Animals
Rats were housed two per cage in plastic cages and maintained in a controlled environment (22 ± 2°C; 12h light/12h dark cycles). The animals were provided a standard diet with ad libitum access to food and water, and allowed to give birth. All animal experimental procedures were approved by the Iowa State University’s Institutional Animal Care and Use Committee.
In vitro ovarian cultures
Ovaries from PND4 Fisher 344 (F344) rats were cultured as described by Parrott and Skinner (1999). Briefly, pups were euthanized by CO2 inhalation followed by decapitation. Ovaries were removed, trimmed of oviduct and other excess tissue and placed onto Millicell-CM membrane floating on 250 μl of DMEM/Ham’s F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 μg/ml ascorbic acid, 5 U/ml penicillin and 27.5 μg/ml transferrin per well in a 48 well plate previously equilibrated to 37°C. One ovary was placed in the control treatment, while the contralateral ovary was exposed to the experimental treatment. A drop of medium was placed on top of each ovary to prevent it from drying. Ovaries were treated with vehicle control medium (1% DMSO), VCD (30 μM) or LY294002 (20 μM) and maintained at 37°C and 5% CO2 for 2–8 d. For chronic VCD exposure, ovaries were continuously exposed to VCD (30 μM) on alternate days and samples were collected accordingly. For the acute VCD exposure, ovaries were exposed for 2d to VCD (30 μM) beginning at the onset of culture and were subsequently incubated in control medium for a further 4d for a total of 6d of culture. The concentration (30 μM) of in vitro VCD exposure was previously determined to cause about 50% loss of both primordial and small primary follicles after 6d (Devine et al., 2002; Keating et al., 2009). The concentration of LY294002 (20 μM) was previously shown to be effective in the rat PND4 ovary culture system (Keating et al., 2009). For cultures lasting for more than 2d, media was changed every alternate day and treatment added.
RNA isolation and polymerase chain reaction (PCR)
Following 2, 4, 6 or 8 d of in vitro culture, ovaries treated with control or VCD (30 μM) were stored in RNAlater at −80°C. Total RNA was isolated from ovaries (n=3; 10 ovaries per pool) using an RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. RNA was eluted in 14ul of RNase-free water and the concentration was determined using a NanoDrop (λ=260/280 nm; ND 1000; Nanodrop Technologies Inc, Wilmington, DE). Total RNA (500 ng) was reverse transcribed to cDNA using Superscript III One-Step RT-PCR System (Invitrogen). Gstm and β-actin (Actb) were amplified on a Rotor-Gene 3000 (Corbett Research, Sydney, Australia). An Eppendorf mastercycler (Hauppauge, NY) was used to amplify Gapdh and Ask1. A Quantitect™ SYBR Green PCR kit (Qiagen Inc.Valencia, CA) was used for amplification. The primers used for Gstm and Actb were those described in (Keating et al., 2008). The Gapdh primers were from Bhattacharya and Keating (2012). Ask1 primers were: forward - 5′-AGAAAAGGACCAAGAAATTAAGCAC-3′; reverse-5′-AACCGACTTATAGTGTCTTCGTCAG-3′. There was no difference in Actb or Gapdh mRNA expression level between vehicle control and VCD treated ovaries.
Protein Isolation
Pools (n = 3 pools; 10 ovaries per pool for Western blotting; 20 ovaries per pool for immunoprecipitation) of whole ovarian protein suspensions were obtained by homogenization in tissue lysis buffer containing protease and phosphatase inhibitors (Thompson et al., 2005). SDS-free lysis buffer was used to isolate protein for immunoprecipitation. After homogenization, samples were placed on ice for 30 min, followed by two rounds of centrifugation at 10,000 rpm for 15 min. Protein quantification was performed using a standard BCA protocol on a 96-well assay plate. Emission absorbance was detected with a λ= 540nm excitation on a Synergy™ HT Multi-Detection 1 Microplate Reader using KC4™ software (Bio-Tek® Instruments Inc., Winooski, VT). Protein concentrations were calculated from a BSA protein standard curve.
Protein immunoprecipitation
Protein immunoprecipitation was performed as previously described (Bhattacharya and Keating, 2012). Briefly, ovarian protein (30 μg) was incubated overnight at 4°C with 15 μl of anti-ASK1 antibody. Protein G agarose beads were washed, added to the protein-antibody mixture and incubated at 4°C for 3 h with shaking, followed by centrifugation at 10,000 rpm for 10 mins. The supernatant containing the unbound protein was saved for Western blotting. After washing with lysis buffer beads were incubated with Laemmli sample buffer (40 μl) at 95°C for 10 min. Thereafter, the beads were centrifuged and the bound protein (supernatant) fraction (40 μl) was collected.
Western blot analysis
10% SDS-PAGE gels were used to separate proteins (n=3; 10 μg total protein or 20 μl unbound or bound immunoprecipitation fractions) followed by transfer onto nitrocellulose membranes as previously described (Thompson et al., 2005). Membranes were blocked for 1 h with shaking at 4°C in 5% milk in Tris-buffered saline with Tween-20 (TTBS). Primary antibody diluted in 5% milk in TTBS was added to the membranes and incubated overnight at 4°C with rocking. The GSTM and ASK1 antibody dilutions used were 1:200 and 1:1000. Membranes were washed three times (10 min) in TTBS and incubated in appropriate HRP-conjugated secondary antibody (1:2000) for 1hr at room temperature. Membranes were washed three times (10 min) in TTBS followed by a single wash for 10 min in Tris Buffered Saline (TBS). Membranes were incubated in ECL plus chemiluminescence detection substrate for 5 min and exposed to X-ray film. Equal total protein loading was confirmed by Ponceau S staining of membranes. Densitometry of the appropriate bands was quantified using ImageJ software (NCBI). Each band was normalized to an appropriate internal control – Ponceau S for total protein measurements and to ASK1 for immunoprecipitation. The treatment values were compared to control values using Prism 5.04 software to determine any difference between experimental treatments.
Statistical analysis
All statistical analyses were performed using Prism 5.04 software (GraphPad Software). One ovary from each animal was placed in control media while the contralateral ovary was placed in the experimental treatment, thus data were analyzed by paired t-tests comparing the treated ovaries to controls. Statistical significance was defined as P < 0.05. For Western blotting, statistical analysis was performed on the raw data comparing experimental treatment to the respective control at each time-point. For each experiment, the n-value equals three groups that consisted of 8–10 ovaries per group, and data are presented as mean value +/− standard error.
Results
Temporal effect of chronic VCD exposure on Gstm mRNA expression in PND4 F344 rat ovaries
To investigate the effect of VCD on ovarian Gstm mRNA, real-time PCR was performed over the time-course of VCD exposure; 2d, 4d, 6d or 8d. Relative to control ovaries, no change in Gstm mRNA expression was observed at any time point (Figure 1).
Figure 1. Temporal effect of chronic VCD exposure on Gstm mRNA expression.
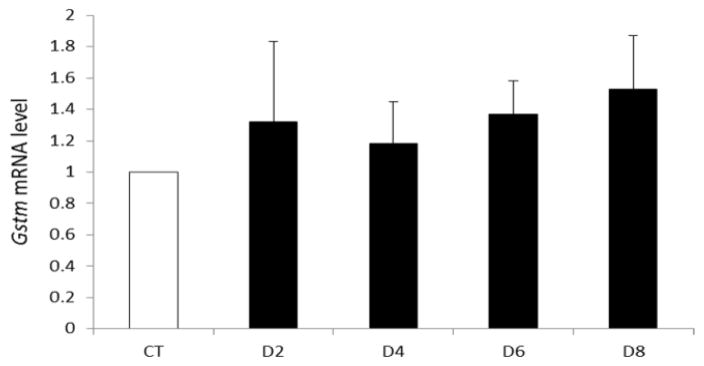
F344 PND4 rat ovaries were cultured in control (CT) media or media containing 30 μM VCD for 2–8 d. Following incubation, total RNA was isolated, reverse transcribed to cDNA and analyzed for Gstm and Actb mRNA expression by RT-PCR as described in materials and methods. Values are expressed as fold-change relative to control ± SE; n=3 (10 ovaries per pool).
Temporal effect of chronic VCD exposure on GSTM protein in F344 rat ovaries
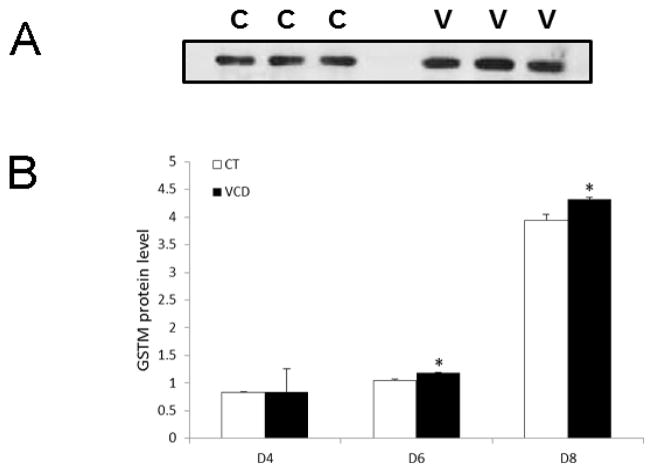
To determine any impact of VCD exposure on GSTM protein level, PND4 rat ovaries were cultured in control media or media containing VCD (30 μM) for 4d, 6d or 8d. There was no effect of VCD on GSTM protein level on d4. Relative to control-treated ovaries, VCD increased (P < 0.05) GSTM protein level on d6 by 13% and on d8 by 9% (Figure 2A, B).
Figure 2. Temporal effect of chronic VCD exposure on GSTM protein level.
F344 PND4 rat ovaries were cultured in control (CT) media or media containing 30 μM VCD for 4–8 d. Following incubation, total protein was isolated and Western blotting was performed to detect GSTM protein as described in materials and methods. (A) Representative GSTM Western blot on d6. (B) Quantification of GSTM protein levels on d4, d6, d8; Values are expressed as a percentage of control; n=3 (10 ovaries per pool). * P < 0.05; different from control.
Induction of Ask1 mRNA in F344 rat ovaries by VCD at the time of follicle loss onset
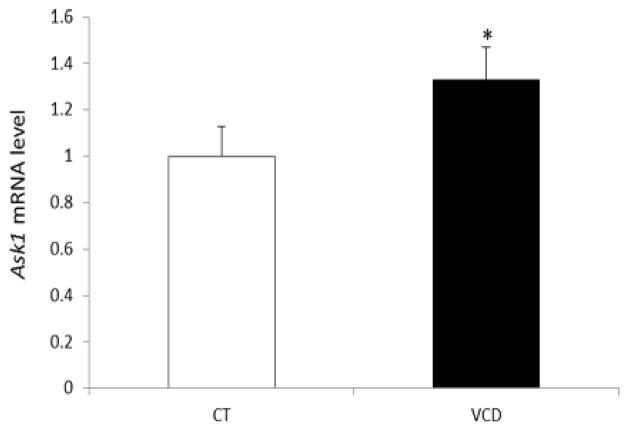
To study if VCD induces Ask1 mRNA, PND4 rat ovaries were cultured in control media or media containing VCD (30 μM) for 6 d. Ask1 expression was measured on d6 since this time point coincides with the beginning of VCD-induced follicle loss. Relative to control-treated ovaries, Ask1 mRNA was increased (0.33-fold; P < 0.05) by VCD exposure (Figure 3).
Figure 3. Effect of chronic VCD exposure on Ask1 mRNA expression.
F344 PND4 rat ovaries were cultured with control (CT) media or media containing 30 μM VCD for 6d. Following incubation, total RNA was isolated, reverse transcribed to cDNA and analyzed for Ask1 mRNA expression by RT-PCR as described in materials and methods. Values are expressed as fold-change relative to control ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control.
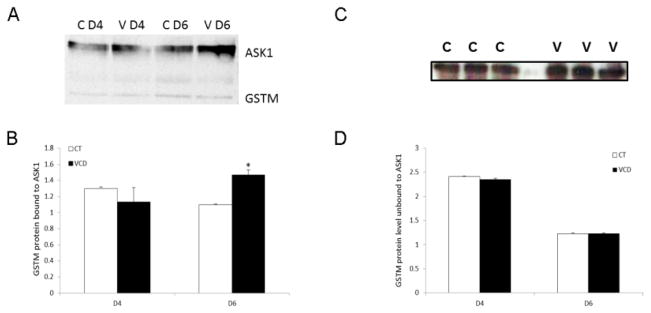
Effect of chronic VCD exposure on GSTM:ASK1 protein complex formation in F344 rat ovaries
To evaluate the presence of a GSTM:ASK1 protein complex in the ovary as well as to investigate the effect of VCD exposure on this complex during VCD exposure, PND4 F344 rat ovaries were collected at the time points prior to (d4) and at the time of (d6) VCD-induced follicle loss. A GSTM:ASK1 protein complex was detected in ovarian tissue (Figure 4A). There was no effect of VCD on the amount of GSTM bound to ASK1 on d4 but this was increased by 33% (P < 0.05) on d6 (Figure 4B). There was no impact of VCD exposure or ASK1 interaction on GSTM protein that was unbound to ASK1 at either time point (Figure 4C, D).
Figure 4. Effect of chronic VCD exposure on the GSTM:ASK1 protein complex.
Ovaries from F344 PND4 rats were cultured in control media (CT) or media containing VCD (30 μM) for 4 or 6 d. Ovaries were exposed to VCD on alternate days from the beginning of culture. Following incubation, total protein was isolated and immunoprecipitation was carried out as described in materials and methods. (A) ASK1 immunoprecipitation followed by detection of GSTM protein on d4 or d6; control = c, VCD = v. (B) Quantification of the amount of GSTM protein bound to ASK1. (C) Representative Western blot for unbound GSTM protein on d6. (D) Quantification of the amount of unbound GSTM protein levels on d4 and d6. Values are expressed as a percentage of control; n=3 (20 ovaries per pool). * P < 0.05; different from control.
Acute VCD exposure effects on GSTM protein level in F344 rat ovaries
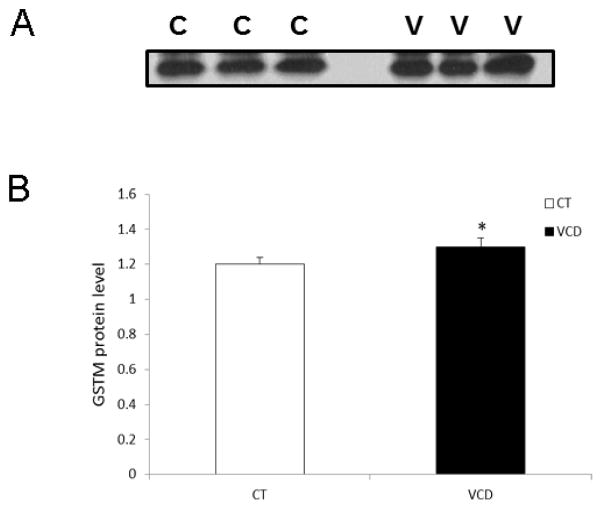
Whether a single (acute) exposure of PND4 rat ovaries for 2d to VCD was sufficient to induced increased GSTM protein levels was determined. Protein was collected 6d after the onset of culture. Relative to control-treated ovaries, GSTM protein was increased (P < 0.05) on d6 by 6% (Figure 5A, B).
Figure 5. Effect of acute VCD exposure on GSTM protein level.
Ovaries from F344 PND4 rats were cultured in control (CT) media or media containing VCD (30 μM) for 6d with exposure to VCD at the onset of culture for 2d. Following incubation, total protein was isolated and Western blotting was performed to detect GSTM protein as described in materials and methods. (A) Representative Western blot on d6; control = c, VCD = v. (B) Quantification of GSTM protein levels, values are expressed as a percentage of control; n=3 (10 ovaries per pool). * P < 0.05; different from control.
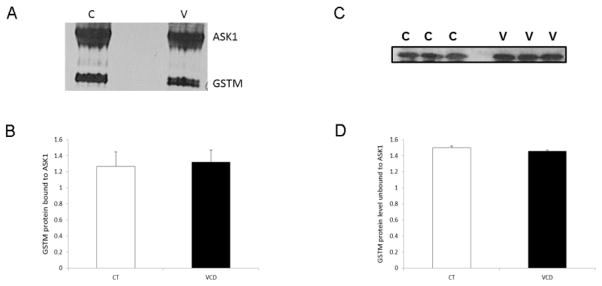
Determination of VCD acute exposure effect on the GSTM:ASK1 protein complex in F344 rat ovaries
To determine if acute VCD exposure could cause dissociation of the GSTM:ASK1 protein complex, ovaries were exposed to VCD for 2d and ovaries collected 6d after the onset of culture. There was no effect of VCD exposure on the amount of GSTM protein bound to ASK1 (Figure 6A, B). Also, no impact on the amount of GSTM in the unbound protein fraction was observed (Figure 6C, D).
Figure 6. Effect of acute VCD exposure on GSTM:ASK1 protein complex formation.
Ovaries from F344 PND4 rats were cultured in control (CT) media or media containing VCD (30 μM) for 6d with exposure to VCD at the onset of culture for 2d. Following incubation, total protein was isolated and immunoprecipitation was carried out as described in materials and methods. (A) ASK1 immunoprecipitation, followed by detection of GSTM protein by Western blotting on d6; control = c, VCD = v. (B) Quantification of the amount of GSTM protein bound to ASK1, values are expressed as a percentage of control; n=3 (10 ovaries per pool). * P < 0.05; different from control. (C) Western blotting was performed on the unbound protein fraction to detect GSTM protein; control = c, VCD = v. (D) Quantification of the amount of unbound GSTM protein levels, values are expressed as a percentage of control; n=3 (20 ovaries per pool). *P < 0.05; different from control.
Effect of PI3K inhibition on ovarian GSTM protein level
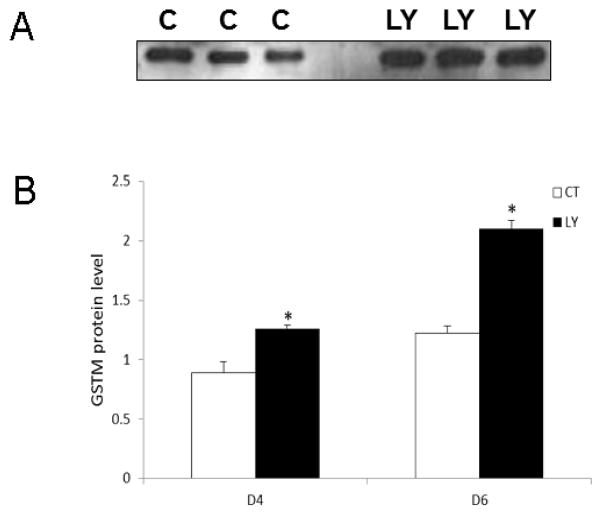
An increase in Gstm mRNA was previously demonstrated after 2d and 4d of PI3K inhibition (Bhattacharya and Keating, 2012). In order to investigate whether altered Gstm mRNA was correlated with GSTM protein after PI3K inhibition, LY294002 treated ovaries were collected on 4d and 6d. Inhibition of PI3K increased (P < 0.05) GSTM protein levels by 40% and 71% on d4 and d6, respectively (Figure 7A, B).
Figure 7. Effect of PI3K inhibition on ovarian GSTM protein level.
F344 PND4 rat ovaries were cultured in media containing control or LY294002 (20 μM) for 4d or 6d. Total protein was isolated and Western blotting was performed to detect GSTM protein levels. (A) Representative Western blot on d6; control = c, LY294002 = LY. (B) Quantification of Western blotting for GSTM protein, values are expressed as a percentage of control; n=3 (20 ovaries per pool). * P < 0.05; different from control.
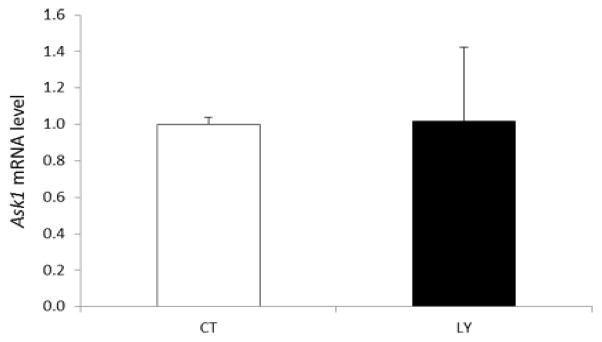
Effect of PI3K inhibition on ovarian Ask1 mRNA level
Any impact of PI3K inhibition on Ask1 mRNA expression was determined in ovaries that had been treated for 6d with LY294002. There was no effect of LY294002 treatment on Ask1 mRNA, relative to control treated ovaries (Figure 8).
Figure 8. Effect of PI3K inhibition on Ask1 mRNA expression.
F344 PND4 rat ovaries were cultured with control (CT) media or media containing 20 μM LY294002 for 6d. Following incubation, total RNA was isolated, reverse transcribed to cDNA and analyzed for Ask1 mRNA expression by RT-PCR as described in materials and methods. Values are expressed as fold-change relative to control ± SE; n=3 (10 ovaries per pool).
Discussion
Ovarian GSTP is increased in response to VCD exposure and inhibits JNK activity through a protein:protein interaction, potentially contributing to the ovarian xenobiotic protective response (Keating et al., 2010). In the PND4 mouse ovary, it has been shown that VCD (30 μM) exposure increases Gstm mRNA expression prior to observed VCD-induced follicle loss (Keating et al., 2008). However, further investigation into a functional role for GSTM during VCD-induced ovotoxicity has been lacking. Therefore, this study investigated the ovarian response of Gstm to VCD exposure in cultured PND4 rat ovaries. Although there was no change at the mRNA level at any time point studied, GSTM protein level was increased at both 6d and 8d, and was highest at the time point at which follicle loss is first observed (d6). These results support an involvement of GSTM in the ovarian protective response to VCD exposure in rats.
Several studies have reported that GSTM inhibits the action of pro-apoptotic ASK1 (Cho et al., 2001; Dorion et al., 2002). This negative regulation of ASK1 by GSTM is through formation of an ASK1:GSTM protein complex. The complex is disrupted following external environmental stress stimulus such as heat shock (Dorion et al., 2002). Such stress releases GSTM from ASK1, and the unbound ASK1 initiates the c-JUN and p38 MAPK signaling pathways in favor of apoptosis (Dorion et al., 2002; Ichijo et al., 1997). Thus, ASK1 is involved in activating apoptotic signaling pathways.
It is known that VCD-induced primordial and small primary follicle loss is via apoptosis (Hu et al., 2001; Devine et al., 2002; Springer et al., 1996a). Moreover, VCD-induced apoptosis involves activation of JNK resulting in phosphorylation of c-JUN in small pre-antral follicles which are the selective target of VCD (Hooser et al., 1994; Hu et al., 2002). Because ASK1 activates JNK signaling, a role for ASK1 during VCD-induced ovotoxicity was hypothesized. Additionally, GSTM is increased in response to VCD exposure, and can form a complex with ASK1 in extra-ovarian tissues as an anti-apoptotic mechanism. Thus, it was logical to investigate whether this was part of an ovarian protective response to VCD exposure. Our data demonstrates induction of Ask1 mRNA at the time of VCD-induced follicle depletion, thus supporting pro-apoptotic Ask1 involvement during VCD-induced ovotoxicity. It was further shown that ovarian GSTM protein is bound to ASK1 and that this complex is increased in response to VCD exposure at the time of follicle loss. This is similar to the response of a GSTP:JNK complex during both VCD (Keating et al., 2010) and DMBA exposures (Bhattacharya and Keating, 2012). There was no disruption of the GSTM:ASK1 protein complex by VCD in terms of changes in the unbound form of GSTM. Since the ovaries were exposed to VCD every alternate day, we surmised that this chronic exposure may be the reason for lack of a clear disruption in the protein complex, since GSTM may be continuously activated, and thus constantly binding ASK1. For this reason an experiment was performed to determine if a single acute VCD exposure would unveil more radical changes in the GSTM:ASK1 complex. GSTM protein was confirmed to increase in this paradigm, albeit marginally, however no major impact on GSTM:ASK1 protein binding was observed. It may however be the case that more distinct changes may have taken place immediately after VCD exposure (d2 or d4). Collectively, from both the chronic and acute VCD exposures, it is evident that GSTM binds pro-apoptotic ASK1. GSTM-induced inactivation of ASK1 may also lead to reduced JNK activity as was evident during VCD-exposure (Keating et al., 2010) since ASK1 is an upstream regulator of JNK (Tobiume et al., 2001). Therefore, it is hypothesized that the complexing of GSTM with ASK1 is part of an ovarian protective response to VCD. That is, in response to VCD-induced apoptosis, GSTM may be involved in an effort to prevent toxicity through binding and suppressing pro-apoptotic action of ASK1 in cultured rat ovaries. Whether GSTM plays an additional role in GSH conjugation cannot be confirmed by these studies.
Inhibition of PI3K prevents VCD-induced primordial follicle loss (Keating et al., 2009) and VCD has been shown to inhibit phosphorylation of the PI3K members c-KIT, pAKT and to decrease total FOXO3 protein (Keating et al., 2011). Inhibition of PI3K signaling increases both mEH (Bhattacharya et al., 2012) and Gstp mRNA and protein expression (Bhattacharya and Keating, 2012), indicating that this pathway regulates ovarian xenobiotic metabolism gene expression. Also, inhibition of PI3K increases Gstm mRNA levels in the rat ovary (Bhattacharya and Keating, 2012). Because a role for GSTM in VCD-induced toxicity was supported by the data herein, an investigation of a regulatory role for PI3K in GSTM translation was made. GSTM protein was increased by PI3K inhibition on d4 and d6 of culture. Although we did not observe any impact of PI3K inhibition on Ask1 mRNA induction, it is possible that increased GSTM binding to ASK1 could result during PI3K inhibition however, this study cannot confirm that this is the case. Thus, these data further add to an understanding of the involvement of PI3K in xenobiotic biotransformation enzyme regulation (Bhattacharya et al., 2012; Bhattacharya and Keating et al., 2012). Gstm mRNA was increased in mouse ovaries exposed to VCD, while protein levels were not affected (Keating et al., 2008), thus there appears to be some species-specific differences in Gstm regulation. Whether this regulation is through control of Gstm transcription or translation remains to be determined, but could potentially involve the action of microRNA’s, as is the case for Gstp (Zhang et al., 2012; Patron et al., 2012).
VCD exposure decreases PI3K signaling (Keating et al., 2011) and it is hypothesized that this leads to impaired follicle viability, and accelerate activation of primordial follicles into the growing follicular pool, both of which would lead to premature ovarian failure (Keating et al., 2009). During the same time frame VCD also increases ovarian expression of mEH (Bhattacharya et al., 2012), Gstp (Keating et al., 2010) and Gstm, likely as a result of PI3K inhibition (summarized in Figure 9). PI3K regulates ovarian arylhydrocarbon receptor (Ahr) and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) mRNA and protein expression (Bhattacharya et al., 2012), both transcription factors that regulate xenobiotic metabolism genes, including Gstm, thus they may be involved in VCD-induced Gstm activation. Why the increased expression of the xenobiotic metabolism enzymes is insufficient to protect against VCD-induced follicular destruction remains unclear, but may result either because the enzymes are overwhelmed by continuous VCD exposure, or because VCD-induced impairment of oocyte viability becomes impossible to counteract.
Figure 9. Proposed mechanistic model.
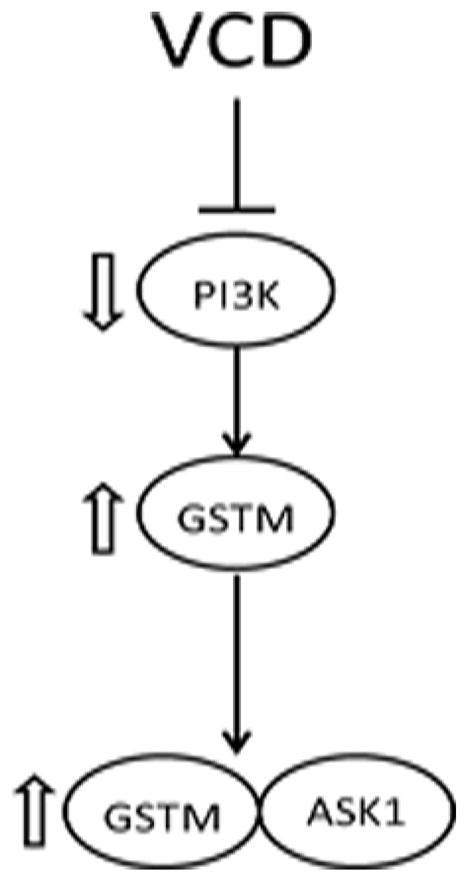
VCD exposure inhibits PI3K signaling, leading to increased GSTM protein, resulting in increased GSTM:ASK1 protein interaction.
In summary, GSTM appears to be involved in the ovarian response to VCD; potentially contributing to both VCD detoxification and prevention of apoptosis. Future studies are aimed at further characterizing Gstm involvement during VCD-induced ovotoxicity.
Highlights.
GSTM protein increases in response to ovarian VCD exposure
VCD increases Ask1 mRNA at the onset of follicle loss
Ovarian GSTM binds more ASK1 protein during VCD-induced ovotoxicity
PI3K regulates ovarian GSTM protein
Footnotes
Conflict of Interest Statement:
The project described was supported by award number R00ES016818 to AFK and R01ES09246 to PBH from the National Institutes of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Protective role for ovarian Glutathione S-transferase isoform pi during 7,12-dimethylbenz[a]anthracene-induced ovotoxicity Toxicol. Appl Pharamacol. 2012;260:201–2088. doi: 10.1016/j.taap.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Sen N, Hoyer PB, Keating AF. Ovarian expressed microsomal epoxide hydrolase: Role in detoxification of 4-vinylcyclohexene diepoxide and regulation by phosphatidylinositol-3 kinase signaling. Toxicol Appl Pharmacol. 2012;258:118–123. doi: 10.1016/j.taap.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochrome P450 2E1, 2A, and 2B in the mouse ovary: The effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Cho SG, Lyy YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, Shim J, Kim Y, Dong MS, Lee MJ, Kim SG, Ichijo H, Choi EJ. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol. 2002;184:107–115. [PubMed] [Google Scholar]
- Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-Transferase Mu from ASK1. J Biol Chem. 2002;277:30792–30797. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hooser SB, Douds DP, DeMerell DG, Hoyer PB, Sipes IG. Long-term ovarian and gonadotropin changes in mice exposed to 4-vinylcyclohexene. Reprod Toxicol. 1994;8:315–323. doi: 10.1016/0890-6238(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monograph on the evaluation of carcinogenic risk of chemicals to humans. International Agency for Research on Cancer; Lyon, France: 1976. Cadmium, nickel, some epoxides, miscellaneous industrial chemicals and general considerations on volatile anaesthetics; pp. 141–145. [Google Scholar]
- Kao SW, Sipes IG, Hoyer PB. Early effects induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Keating AF, Fernandez SM, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2011;84:743–751. doi: 10.1095/biolreprod.110.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol. 2009;241:127–134. doi: 10.1016/j.taap.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sen N, Sipes IG, Hoyer PB. Dual protective role for glutathione S-transferase class pi against VCD-induced ovotoxicity in the rat ovary. Toxicol Appl Pharmacol. 2010;247:71–75. doi: 10.1016/j.taap.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sipes IG, Hoyer PB. Expression of ovarian microsomal epoxide hydrolase and glutathione S-transferase during onset of VCD-induced ovotoxicity in B6C3F(1) mice. Toxicol Appl Pharmacol. 2008;230:109–116. doi: 10.1016/j.taap.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Jakoby WB. Glutathione transferases. Catalysis of nucleophilic reactions of glutathione. J Biol Chem. 1978;253:5654–5657. [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamundi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Luderer U, Kavanagh TJ, White CC, Faustman EM. Gonadotropin regulation of glutathione synthesis in the rat ovary. Reprod Toxicol. 2001;15:495–504. doi: 10.1016/s0890-6238(01)00157-5. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Schulman JD. How xenobiotic chemicals can destroy oocytes. Contemp Obstet Gynecol. 1980;15:157. [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinol. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Patron JP, Fendler A, Bild M, Jung U, Muller H, Arntzen MO, Piso C, Stephan C, Thiede B, Mollenkopf HJ, Jung K, Kaufmann SH, Schreiber J. PiR-133b targets antiapoptotic genes and enhances death receptor-induced apoptosis. PLoS One. 2012;7:e35345. doi: 10.1371/journal.pone.0035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol Appl Pharmacol. 2007;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–8. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol-3 kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Xing R, Tie Y, Fu H, Zheng X, Yu B. MiR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung Canc. 2012;77:488–494. doi: 10.1016/j.lungcan.2012.05.107. [DOI] [PubMed] [Google Scholar]