Abstract
Background
The aim of this open-label, randomized, parallel-group pilot study was to evaluate the efficacy of cefditoren pivoxil and levofloxacin in terms of speed of reduction in inflammatory parameters, clinical recovery, and microbiological eradication.
Methods
Forty eligible patients with acute exacerbation of chronic bronchitis (AECB) were randomized to receive cefditoren 200 mg twice a day for 5 days (n = 20) or levofloxacin 500 mg once daily for 7 days (n = 20).
Results
The inflammatory parameters which were significantly reduced at test-of-cure with respect to visit 1 were Krebs von den Lundgen-6 (KL-6) and interleukin-6. KL-6 decreased both in the overall study population (from 19 ± 11 UI/mL to 6 ± 8 UI/mL, P = 0.000) and in the cefditoren (from 19 ± 13 UI/mL to 8 ± 10 UI/mL, P = 0.006) and levofloxacin (from 19 ± 10 UI/mL to 5 ± 5 UI/mL, P = 0.000) arms. Similarly, interleukin-6 decreased both in the overall study population (from 13.35 ± 16.41 pg/mL to 3 ± 4.7 pg/mL, P = 0.000) and in the cefditoren (from 15.90 ± 19.54 pg/mL to 4.13 ± 6.42 pg/mL, P = 0.015) and levofloxacin (from 10.80 ± 12.55 pg/mL to 1.87 ± 1.16 pg/mL, P = 0.003) arms. At the end of treatment (test-of-cure, 6–9 days after drug initiation), the clinical success rate in the overall study population was 78%; the clinical cure rate was 80% in the cefditoren arm and 75% in the levofloxacin arm. Globally, bacteriological eradication at test-of-cure was obtained in 85% of the overall study population. Both treatments were well tolerated.
Conclusion
Cefditoren represents a valid option in the treatment of mild to moderately severe cases of AECB in the outpatient care setting. Moreover, the use of this cephalosporin is associated with a significant reduction of interleukin-6 and KL-6, two key mediators of lung inflammation and epithelial damage.
Keywords: cefditoren pivoxil, levofloxacin, serum inflammatory biomarkers, chronic bronchitis, acute exacerbations
Introduction
Chronic respiratory diseases like chronic bronchitis and chronic obstructive pulmonary disease (COPD) place a huge burden on economic resources and on health care systems.1–3 Acute exacerbation of chronic bronchitis (AECB) represents a major public health concern and causes significant morbidity and mortality in these patients, contributing to a long-term decline in lung function and increasing the risk of cardiovascular events.4–9 It is estimated that up to 50%–80% of AECBs are caused by bacterial infections which may respond to antimicrobial therapy.10–13
Inflammation also plays a central role in the pathogenesis of AECB.14–16 More specifically, if bacteria are thought to be the trigger for AECB, a neutrophilic inflammation would be expected as a response to the acute infection. Sethi et al showed that increased airway inflammation, ie, higher levels of tumor necrosis factor alpha and interleukin (IL)-8, was associated with Haemophilus influenzae and Moraxella catarrhalis isolates in sputum, thus supporting an etiologic role for these pathogens in AECB.16 Cytokines titers/levels have often been used to describe inflammatory patterns in lung diseases, both in serum and respiratory tract/airway specimens, such as sputum or bronchoalveolar lavage fluid. Krebs von den Lundgen-6 (KL-6), instead, is a circulating mucin-like high molecular weight glycoprotein that is strongly expressed on type II pneumocytes and bronchiolar epithelial cells, and is a useful serum marker of epithelial damage in the lung.17 KL-6 is elevated in bronchoalveolar lavage fluid and in the serum of patients with different types of interstitial pneumonia, and is a useful serum marker in the management of pneumonia.17–19
AECB is usually classified based on severity. A significant number of individuals are affected by mild to moderately severe cases of AECB and can therefore be treated in an outpatient setting, with institution of an oral therapy, often decided by general practitioners.20 Given that at least half of AECBs are caused by bacterial infections, early institution of an adequate/appropriate antimicrobial regimen is crucial in order to ensure quick recovery. GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines suggest the use of amoxicillin-clavulanic acid, advanced macrolides, and second-generation and third-generation cephalosporins for uncomplicated AECB, but the prevalence of macrolide resistance is increasing worldwide. Respiratory fluoroquinolones (levofloxacin, moxifloxacin) are a common choice for patients with severe airway obstruction, comorbidity, and/or recurrent exacerbations, but could be spared in less severe cases.11
Cephalosporins may be useful for the treatment of mild to moderately severe cases of AECB. Several studies have demonstrated both the effectiveness and safety of these drugs in this population, also in comparison with fluoroquinolones.21–25 Among the cephalosporins, cefditoren pivoxil, an oral, β-lactamase-stable, expanded-spectrum cephalosporin, was shown to have good in vitro activity not only against methicillin-susceptible Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, including penicillin-resistant strains, but also against H. influenzae and M. catarrhalis,26–31 with minimum inhibitory concentrations of 90% for AECB isolates that are lower than those for other oral cephalosporins,22 and its clinical efficacy and safety have been well established.32–35 In comparative studies with cephalosporins, levofloxacin was demonstrated to be more favorable in the treatment of AECB21,24,36 but, to the best of our knowledge, no trial has been conducted comparing cefditoren and levofloxacin.
The current study was designed to compare cefditoren and levofloxacin in order to assess their efficacy in terms of speed of reduction of inflammatory parameters, clinical recovery, and microbiological eradication.
Materials and methods
Study design
An open-label, randomized, levofloxacin-controlled, parallel-group pilot study of cefditoren was performed from January 2012 to April 2012 in the Respiratory Department, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. The protocol was approved by the ethics committee of the participating center (EudraCT 2011-000531-88, ClinicalTrial.gov identifier NCT01467297). All patients gave written informed consent prior to study entry.
Patient selection
All outpatients aged 40–75 years with a diagnosis of chronic bronchitis (defined as history of cough, excessive mucus secretion, and production of sputum on most days over at least three consecutive months for more than two successive years), an acute exacerbation (characterized by the presence of three symptoms or at least two symptoms, including purulence, increased dyspnea, increased sputum volume, increased sputum purulence) and a valid sputum specimen from the lower airways for microbiological evaluation (<10 squamous epithelial cells and >25 polymorph nuclear leukocytes per low power magnification 100 × field) were screened. Patients with hypersensitivity/allergy to any component of the study medications, underlying asthma, systemic corticosteroids, pregnancy, history of tendinopathy, psychiatric disease, epilepsy, recent cardiovascular disease, forced expiratory volume in one second (FEV1) <50%, any electrocardiographic abnormalities, any chest x-ray abnormalities, deficient glucose-6-phosphatase dehydrogenase activity, hepatic failure, renal failure, lower respiratory tract illness other than chronic bronchitis or COPD, concurrent infections and/or neoplasm, concurrent treatment with hypoglycemic drugs, fenbrufen or xanthines, treatment with antibiotics within the previous week, or treatment with experimental drugs in the previous 4 weeks were excluded.
Treatment
Patients who fulfilled all the inclusion and none of the exclusion criteria were randomized (1:1) to receive cefditoren 200 mg twice a day for 5 days or levofloxacin 500 mg once daily for 7 days, according to the manufacturer’s indications.
Clinical assessment
Patients were examined at the time of study enrolment (visit 1 on day 1 of treatment), and evaluations were performed during treatment (visit 2, days 2–4), at the end of treatment (visit 3, test-of-cure visit, days 6–9), and at follow-up (visit 4, late post therapy assessment, days 28–30). At visit 1, inclusion/exclusion criteria, age, gender, underlying comorbidities, randomization number, medical history, physical examination, vital signs, pregnancy test in urine (for females in child-bearing age), electrocardiogram, and chest x-rays were assessed, and a sputum sample was obtained by expectoration and forwarded to the laboratory for microscopic examination, Gram staining, and culture of valid specimens for pathogen identification. At all visits, the following variables were evaluated by the investigator: physical examination, vital signs, dyspnea (0, absent; 1, mild; 2, moderate; 3, severe), cough (0, absent; 1, mild, morning only; 2, moderate, night and day, but not disturbing sleep; 3, severe, disturbing sleep), fever > 37.5°C (0, absent; 1, present), sputum purulence (0, mucoid, white to gray; 1, mucopurulence, yellowish; 2, purulence, greenish; 3, severe, brownish), sputum volume (0, absent; 1, mild; 2, moderate; 3, severe), lung function test (FEV1, FVC [forced vital capacity]), microbiological assessment (if a sputum specimen was available) and occurrence of adverse events. Blood samples were collected for analysis of biomarkers (KL-6, cytokines) at visit 1, 2 and 3, and for laboratory tests at visit 1 and 3. Clinical and microbiological data and biomarkers were compared at visits 2 and 3 with those present at baseline to evaluate clinical response; a further microbiological assessment (in case of clinical worsening) was performed at visit 4. A standardized case report form was used to record patient characteristics.
Titration of cytokines
The Evidence Investigator™ cytokine and growth factors high sensitivity array with Randox biochip technology (solid-state device containing an array of discrete test regions of immobilized antibodies specific to different cytokines and growth factors) was used for the simultaneous quantitative detection of multiple related immunoassays from a single sample. A sandwich chemiluminescent immunoassay was used for the cytokine array. Increased cytokine levels in a clinical specimen leads to increased binding of antibody labeled with horseradish peroxidase and an increase in the chemiluminescence signal emitted. The light signal generated from each of the test regions on the biochip is detected using digital imaging technology and compared with that from a stored calibration curve. The concentration of analyte present in the sample was calculated from the calibration curve.
KL-6 determination
A human KL-6 enzyme-linked immunosorbent assay kit can be used for in vitro quantitative determination of human KL-6 concentrations in serum, plasma, and other biological fluids. The microtiter plate provided in the kit is precoated with an antibody specific to KL-6. Standards or samples were added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for KL-6, and avidin conjugated with horseradish peroxidase was added to each microplate well and incubated. A tetramethyl-benzidine substrate solution was added to each well. Only wells containing KL-6, biotin-conjugated antibody, and enzyme-conjugated avidin showed a change in color. The enzyme-substrate reaction was terminated by addition of a sulfuric acid solution and the color change was measured spectrophotometrically at a wavelength of 450 ± 2 nm. The concentration of KL-6 in the samples was determined by comparing the OD of the samples with the standard curve.
Efficacy evaluation
The clinical score was calculated by adding the single scores of the semiquantitative scales of dyspnea, cough, fever, sputum purulence, and sputum volume. The total scores obtained at visit 2 and visit 3 (test-of-cure) were compared with those obtained at visit 1. Clinical efficacy, evaluated at visit 2 and 3, was defined as cure (disappearance of all signs and symptoms of AECB reported at visit 1, or reduction of at least 3 points on the total score calculated at visit 1 and no further antibiotic therapy required) or failure (worsening of at least one sign and symptom of AECB reported at visit 1, or no change, increase, or reduction of <3 points on the total score calculated at visit 1, or additional antimicrobial therapy required). Bacteriological efficacy, evaluated at visit 2 and 3, was defined as eradication (original causative organism absent on sputum culture), persistence (original organism still present on sputum culture), superinfection (isolation of new organism judged to be causing an infectious process in the respiratory tract associated with clinical signs), presumed eradication (absence of appropriate culture material for evaluation because the patient improved clinically and was unable to produce sputum), presumed persistence (absence of appropriate culture material for evaluation and patient assessed as clinical failure), or indeterminate (bacteriological response of the study drug not evaluable for any other reason). At the follow-up visits, in cases where a worsening of key signs and symptoms was observed, bacteriological efficacy was defined as relapse (presence of the original causative organism in the sputum), eradication with reinfection (elimination of the initial causative organism followed by replacement with new species), or not evaluable.
Statistical analysis
Baseline characteristics of the population and biomarkers levels on visits 1, 2, and 3 were considered for statistical analysis. Continuous data were expressed as the mean ± standard deviation and compared using the unpaired Student’s t-test. Categorical data were expressed as the number and percentage of events and compared using Fisher’s exact test. In all the comparisons, a P value < 0.05 was considered to be statistically significant. The data were analyzed using the Statistical Package for Social Sciences version 18.0 (SPSS Inc, Chicago, IL, USA) for MAC OS.
Results
Patient demographics
A total of 40 patients (median age 63 ± 7 years) were enrolled in the study. Twenty patients were assigned to the cefditoren arm and 20 patients to the levofloxacin arm. Demographic characteristics, concomitant diseases, and baseline FEV1 values and blood oxygen saturation are shown in Table 1. There were no differences in baseline characteristics between the two treatment groups; all patients were either smokers or ex-smokers. Causative pathogen(s) were isolated in 29 patients (72.5%), comprising 15 treated with cefditoren and 14 treated with levofloxacin. The most frequently isolated pathogens were H. influenzae (n = 19), S. pneumoniae (n = 6), and M. catarrhalis (n = 2); infection was caused by multiple pathogens in two cases (S. pneumoniae plus M. catarrhalis, n = 1; H. influenzae plus M. catarrhalis, n = 1).
Table 1.
Baseline characteristics of the study population
| Overall study population (n = 40) | Group 1 – cefditoren (n = 20) | Group 2 – levofloxacin (n = 20) | P | |
|---|---|---|---|---|
| Female, n (%) | 14 (35) | 9 (45) | 5 (25) | 0.320 |
| AGE, years (SD) | 63 ± 7 | 65 ± 7 | 62 ± 7 | 0.183 |
| Cardiovascular disease, n (%) | 7 (18) | 3 (15) | 4 (20) | 1.000 |
| GERD, n (%) | 5 (13) | 3 (15) | 2 (10) | 1.000 |
| Diabetes, n (%) | 4 (10) | 1 (5) | 3 (15) | 0.605 |
| FEV1 %, mean (SD) | 76 ± 12 | 76 ± 14 | 75 ± 8 | 0.783 |
| SpO2 %, mean (SD) | 95 ± 1 | 95 ± 1 | 94 ± 1 |
Abbreviations: GERD, Gastroesophageal reflux disease; FEV1, Forced Expiratory Volume in 1 second; SpO2, blood oxygen saturation; SD, standard deviation.
Inflammatory parameters
Table 2 lists the inflammatory parameters evaluated at visit 1 and at test-of-cure (visit 3). In general terms, there were no significant differences between groups both at visit 1 and the test-of-cure visit for any of the parameters, except for IL-4 (visit 1 and test-of-cure), interferon gamma (visit 1), and KL-6 (test-of-cure). The inflammatory parameters which were significantly reduced at test-of-cure with respect to visit 1 were KL-6 and IL-6. KL-6 decreased in the overall study population (from 19 ± 11 UI/mL to 6 ± 8 UI/mL, P = 0.000) and in the cefditoren (from 19 ± 13 UI/mL to 8 ± 10 UI/mL, P = 0.006) and levofloxacin (from 19 ± 10 UI/mL to 5 ± 5 UI/mL, P = 0.000) arms. Similarly, IL-6 decreased both in the overall study population (from 13.35 ± 16.41 pg/mL to 3 ± 4.7 pg/mL, P = 0.000) and in the cefditoren (from 15.90 ± 19.54 pg/mL to 4.13 ± 6.42 pg/mL, P = 0.015) and levofloxacin (from 10.80 ± 12.55 pg/mL to 1.87 ± 1.16 pg/mL, P = 0.003) arms (Figure 1A and B). C-reactive protein levels showed a similar pattern (Figure 1C). The delta values for reduction of KL-6 and IL-6 were similar for the overall study population, cefditoren arm, and levofloxacin arm, with no statistically significant differences between groups (Table 3).
Table 2.
Inflammatory biomarkers on visit 1 and on test of cure (TOC) in the overall study population and in the study groups
| Biomarker | Overall study population (n = 40) | Group 1 – cefditoren (n = 20) | Group 2 – Levofloxacin (n = 20) | P |
|---|---|---|---|---|
| VISIT 1 | ||||
| KL6, UI/mL | 19 ± 11 | 19 ± 13 | 19 ± 10 | 0.849 |
| IL2 | 3.04 ± 2.77 | 3.28 ± 3.11 | 2.80 ± 2.44 | 0.586 |
| IL4 | 1.99 ± 1.57 | 1.39 ± 1.36 | 2.60 ± 1.56 | 0.013 |
| IL6 | 13.35 ± 16.42 | 15.90 ± 19.54 | 10.80 ± 12.55 | 0.332 |
| IL8 | 17.00 ± 26.48 | 17.09 ± 25.70 | 16.91 ± 27.90 | 0.983 |
| IL10 | 3.04 ± 9.72 | 1.53 ± 1.65 | 4.56 ± 13.65 | 0.331 |
| VEGF | 133.75 ± 199.63 | 152.07 ± 211.32 | 115.44 ± 190.90 | 0.568 |
| IFNβ | 1.44 ± 2.08 | 2.14 ± 2.63 | 0.75 ± 0.96 | 0.032 |
| TNFα | 4.41 ± 2.26 | 4.54 ± 2.54 | 4.28 ± 2.00 | 0.722 |
| IL1α | 0.39 ± 0.52 | 0.35 ± 0.49 | 0.43 ± 0.56 | 0.653 |
| IL1β | 1.63 ± 1.17 | 1.73 ± 1.29 | 1.54 ± 1.07 | 0.617 |
| MCP1 | 228.53 ± 170.58 | 254.38 ± 210.72 | 202.67 ± 117.98 | 0.344 |
| EGF | 46.29 ± 87.87 | 49.89 ± 80.87 | 47.70 ± 96.46 | 0.921 |
| TOC | ||||
| KL6, UI/mL | 6 ± 8a | 8 ± 10a | 5 ± 5a | 0.016 |
| IL2 | 4.79 ± 8.35 | 6.71 ± 11.23 | 2.87 ± 3.00 | 0.147 |
| IL4 | 2.07 ± 1.37 | 1.55 ± 1.20 | 2.58 ± 1.37 | 0.016 |
| IL6 | 3.00 ± 4.70a | 4.13 ± 6.42a | 1.87 ± 1.16a | 0.130 |
| IL8 | 14.29 ± 20.93 | 12.09 ± 17.68 | 16.48 ± 24.01 | 0.514 |
| IL10 | 11.69 ± 63.91 | 2.15 ± 1.89 | 21.24 ± 90.49 | 0.351 |
| VEGF | 112.53 ± 147.90 | 116.97 ± 166.16 | 108.09 ± 131.35 | 0.852 |
| IFNγ | 4.40 ± 11.03 | 3.36 ± 6.95 | 5.44 ± 14.11 | 0.558 |
| TNFα | 9.39 ± 17.64 | 13.25 ± 24.32 | 5.53 ± 3.93 | 0.169 |
| IL1α | 0.50 ± 0.66 | 0.55 ± 0.69 | 0.44 ± 0.64 | 0.605 |
| IL1β | 1.19 ± 1.01 | 1.31 ± 1.08 | 1.06 ± 0.93 | 0.436 |
| MCP1 | 234.20 ± 156.77 | 248.08 ± 181.61 | 220.31 ± 130.62 | 0.582 |
| EGF | 54.48 ± 93.46 | 59.33 ± 104.67 | 46.63 ± 83.21 | 0.747 |
Notes:
P < 0.05 TOC vs visit 1. Levels of biomarkers are expressed as pg/mL, unless otherwise indicated. All data are expressed as Mean ± SD; Group 1 and Group 2 were compared using the unpaired Student t-test. P values ≤ 0.05 were considered significan. TOC is 6–9 days after the drug initiation, at the end of treatment.
Abbreviations: KL-6, Krebs von den Lundgen-6; IL-2, interleukin 2; IL-4, interleukin 4; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; VEGF, Vascular Endothelial Growth Factor; IFN, Interferon; TNFα, Tumor Necrosis factor alpha; IL1α, interleukin 1 alpha; IL1β, interleukin 1 beta; MCP1, Monocyte Chemoattractant Protein 1; EGF, Epidermal Growth Factor; TOC, test of cure.
Figure 1.
Levels of KL6 (A) and IL6 (B) and C-reactive protein (C), cefditoren and levofloxacin arms at visit 1, 2 and TOC.
Note: *P = 0.05 TOC vs visit 1.
Abbreviations: TOC, test of cure; KL-6, Krebs von den Lundgen-6; IL-6, interleukin 6; CRP, C-reactive protein.
Table 3.
Speed of reduction of inflammatory biomarkers
| ΔBiomarker | Overall study population | Group 1 – cefditoren | Group 2 – levofloxacin | P |
|---|---|---|---|---|
| ΔKL6, UI/mL | 12.63 ± 10.98 | 10.53 ± 10.84 | 14.73 ± 10.99 | 0.231 |
| ΔIL6, pg/mL | 10.36 ± 13.09 | 11.76 ± 14.07 | 8.93 ± 12.23 | 0.499 |
Notes: Comparison between treatments was performed at TOC. Variations (Δ) between visit 1 and TOC were calculated for each parameter. All Δs are expressed as Mean ± SD. Group 1 and Group 2 were compared using the unpaired Student’s t-test. P values ≤ 0.05 were considered significant. TOC is 6–9 days after the drug initiation, at the end of treatment.
Abbreviations: IL-6, interleukin 6; KL-6, Krebs von den Lundgen-6; TOC, test of cure.
Clinical and bacteriological response
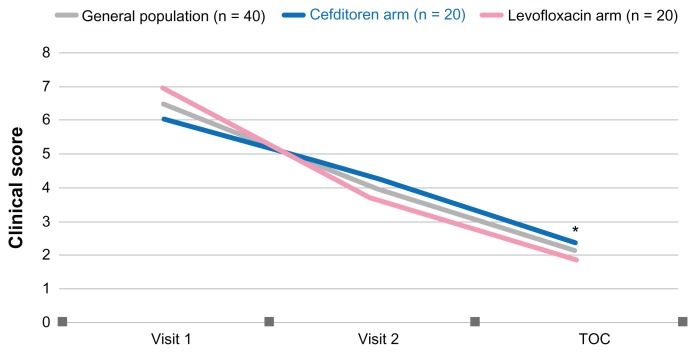
The clinical score, calculated based on the single scores of dyspnea, cough, fever, sputum purulence, and sputum volume, decreased progressively during the study, both in the overall study population (from 6.48 ± 1.59 at visit 1 to 2.05 ± 1.62 at test-of-cure, P = 0.000) and in the cefditoren (from 6 ± 1.59 at visit 1 to 2.30 ± 1.75 at test-of-cure, P = 0.000) and levofloxacin (from 6.95 ± 1.47 at visit 1 to 1.80 ± 1.47 at test-of-cure, P = 0.000) arms (Figure 2). Differences between groups were not statistically significant.
Figure 2.
Clinical score in the overall population, the cefditoren and levofloxacin arms at visit 1, 2 and test of cure (TOC).
Note: *P = 0.05 TOC vs visit 1 for general population, cefditoren and levofloxacin.
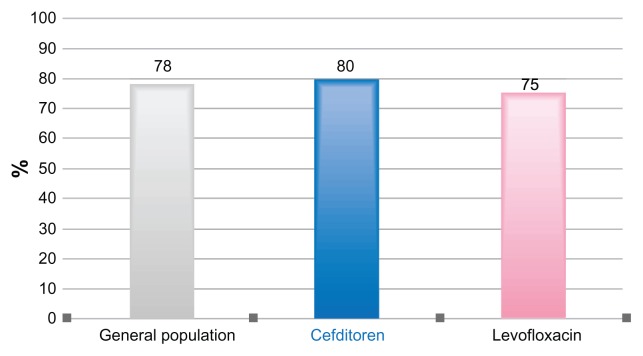
At the end of treatment (test-of-cure, 6–9 days after drug initiation), the clinical success rate in the overall study population was 78%, and the clinical cure was similar in both study groups (80% and 75% in the cefditoren and levofloxacin arms, respectively, Figure 3). Globally, bacteriological eradication at test-of-cure was obtained in 85% of the overall study population; eradication rate was higher in the levofloxacin arm compared with the cefditoren arm (95% versus 75%), although the difference was not statistically significant.
Figure 3.
Clinical efficacy in the overall population, the cefditoren and levofloxacin arms at test of cure (TOC).
Safety
Four patients in the levofloxacin arm and two patients in the cefditoren arm reported gastrointestinal side effects (either mild nausea or diarrhea).
Discussion
COPD is characterized by an abnormal inflammatory response which extends beyond the lungs, with evidence of low-grade systemic inflammation. Increased levels of acute phase proteins such as proinflammatory cytokines, including IL-6, were found in the circulation of patients with stable COPD, and have been shown to be associated with impaired functional capacity, reduced daily physical activity, and decreased health status.37 IL-6 is now emerging as a potentially pivotal player in the pathogenesis of lung disease. Further, in the lung, IL-6 dissociates from other inflammatory markers and could be a target for treatment of asthma, COPD, or other pulmonary diseases.38 A recent study by Grubek-Jaworska et al, which sought a relationship between IL-6 and IL-13 concentrations in induced sputum and respiratory function of patients with COPD or asthma, showed a negative correlation between IL-6 level, but not IL-13 level, and respiratory disorders, determined by a temporal decay in FEV1 and FVC in COPD patients.39 Two recently published studies of inflammatory biomarkers in patients with COPD showed that elevated IL-6 serum levels, but not tumor necrosis factor alpha or IL-8 levels, are predictive of increased mortality, and are associated with poor clinical outcomes.40,41 Moreover, acute exacerbations of chronic obstructive pulmonary disease are associated with elevations of plasma fibrinogen and serum IL-6 levels.42 Our results show that both cefditoren and levofloxacin are effective in decreasing inflammatory biomarkers in AECB. Serum IL-6 levels were significantly different between visit 1 and test-of-cure, and the reduction was similar in the cefditoren and levofloxacin groups (11.76 ± 14.07 pg/mL and 8.93 ± 12.23 pg/mL, respectively).
Our results show that both cefditoren and levofloxacin are also significantly effective in decreasing KL-6 baseline levels in AECB (delta value between visit 1 and test-of-cure was 10.53 ± 10.84 UI/mL and 14.73 ± 10.99 UI/mL, respectively; difference not statistically significant). KL-6 has already been described as a useful serum marker of lung epithelial damage. In fact, KL-6 is elevated in the plasma and bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Plasma levels of KL-6 reflect the severity of lung injury and show that bronchoalveolar lavage fluid levels correlate with indices of inflammation.43 Elevated levels of KL-6 in plasma and epithelial lining fluid may provide a useful marker for acute respiratory distress syndrome in ventilated patients and have a possible negative prognostic significance.44,45 Extensive investigations showed that serum KL-6 is elevated in 70%–100% of patients with various interstitial lung diseases (idiopathic interstitial pneumonias, collagen vascular disease-associated interstitial pneumonia, hypersensitivity pneumonia, radiation pneumonitis, drug-induced interstitial lung diseases, acute respiratory distress syndrome, pulmonary sarcoidosis, and pulmonary alveolar proteinosis) and support its utility both as a serum biomarker for detecting the presence of disease and as an index of disease activity.19,46 KL-6 is currently one of the best and most reliable serum biomarkers available for management of interstitial lung disease.19 In these cases, initial elevated serum KL-6 levels can identify patients at increased risk for subsequent mortality.47,48 Furthermore, Ishikawa et al demonstrated that aging and long-term smoking lead to an increase in KL-6 levels in the lung, induced sputum, and plasma.49 A recently published study showed that both KL-6 and surfactant protein D, and particularly the product of surfactant protein D and KL-6, are good indicators of the presence of fibrotic lesions in the lungs of patients with combined pulmonary fibrosis and emphysema.17 Finally, levels of surfactant proteins A and D and KL-6 are elevated in the induced sputum of patients with COPD.50 To the best of our knowledge, no other trial comparing levofloxacin and cephalosporins has focused on KL-6; in general, no studies have evaluated KL-6 in AECB. Our results confirm that although levofloxacin is associated with a more rapid reduction in KL-6 levels, treatment with cefditoren is also associated with a decrease in KL-6 levels. These patterns of response were also confirmed by analysis of C-reactive protein levels across time.
The effect of the treatments on inflammation is probably related to their antibacterial efficacy, even if immunomodulation/anti-inflammatory properties of the two antibiotics cannot be ruled out. Both cefditoren and levofloxacin showed very good clinical efficacy (75%–80%). Levofloxacin offered a quicker rate of symptom resolution at visit 2, but at test-of-cure, all patients treated with cefditoren recovered fully. The clinical value of cefditoren in AECB has been demonstrated previously by other clinical studies.22,51 The clinical efficacy of these trials was in line with that observed in our study. Other randomized studies compared levofloxacin 500 mg once daily with cephalosporins, especially cefuroxime 250 mg twice daily in patients with AECB. Generally, the clinical cure rates were higher in the levofloxacin group.21,24,36
Antimicrobial prescribing should aim to eradicate or maximally reduce the pathogen bacterial load. In our study, the microbiological data were consistent with traditional findings in AECB; in particular, isolates from sputum on visit 1 are similar to those of other studies.16,22,52 Both antibiotics showed good activity against the microorganisms isolated.
Cefditoren was very well tolerated. Only a few adverse events were reported in our study and did not cause treatment discontinuation. Moreover, cefditoren had an adverse event profile comparable with that of other cephalosporins (gastrointestinal system disorders being the most frequently reported adverse events).21,22
In an era characterized by a limited number of new antibiotics in the pipeline, and overuse and misuse of current antibiotics, appropriate selection of available antimicrobial agents can reduce treatment failures (and subsequently health care costs) and help to prevent the spread of resistant bacterial strains. In 2005, the Council for Appropriate and Rational Antibiotic Therapy (CARAT), an independent and multidisciplinary panel of health care professionals, clinicians, and scientists, developed criteria to guide appropriate and accurate antibiotic selection aimed at optimizing antibiotic therapy. These criteria are evidence-based results, therapeutic benefits, safety, optimal drug for the optimal duration, and cost-effectiveness.53 Martinez et al used CARAT criteria for choosing the optimal drug at its optimal dose and duration for antimicrobial treatment in outpatients with AECB due to bacterial infection. Patients were stratified according to risk factors to improve selection of targeted antimicrobial therapy. In chronic bronchitis without risk factors (simple), the first-line drugs are second-generation macrolides, second-generation or third-generation cephalosporins, amoxicillin, doxycycline, or cotrimoxazole; fluoroquinolones are recommended as first-line therapy for patients with chronic bronchitis who have risk factors.54 Recently, the appropriate and accurate use of antibiotics has been emphasized by International and National Scientific Society, including the Italian Society of Chemotherapy.55 In 2011, a multinational working group on antibiotic stewardship from the International Society of Chemotherapy put together ten recommendations for physicians prescribing antibiotics for outpatients. The first two recommendations are strictly linked to appropriate use, ie, use antibiotics only when needed and select the appropriate antibiotic.56
Canut et al used a probability (therapeutic outcomes) model to predict the likelihood of clinical success with particular antimicrobial agents in the treatment of patients with AECB. According to this model, fluoroquinolones (levofloxacin, ciprofloxacin, moxifloxacin), cefditoren, and amoxicillin-clavulanate are the most appropriate antibiotics for treatment of patients with AECB in terms of predicted clinical efficacy.57 In our study, cefditoren was shown to be therapeutically comparable with levofloxacin in terms of efficacy and safety for management of cases of AECB. Although levofloxacin had excellent activity in almost all cases, oral cephalosporins should be considered for less severely unwell patients and uncomplicated AECB, as recommended by some Italian scientific groups (Federazione delle Associazioni dei Dirigenti Ospedalieri Internisti; Gruppo Italiano per lo Studio delle Antibiotico Resistenze nelle Infezioni Respiratorie), reserving fluoroquinolones for more severe cases (moderate to severe AECB with or without risk factors for multiresistant pathogens) or for intolerance to beta-lactams/macrolides or treatment failure (second-line therapy).20,58 Another point to consider in the choice of the appropriate antibiotic for AECB is safety. Cardiac alterations,59,60 blood glucose impairment,59 hepatic injury,61 and ocular events (such as retinal detachment62) have been reported with fluoroquinolones, especially in patients with predisposing factors, such as diabetes and heart disease.59 In addition, because of physiological changes in renal function and when certain comorbidities are present, some special considerations are necessary when elderly patients are treated with these drugs.63 For this reason, Lode et al, evaluating the safety and tolerability of oral antibiotics commonly prescribed for respiratory tract infections, devote considerable attention to the fluoroquinolone agents.64 According to the recently published European Respiratory Society guidelines on lower respiratory infections, fluoroquinolones are highly active and efficacious against respiratory pathogens and should be used in well defined circumstances. If the prevalence of first-step mutants is low, use of the most potent fluoroquinolones is a logical choice if resistance has to be avoided/delayed.65
To the best of our knowledge, this is the first study comparing levofloxacin and cefditoren in the treatment of AECB. The most important strength of our study is the assessment of the airway inflammatory pattern. We did not just describe outcomes, but we also examined inflammation and lung damage during an acute infection. Assessment of the inflammatory pattern should also be extended to distal airways specimens, like sputum or possibly exhaled breath condensate. Further studies are needed to understand better the potential role played by oral cephalosporins in the management of AECB.
Conclusion
Cefditoren represents a valid option in the treatment of mild to moderately severe cases of AECB in the outpatient care setting. In this study, cefditoren showed very good clinical efficacy and activity against the microorganisms isolated. Moreover, use of this cephalosporin was associated with a significant reduction of IL-6 and KL-6, two key mediators of lung inflammation and epithelial damage.
Footnotes
Disclosure
The study was partially funded by a research grant from Zambon Italy srl. The authors have no other conflicts of interest in this work.
References
- 1.Kim N, Leeper KV., Jr Epidemiology of chronic bronchitis and acute infective exacerbations of chronic bronchitis. Semin Respir Crit Care Med. 2000;21(2):73–78. doi: 10.1055/s-2000-9845. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Kiley J, Bateman ED, et al. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J. 2010;36(5):995–1001. doi: 10.1183/09031936.00012610. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Anthonisen NR, Manfreda J, Warren PC, Hershfield EC, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 5.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 6.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanner RE, Anthonisen NR, Connett JE Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds HY. Chronic obstructive pulmonary disease, chronic bronchitis, and acute exacerbations. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Disease. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 11.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. [Accessed November 10, 2012]. Available from: http://www.goldcopd.org.
- 12.Reilly JJ. COPD and declining FEV1 – time to divide and conquer? N Engl J Med. 2008;359(15):1616–1618. doi: 10.1056/NEJMe0807387. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 14.Hayes D, Jr, Meyer KC. Acute exacerbations of chronic bronchitis in elderly patients: pathogenesis, diagnosis and management. Drugs Aging. 2007;24(7):555–572. doi: 10.2165/00002512-200724070-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mensa J, Trilla A. Should patients with acute exacerbation of chronic bronchitis be treated with antibiotics? Advantages of the use of fluoroquinolones. Clin Microbiol Infect. 2006;12( Suppl 3):42–54. doi: 10.1111/j.1469-0691.2006.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S. Pathogenesis and treatment of acute exacerbations of chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2005;26(2):192–203. doi: 10.1055/s-2005-869538. [DOI] [PubMed] [Google Scholar]
- 17.Chiba S, Ohta H, Abe K, et al. The diagnostic value of the interstitial biomarkers KL-6 and SP-D for the degree of fibrosis in combined pulmonary fibrosis and emphysema. Pulm Med. 2012;2012:492960. doi: 10.1155/2012/492960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai S, Kurasawa K, Maezawa R, Owada T, Okada H, Fukuda T. Marked increase in serum KL-6 and surfactant protein D levels during the first 4 weeks after treatment predicts poor prognosis in patients with active interstitial pneumonia associated with polymyositis/dermatomyositis. Mod Rheumatol. 2012 Sep 17; doi: 10.1007/s10165-012-0756-0. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50(1):3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Blasi F, Bulfoni A, Concia C, et al. Attualita’ nella gestione delle infezioni delle basse vie respiratorie in medicina interna. [Management of lower respiratory tract infections in internal medicine]. Italian Journal of Medicine. 2012;4(1):42–53. Italian. [Google Scholar]
- 21.Petitpretz P, Choné C, Trémolières F Investigator Study Group. Levofloxacin 500 mg once daily versus cefuroxime 250 mg twice daily in patients with acute exacerbations of chronic obstructive bronchitis: clinical efficacy and exacerbation-free interval. Int J Antimicrob Agents. 2007;30(1):52–59. doi: 10.1016/j.ijantimicag.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Sala JL, Kardos P, Martínez-Beltrán J, Coronel P, Aguilar L. Clinical and bacteriological efficacy in treatment of acute exacerbations of chronic bronchitis with cefditoren-pivoxil versus cefuroxime-axetil. Antimicrob Agents Chemother. 2006;50(5):1762–1767. doi: 10.1128/AAC.50.5.1762-1767.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson R, Langan C, Ball P, Bateman K, Pypstra R Gemifloxacin 207 Clinical Study Group. Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis. Respir Med. 2003;97(3):242–249. doi: 10.1053/rmed.2003.1435. [DOI] [PubMed] [Google Scholar]
- 24.Weiss LR. Open-label, randomized comparison of the efficacy and tolerability of clarithromycin, levofloxacin, and cefuroxime axetil in the treatment of adults with acute bacterial exacerbations of chronic bronchitis. Clin Ther. 2002;24(9):1414–1425. doi: 10.1016/s0149-2918(02)80045-5. [DOI] [PubMed] [Google Scholar]
- 25.Anzueto A, Bishai WR, Pottumarthy S. Role of oral extended-spectrum cephems in the treatment of acute exacerbation of chronic bronchitis. Diagn Microbiol Infect Dis. 2007;57(Suppl 3):31S–38S. doi: 10.1016/j.diagmicrobio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Dubois J, St-Pierre C. In vitro study of the post-antibiotic effect and the bactericidal activity of Cefditoren and ten other oral antimicrobial agents against upper and lower respiratory tract pathogens. Diagn Microbiol Infect Dis. 2000;37(3):187–193. doi: 10.1016/s0732-8893(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 27.Gracia M, Díaz C, Coronel P, et al. Antimicrobial susceptibility of Streptococcus pyogenes in Central, Eastern, and Baltic European Countries, 2005 to 2006: the cefditoren surveillance program. Diagn Microbiol Infect Dis. 2009;64(1):52–56. doi: 10.1016/j.diagmicrobio.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Tempera G, Furneri PM, Carlone NA, et al. Antibiotic susceptibility of respiratory pathogens recently isolated in Italy: focus on cefditoren. J Chemother. 2010;22(3):153–159. doi: 10.1179/joc.2010.22.3.153. [DOI] [PubMed] [Google Scholar]
- 29.Tempera G, Furneri PM, Ferranti C, et al. In vitro activity of cefditoren versus other antibiotics against S. pneumoniae clinical strains isolated in Italy. Int J Immunopathol Pharmacol. 2010;23(3):833–840. doi: 10.1177/039463201002300318. [DOI] [PubMed] [Google Scholar]
- 30.Gracia M, Díaz C, Coronel P, et al. Antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis isolates in eight Central, East and Baltic European countries in 2005–2006: results of the Cefditoren Surveillance Study. J Antimicrob Chemother. 2008;61(5):1180–1181. doi: 10.1093/jac/dkn083. [DOI] [PubMed] [Google Scholar]
- 31.García-de-Lomas J, Lerma M, Cebrián L, et al. Influence of Haemophilus influenzae beta-lactamase production and/or ftsI gene mutations on in vitro activity of and susceptibility rates to aminopenicillins and second- and third-generation cephalosporins. Int J Antimicrob Agents. 2007;30(2):190–192. doi: 10.1016/j.ijantimicag.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Barberán J, Aguilar L, Giménez MJ. Update on the clinical utility and optimal use of cefditoren. Int J Gen Med. 2012;5:455–464. doi: 10.2147/IJGM.S25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano F, Giménez MJ, Aguilar L. Cefditoren in upper and lower community-acquired respiratory tract infections. Drug Des Devel Ther. 2011;5:85–94. doi: 10.2147/DDDT.S9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granizo JJ, Giménez MJ, Barberán J, Coronel P, Gimeno M, Aguilar L. Efficacy of cefditoren in the treatment of upper respiratory tract infections: a pooled analysis of six clinical trials. Spanish. Rev Esp Quimioter. 2008;21(1):14–21. Spanish. [PubMed] [Google Scholar]
- 35.Granizo JJ, Aguilar L, Gimenez MJ, Coronel P, Gimeno M, Prieto J. Safety profile of cefditoren. A pooled analysis of data from clinical trials in community-acquired respiratory tract infections. Rev Esp Quimioter. 2009;22(2):57–61. Spanish. [PubMed] [Google Scholar]
- 36.Shah PM, Maesen FP, Dolmann A, Vetter N, Fiss E, Wesch R. Levofloxacin versus cefuroxime axetil in the treatment of acute exacerbation of chronic bronchitis: results of a randomized, double-blind study. J Antimicrob Chemother. 1999;43(4):529–539. doi: 10.1093/jac/43.4.529. [DOI] [PubMed] [Google Scholar]
- 37.Yanbaeva DG, Dentener MA, Spruit MA, et al. IL6 and CRP haplotypes are associated with COPD risk and systemic inflammation: a case-control study. BMC Med Genet. 2009;10:23. doi: 10.1186/1471-2350-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8(9):1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubek-Jaworska H, Paplińska M, Hermanowicz-Salamon J, et al. IL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory tests. Respiration. 2012;84(2):101–107. doi: 10.1159/000334900. [DOI] [PubMed] [Google Scholar]
- 40.Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 41.Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(2):210–215. [PubMed] [Google Scholar]
- 43.Nathani N, Perkins GD, Tunnicliffe W, Murphy N, Manji M, Thickett DR. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care. 2008;12(1):R12. doi: 10.1186/cc6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato H, Callister ME, Mumby S, et al. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23(1):142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- 45.Ishizaka A, Matsuda T, Albertine KH, et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 46.Doishita S, Inokuma S, Asashima H, et al. Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases. Intern Med. 2011;50(23):2889–2892. doi: 10.2169/internalmedicine.50.5866. [DOI] [PubMed] [Google Scholar]
- 47.Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med. 2006;260(5):429–434. doi: 10.1111/j.1365-2796.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 48.Yokoyama A, Kondo K, Nakajima M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11(2):164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa N, Mazur W, Toljamo T, et al. Ageing and long-term smoking affects KL-6 levels in the lung, induced sputum and plasma. BMC Pulm Med. 2011;11:22. doi: 10.1186/1471-2466-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa N, Hattori N, Tanaka S, et al. Levels of surfactant proteins A and D and KL-6 are elevated in the induced sputum of chronic obstructive pulmonary disease patients: a sequential sputum analysis. Respiration. 2011;82(1):10–18. doi: 10.1159/000324539. [DOI] [PubMed] [Google Scholar]
- 51.Henry DC, Poling TL, Bettis RB. A double-blind, randomized study of cefditoren vs cefuroxime for AECB. J Respir Dis. 2001;22( Suppl 8):69–74. [Google Scholar]
- 52.Pfaller MA, Ehrhardt AF, Jones RN. Frequency of pathogen occurrence and antimicrobial susceptibility among community-acquired respiratory tract infections in the respiratory surveillance program study: microbiology from the medical office practice environment. Am J Med. 2001;111(Suppl 9A):4S–12S. doi: 10.1016/s0002-9343(01)01025-7. [DOI] [PubMed] [Google Scholar]
- 53.Slama TG, Amin A, Brunton SA, et al. A clinician’s guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(Suppl 7A):1S–6S. doi: 10.1016/j.amjmed.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Martinez FJ, Anzueto A. Appropriate outpatient treatment of acute bacterial exacerbations of chronic bronchitis. Am J Med. 2005;118(Suppl 7A):39S–44S. doi: 10.1016/j.amjmed.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Scaglione F, Bertazzoni Minelli E, De Sarro A, et al. The Charta of Milan: basic criteria for the appropriate and accurate use of antibiotics: recommendations of the Italian Society of Chemotherapy. J Chemother. 2009;21(5):475–481. doi: 10.1179/joc.2009.21.5.475. [DOI] [PubMed] [Google Scholar]
- 56.Levy-Hara G, Amábile-Cuevas CF, Gould I, et al. “Ten Commandments” for the appropriate use of antibiotics by the practicing physician in an outpatient setting. Front Microbiol. 2011;2:230. doi: 10.3389/fmicb.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canut A, Martın-Herrero JE, Labora A, Maortua H. What are the most appropriate antibiotics for the treatment of acute exacerbation of chronic obstructive pulmonary disease? A therapeutic outcomes model. J Antimicrob Chemother. 2007;60(3):605–612. doi: 10.1093/jac/dkm228. [DOI] [PubMed] [Google Scholar]
- 58.Blasi F, Concia E, Mazzei T, et al. Role of the oral beta-lactams in the treatment of exacerbations of chronic bronchitis: critical analysis and therapeutic recommendations. J Chemother. 2010;22( Suppl 1):3–30. doi: 10.1179/joc.2010.22.Supplement-1.3. [DOI] [PubMed] [Google Scholar]
- 59.Pugi A, Longo L, Bartoloni A, et al. Cardiovascular and metabolic safety profiles of the fluoroquinolones. Expert Opin Drug Saf. 2012;11(1):53–69. doi: 10.1517/14740338.2011.624512. [DOI] [PubMed] [Google Scholar]
- 60.Lapi F, Wilchesky M, Kezouh A, Benisty JI, Ernst P, Suissa S. Fluoroquinolones and the risk of serious arrhythmia: a population-based study. Clin Infect Dis. 2012;55(11):1457–1465. doi: 10.1093/cid/cis664. [DOI] [PubMed] [Google Scholar]
- 61.Paterson JM, Mamdani MM, Manno M, Juurlink DN for the Canadian Drug Safety and Effectiveness Research Network. Fluoroquinolone therapy and idiosyncratic acute liver injury: a population-based study. CMAJ. 2012;184(14):1565–1570. doi: 10.1503/cmaj.111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307(13):1414–1419. doi: 10.1001/jama.2012.383. [DOI] [PubMed] [Google Scholar]
- 63.Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27(3):193–209. doi: 10.2165/11531490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Lode H. Safety and tolerability of commonly prescribed oral antibiotics for the treatment of respiratory tract infections. Am J Med. 2010;123(Suppl 4):S26–S38. doi: 10.1016/j.amjmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections – summary. Clin Microbiol Infect. 2011;17( Suppl 6):1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]