Abstract
Cerebrospinal fluid (CSF) is a protein rich fluid contained within the brain ventricles. It is present during early vertebrate embryonic development and persists throughout life. Adult CSF is thought to cushion the brain, remove waste, and carry secreted molecules1,2. In the adult and older embryo, the majority of CSF is made by the choroid plexus, a series of highly vascularized secretory regions located adjacent to the brain ventricles3-5. In zebrafish, the choroid plexus is fully formed at 144 hours post fertilization (hpf)6. Prior to this, in both zebrafish and other vertebrate embryos including mouse, a significant amount of embryonic CSF (eCSF) is present . These data and studies in chick suggest that the neuroepithelium is secretory early in development and may be the major source of eCSF prior to choroid plexus development7.
eCSF contains about three times more protein than adult CSF, suggesting that it may have an important role during development8,9. Studies in chick and mouse demonstrate that secreted factors in the eCSF, fluid pressure, or a combination of these, are important for neurogenesis, gene expression, cell proliferation, and cell survival in the neuroepithelium10-20. Proteomic analyses of human, rat, mouse, and chick eCSF have identified many proteins that may be necessary for CSF function. These include extracellular matrix components, apolipoproteins, osmotic pressure regulating proteins, and proteins involved in cell death and proliferation21-24. However, the complex functions of the eCSF are largely unknown.
We have developed a method for removing eCSF from zebrafish brain ventricles, thus allowing for identification of eCSF components and for analysis of the eCSF requirement during development. Although more eCSF can be collected from other vertebrate systems with larger embryos, eCSF can be collected from the earliest stages of zebrafish development, and under genetic or environmental conditions that lead to abnormal brain ventricle volume or morphology. Removal and collection of eCSF allows for mass spectrometric analysis, investigation of eCSF function, and reintroduction of select factors into the ventricles to assay their function. Thus the accessibility of the early zebrafish embryo allows for detailed analysis of eCSF function during development.
Keywords: Neuroscience, Issue 70, Developmental Biology, Medicine, Anatomy, Physiology, Zebrafish, Danio rerio, eCSF, neuroepithelium, brain ventricular system, brain, microsurgery, animal model
Protocol
1. Preparing Microinjection Needles and Cell Tram
Fill Eppendorf CellTram oil microinjector apparatus with mineral oil according to manufacturer's instructions.
Prepare microinjection needles by pulling capillary tubes using Sutter instruments needle puller.
Mount needle on micromanipulator connected to Eppendorf CellTram.
Carefully break the tip of the needle. For uniform tip size, measure with a micrometer, or compare to a reference needle.
Fill the length of the needle with oil, using the CellTram to push oil down the length, being careful to avoid any bubbles.
2. Preparing the Embryos
Poke holes in a 1% agarose coated dish with a 1-200 μl pipette tip, and remove agarose plugs. Fill dish with embryo media (made according to Westerfield25).
Using forceps, dechorionate embryos under stereomicroscope.
Transfer dechorionated embryos into agarose coated dish.
To anesthetize embryos, add Tricaine (0.1 mg/ml) to agarose dish until embryos stop moving (made according to Westerfield25).
3. Draining the eCSF
Orient the embryos with their tails in the holes of the agarose and their posterior sides closest to the micromanipulator, allowing for visualization of the dorsal side of the brain.
Position the needle at the rhombomere 0/1 hinge-point or the widest point of hindbrain ventricle (Figure 1A).
Carefully pierce roof plate of hindbrain ventricle being sure not to go through the depth of the brain into the yolk.
Drain the eCSF using the CellTram and collect fluid in microinjection needle. Be careful to avoid any cells.
4. Collecting the eCSF for Composition Analysis
Once the eCSF is collected in the needle, carefully stop suction from the CellTram.
Move the dish out from under the needle and carefully position the needle into a tube containing the desired buffer.
Using the CellTram, drain the eCSF out of the needle into the buffer trying to avoid contaminating the solution with oil.
In order to remove cells that you have aspirated along with the eCSF, spin eCSF at 10,000 x g. Collect uncontaminated eCSF (supernatant) and store at -80 °C until ready for further analysis.
5. Reintroduction of Selected Factors
Drain the eCSF every 2 hr during desired time interval, since the fluid is replenished within that time period. Between drainage time-points, remove the needle and store the embryos at 28.5 °C or desired temperature.
Load second micropipette needle with test factor.
Place needle into micromanipulator attached to microinjector.
Adjust injection volume to 1 nl by measuring the drop size in oil and adjusting injection time and pressure.
Inject 1-2 nl into brain ventricles as described previously26.
Representative Results
An example of a drained brain ventricle is shown in Figure 1B-C. Brain ventricles are collapsed as they lack eCSF (Figure 1B vs. C). As seen in dorsal images (Figure 1B-C, and Figure 2A-D) the hindbrain neuroepithelium does retain its characteristic morphology and seems to be open despite lack of eCSF likely due to robust hinge-points. However, lateral views (Figure 2A'-D') demonstrate that the hindbrain ventricle has been drained, consistent with the presence of a thin flexible roof plate epithelium which collapses ventrally. In wild type embryos, eCSF is continuously produced and refills the brain ventricles 2-3 hr post draining (Figure 2). Therefore, CSF needs to be continually depleted over the time of interest.
While removal of the fluid at 24 hpf does not disrupt gross morphology or development, removal of eCSF over a longer period of development may have adverse effects to whole body development. This should be taken into consideration when determining the efficacy of factors injected into the brain ventricles. Further, to determine whether developmental defects are a result of loss of eCSF or due to a needle puncturing the brain, an important control is insertion of a needle into the brain without removing eCSF.
We demonstrated that eCSF has a different protein profile than whole embryo extract, and analysis on a SDS-PAGE protein gel demonstrates that a detectable amount of protein can be collected from zebrafish eCSF (Figure 3). This demonstrates that neuroepithelial cellular contaminants are at lower levels than eCSF specific proteins, and further that a substantial amount of protein can be collected that is sufficient for mass spectrometry analysis.
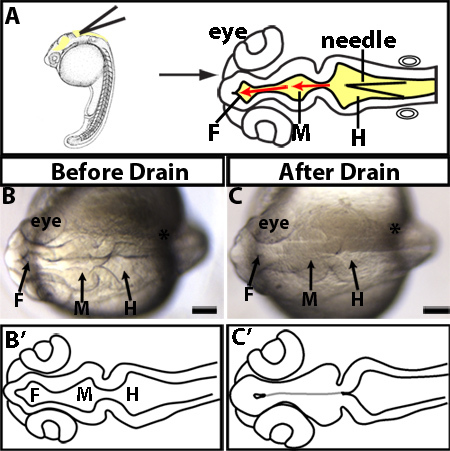 Figure 1. Manually drained brain ventricles. (A) Experimental design. Lateral (left) and dorsal (right) views of where to insert microinjection needle. Needle (black triangle) is inserted into the roof plate of the hindbrain ventricle at the widest point of the hindbrain. The needle is then further inserted into the midbrain and forebrain ventricles as designated by the red arrows. (B-C) Examples of 24 hpf manually drained embryo. The same embryo imaged before draining (B) or after drain (C). (B'-C') Tracings of B-C. Grey line indicates morphology present but not visible. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
Figure 1. Manually drained brain ventricles. (A) Experimental design. Lateral (left) and dorsal (right) views of where to insert microinjection needle. Needle (black triangle) is inserted into the roof plate of the hindbrain ventricle at the widest point of the hindbrain. The needle is then further inserted into the midbrain and forebrain ventricles as designated by the red arrows. (B-C) Examples of 24 hpf manually drained embryo. The same embryo imaged before draining (B) or after drain (C). (B'-C') Tracings of B-C. Grey line indicates morphology present but not visible. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
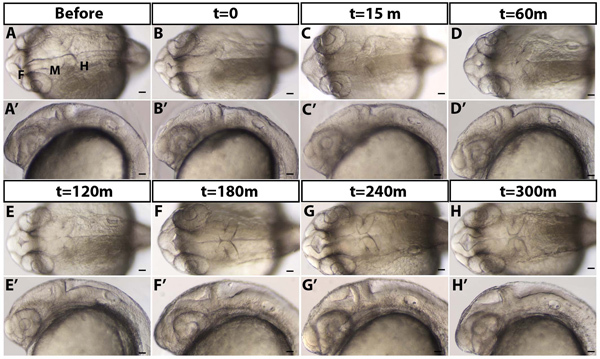 Figure 2. eCSF refills the brain ventricles over time. (A) 24 hpf embryo before draining (B) immediately after t=0 (C) 15, (D) 60, (E) 120, (F) 180, (G) 240, and (H) 300 min (m) after draining. (A-H) Dorsal and (A'-H') lateral brightfield images with anterior to the left. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
Figure 2. eCSF refills the brain ventricles over time. (A) 24 hpf embryo before draining (B) immediately after t=0 (C) 15, (D) 60, (E) 120, (F) 180, (G) 240, and (H) 300 min (m) after draining. (A-H) Dorsal and (A'-H') lateral brightfield images with anterior to the left. F = forebrain, M = midbrain, H = hindbrain. Scale bars = 50 μm.
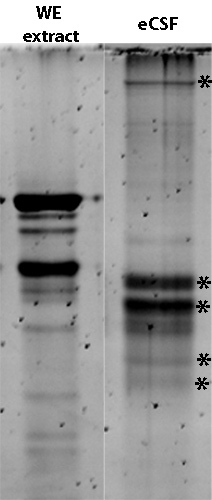 Figure 3. Protein profile differs between 24 hpf whole zebrafish embryo (WE) extract (0.5 μg total protein) and 24 hpf eCSF from 50 embryos. SDS loading buffer added to collected eCSF to denature proteins, sample run on an 8% Tris-HCl PAGE gel, and detected with Sypro Ruby. * indicate bands unique to eCSF.
Figure 3. Protein profile differs between 24 hpf whole zebrafish embryo (WE) extract (0.5 μg total protein) and 24 hpf eCSF from 50 embryos. SDS loading buffer added to collected eCSF to denature proteins, sample run on an 8% Tris-HCl PAGE gel, and detected with Sypro Ruby. * indicate bands unique to eCSF.
Discussion
Use of this technique to manually drain eCSF from zebrafish brain ventricles will be useful for determining the requirement for eCSF during development. In addition, this technique will allow description of the eCSF protein profile over the course of embryonic development. Identification of different proteins during this time will enable further investigation into the function of the CSF and its potential role during brain development. In amniotes, some factors identified in eCSF (IGF2, FGF2, retinoic acid, and apolipoproteins) have a demonstrated role in neuroepithelial cell survival, proliferation and neurogenesis13,17,20,23,27,28. However, there appear to be hundreds of uncharacterized proteins in the eCSF, which was obtained after choroid plexus formation, later than the time-points demonstrated here. Additionally, our lab and others have identified zebrafish mutants with abnormal brain ventricle size or brain defects29-31, and may have abnormalities in eCSF composition and function. This method readily allows for isolating and testing putative abnormal eCSF from mutants or under different environmental conditions.
A powerful use of the zebrafish system is to replace selected factors via injection into the brain ventricles after removal of eCSF, thereby testing their function during brain development. Small molecules, proteins, and eCSF obtained after genetic or chemical perturbation can be tested. Reintroduction of eCSF from other species will allow comparison of factors and functions across species and under different pathological conditions, such as hydrocephalus, complementing and interfacing with mammalian studies. In summary, use of the zebrafish to remove eCSF, analyze its composition and reintroduce eCSF or specific factors into the brain ventricles, will significantly increase understanding the role and regulation of eCSF function and that of the brain ventricular system.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the National Institute for Mental Health, and National Science Foundation. Special thanks to Dr. Jen Gutzman, Dr. Amanda Dickinson and other Sive lab members for many useful discussions and constructive criticism, and to Olivier Paugois for expert fish husbandry.
References
- Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc. Res. Tech. 2001;52:65–82. doi: 10.1002/1097-0029(20010101)52:1<65::AID-JEMT9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr. Top. Dev. Biol. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454:1–18. doi: 10.1007/s00424-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Speake T, Whitwell C, Kajita H, Majid A, Brown PD. Mechanisms of CSF secretion by the choroid plexus. Microsc. Res. Tech. 2001;52:49–59. doi: 10.1002/1097-0029(20010101)52:1<49::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Garcia-Lecea M, Kondrychyn I, Fong SH, Ye ZR, Korzh V. In vivo analysis of choroid plexus morphogenesis in zebrafish. PLoS One. 2008;3:e3090. doi: 10.1371/journal.pone.0003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welss P. Secretory activity of the inner layer of the embryonic mid-brain of the chick, as revealed by tissue culture. The Anatomical Record. 1934;58:299–302. [Google Scholar]
- Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin. Exp. Pharmacol. Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chodobski A. The blood-cerebrospinal fluid barrier. Boca Raton, Fl: Taylor and Francis; 2005. [Google Scholar]
- Salehi Z, Mashayekhi F. The role of cerebrospinal fluid on neural cell survival in the developing chick cerebral cortex: an in vivo study. Eur. J. Neurol. 2006;13:760–764. doi: 10.1111/j.1468-1331.2006.01358.x. [DOI] [PubMed] [Google Scholar]
- Parada C, et al. Embryonic cerebrospinal fluid collaborates with the isthmic organizer to regulate mesencephalic gene expression. J. Neurosci. Res. 2005;82:333–345. doi: 10.1002/jnr.20618. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Salehi Z. The importance of cerebrospinal fluid on neural cell proliferation in developing chick cerebral cortex. Eur. J. Neurol. 2006;13:266–272. doi: 10.1111/j.1468-1331.2006.01208.x. [DOI] [PubMed] [Google Scholar]
- Martin C, et al. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev. Biol. 2006;297:402–416. doi: 10.1016/j.ydbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Martin C, et al. Early embryonic brain development in rats requires the trophic influence of cerebrospinal fluid. Int. J. Dev. Neurosci. 2009;27:733–740. doi: 10.1016/j.ijdevneu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Gato A, et al. Embryonic cerebrospinal fluid regulates neuroepithelial survival, proliferation, and neurogenesis in chick embryos. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;284:475–484. doi: 10.1002/ar.a.20185. [DOI] [PubMed] [Google Scholar]
- Desmond ME, Levitan ML, Haas AR. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;285:737–747. doi: 10.1002/ar.a.20211. [DOI] [PubMed] [Google Scholar]
- Alonso MI, Martin C, Carnicero E, Bueno D, Gato A. Cerebrospinal fluid control of neurogenesis induced by retinoic acid during early brain development. Dev. Dyn. 2011;240:1650–1659. doi: 10.1002/dvdy.22657. [DOI] [PubMed] [Google Scholar]
- Miyan JA, Zendah M, Mashayekhi F, Owen-Lynch PJ. Cerebrospinal fluid supports viability and proliferation of cortical cells in vitro, mirroring in vivo development. Cerebrospinal Fluid Res. 2006;3:2. doi: 10.1186/1743-8454-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi F, Bannister CM, Miyan JA. Failure in cell proliferation in the germinal epithelium of the HTx rats. Eur. J. Pediatr. Surg. 2001;11(1):S57–S59. [PubMed] [Google Scholar]
- Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappaterra MD, et al. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J. Proteome. Res. 2007;6:3537–3548. doi: 10.1021/pr070247w. [DOI] [PubMed] [Google Scholar]
- Parvas M, Parada C, Bueno D. A blood-CSF barrier function controls embryonic CSF protein composition and homeostasis during early CNS development. Dev. Biol. 2008;321:51–63. doi: 10.1016/j.ydbio.2008.05.552. [DOI] [PubMed] [Google Scholar]
- Parada C, Gato A, Bueno D. Mammalian embryonic cerebrospinal fluid proteome has greater apolipoprotein and enzyme pattern complexity than the avian proteome. J. Proteome Res. 2005;4:2420–2428. doi: 10.1021/pr050213t. [DOI] [PubMed] [Google Scholar]
- Gato A, et al. Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J. Exp. Zool. A Comp. Exp. Biol. 2004;301:280–289. doi: 10.1002/jez.a.20035. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Sprague J, Doerry E, Douglas S, Grp Z. The Zebrafish Information Network (ZFIN): a resource for genetic, genomic and developmental research. Nucleic Acids Res. 2001;29:87–90. doi: 10.1093/nar/29.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman JH, Sive H. Zebrafish Brain Ventricle Injection. J. Vis. Exp. 2009. p. e1218. [DOI] [PMC free article] [PubMed]
- Parada C, Gato A, Bueno D. All-trans retinol and retinol-binding protein from embryonic cerebrospinal fluid exhibit dynamic behaviour during early central nervous system development. Neuroreport. 2008;19:945–950. doi: 10.1097/WNR.0b013e3283021c94. [DOI] [PubMed] [Google Scholar]
- Parada C, Escola-Gil JC, Bueno D. Low-density lipoproteins from embryonic cerebrospinal fluid are required for neural differentiation. J. Neurosci. Res. 2008;86:2674–2684. doi: 10.1002/jnr.21724. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057–2067. doi: 10.1242/dev.01791. [DOI] [PubMed] [Google Scholar]
- Lowery LA, De Rienzo G, Gutzman JH, Sive H. Characterization and classification of zebrafish brain morphology mutants. Anat. Rec. (Hoboken) 2009;292:94–106. doi: 10.1002/ar.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]


