Abstract
Clinical trials have shown the benefits of acetylcholinesterase inhibitors, such as donepezil and galantamine, and an N-methyl-D-aspartate receptor antagonist, memantine, in patients with Alzheimer’s disease (AD). However, little is known regarding the effects of switching from donepezil 5 mg/day to galantamine 16 or 24 mg/day, or regarding the effects of adding memantine to established therapy compared with increasing the dose of donepezil. This report discusses two studies conducted to evaluate treatment with galantamine and memantine with respect to cognitive benefits and caregiver evaluations in patients with AD receiving donepezil 5 mg/day for more than 6 months. Patients with mild or moderate AD (scores 10–22 on the Mini-Mental State Examination) were enrolled in the Galantamine Switch study and switched to galantamine (maximum doses 16 mg versus 24 mg). Patients with moderate to severe AD (Mini-Mental State Examination scores 3–14) were enrolled in the Donepezil Increase versus Additional Memantine study and either had their donepezil dose increased to 10 mg/day or memantine 20 mg/day added to their existing donepezil dose. Patients received the study treatment for 28 weeks and their Disability Assessment for Dementia, Mental Function Impairment Scale, Cohen-Mansfield Agitation Inventory, and Neuropsychiatric Inventory scores were assessed with assistance from their caregivers. For the Galantamine Switch study after 8 weeks, agitation evaluated by the Cohen-Mansfield Agitation Inventory improved in both the 16 mg and 24 mg groups compared with baseline. However, there were no significant differences between the two galantamine groups. Agitation was also less in patients in the additional memantine group than in the donepezil increase group. In summary, switching to galantamine from donepezil and addition of memantine in patients with AD receiving donepezil were both safe and meaningful treatment options, and particularly efficacious for suppression of agitation.
Keywords: Alzheimer’s disease, galantamine, memantine, donepezil, agitation, acetylcholinesterase inhibitors
Introduction
Alzheimer’s disease (AD) is a progressive illness of the elderly. The loss of cholinergic neurotransmission in the cerebral cortex, hippocampus, and other areas of the brain is thought to contribute to the pathogenesis of AD. Patients show increasing decline in cognition and behavioral symptoms at all stages of AD. While all patients with AD can present with depression, agitation, and aggressive behaviors, behavioral difficulties are most pronounced during more advanced stages of the disease.1,2
Acetylcholinesterase inhibitors, such as donepezil and galantamine, are widely used to slow the rate of cognitive dysfunction and can also provide behavioral and functional benefits in patients with mild to moderate AD.3,4 In addition, galantamine has allosteric-modulating activity at the nicotinic receptors,5 so may benefit patients who are not responding to donepezil. An N-methyl-D-aspartate receptor antagonist such as memantine is effective primarily in patients with moderate to severe AD.6 Glutamate is the principal excitatory neurotransmitter in the brain, and glutamatergic overstimulation may induce neuronal damage. Glutamate stimulates a number of postsynaptic receptors, including the N-methyl-D-aspartate receptor, which has been particularly implicated in memory processes, dementia, and the pathogenesis of AD.7,8 However, the effects of switching from donepezil 5 mg/day to galantamine 16 or 24 mg/day and the effects of adding memantine to established therapy, compared with increasing the dose of donepezil, have not been fully demonstrated.6,9,10
This report describes two studies in which the cognitive benefits and changes in caregiver evaluations were evaluated. In both studies, all patients with AD were undergoing treatment with donepezil 5 mg/day for more than 6 months. Patients were assigned to one of two studies based on the severity of their AD, measured using the Mini-Mental State Examination (MMSE). The purpose of these studies was to investigate the efficacy of switching from donepezil to galantamine and to compare the effects of increasing the dose of donepezil with adding memantine to the established donepezil dose.
Materials and methods
Patients were recruited from August 2011 to April 2012. All subjects included were outpatients who lived with, or had regular daily visits from, a responsible caregiver and had a diagnosis of AD, according to the criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.11 All patients (n = 67) had been treated with donepezil 5 mg/day for more than 6 months. Patients with AD were excluded if they were undergoing therapy with psychotropic, antidepressant, and/or sleep medications. Patients were enrolled and divided into two studies, ie, the Galantamine Switch study or the Donepezil Increase versus Additional Memantine (DIAM) study (Figure 1), based on their score on the MMSE at baseline.12 If the MMSE score fell within the 10–14 range, patients were randomly assigned to either the Galantamine Switch or the DIAM study. After study assignment, each patient was enrolled in a 28-week open-label study. For the Galantamine Switch study, patients were divided into one of two groups, ie, a 16 mg group or a 24 mg group. All Galantamine Switch study patients started with an initial 8 mg/day dose of galantamine and the dose was titrated up to a maximum of either 16 mg/day or 24 mg/day. All dose titrations were performed every 4 weeks using 8 mg/day increments to reach the maximum dose for that group. Patients enrolled in the DIAM study were randomly assigned to either the donepezil increase group or to the additional memantine group. The donepezil increase group was prescribed donepezil 10 mg/day at baseline. The additional memantine group began with memantine 5 mg/day and the dose was titrated up by 5 mg/day on a weekly basis to a maximum dose of 20 mg/day (5 → 10 → 15 → 20 mg/day).
Figure 1.
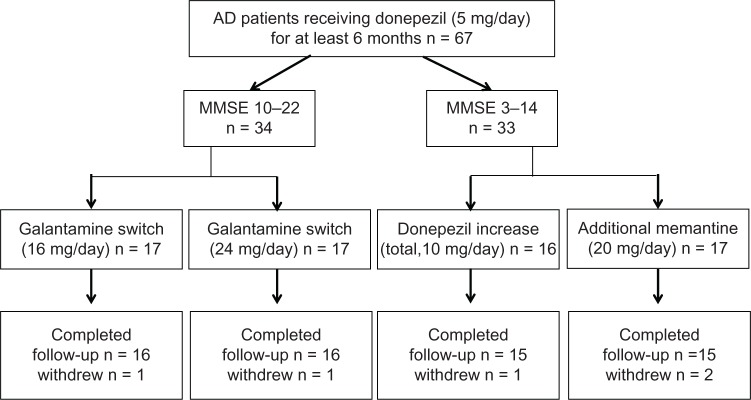
Subject disposition.
All subjects in both studies underwent MMSE, and a care-giver evaluation was conducted at baseline and at weeks 4, 8, 12, 16, 20, 24, and 28. Caregiver evaluations consisted of the Disability Assessment for Dementia (DAD),13 Mental Function Impairment Scale (MENFIS),14 Cohen-Mansfield Agitation Inventory (CMAI),15,16 and Neuropsychiatric Inventory (NPI),17,18 obtained via interviews with caregivers.
The DAD is a 46-item structured interview or questionnaire (scored 0–100) designed to evaluate activities of daily living. The MENFIS is one of the subscales of the Clinician’s Interview-Based Impression of Change-Plus19 and evaluates core symptoms of dementia, including cognitive, motivational, and emotional aspects, based on interviews with the patient and information from the caregiver (score 0–78). For the MENFIS, the score is directly related to the degree of functional deficit. The CMAI involves measurement of agitation in patients with dementia and consists of 29 items rated 0–7 (0 = none and 7 = several times an hour), with a total score ranging 0 from 203. The NPI was developed to assess behavioral disturbance occurring in patients with dementia. It consists of 12 domains/subscales that are designed to rate specific behavioral characteristics (delusions, hallucinations, agitation, dysphonia, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, night-time behavior disturbances, and appetite and eating abnormalities). For each domain, the frequency and severity of each behavior type is scored and the domain score is calculated by multiplying severity by frequency. Severity is rated 0–3 (0 = none and 3 = severe) and frequency is rated 0–4 (0 = none and 4 = very frequently). Caregiver distress is rated by the caregiver on a 0–5 scale (0 = none and 5 = very severe or extreme distress). Statistical analysis was carried out with the Mann-Whitney U-test, with P < 0.05 used as the threshold for statistical significance.
This study was approved by the ethics committee of Toho University Omori Medical Center. All patients and legally acceptable caregivers participating in the trial were given an explanation of the trial by the investigators before the start of the study, and informed consent for participation in the study was obtained. Consent of patients was obtained verbally or in writing, and the consent of legally acceptable representatives was always obtained in writing.
Results
Subject disposition
A total of 67 patients were screened. For the Galantamine Switch study, 34 patients were enrolled. The other 33 patients were enrolled in the DIAM study. Within each study, patients were randomly assigned to one of two groups. For the Galantamine Switch study, two patients discontinued due to hospitalization at another hospital (n = 1) or relocation (n = 1). In the DIAM study, three patients discontinued due to agitation (donepezil increase group, n = 1) or dizziness (additional memantine group, n = 2).
Galantamine Switch study
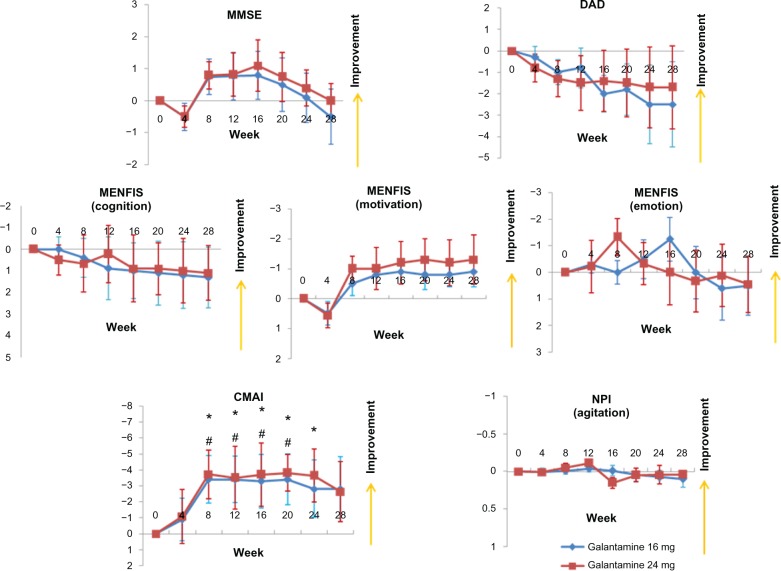
A total of 32 patients (16 mg group, n = 16; 24 mg group, n = 16) completed the study. The patient demographic data are shown in Table 1. Compared with baseline scores, the MMSE score did not improve and no significant differences were observed between the 16 mg and 24 mg groups (Figure 2). With respect to the caregiver evaluations, the DAD and MENFIS scores did not change from baseline and there were no significant differences observed between the 16 mg and 24 mg groups. However, scores on the CMAI demonstrated an improvement in agitation after 8 weeks, in both the 16 mg and the 24 mg groups, whereas there were no differences in agitation as one of the domains of the NPI, when compared with baseline and when the two groups were compared.
Table 1.
Demographic data
| Galantamine switch
|
Donepezil increase
|
Additional memantine
|
||
|---|---|---|---|---|
| 16 mg (n = 16) | 24 mg (n = 16) | total, 10 mg/day (n = 15) | 20 mg/day (n = 15) | |
| Age (years), mean (SD) | 75.4 (8.8) | 78.8 (9.2) | 76.8 (6.2) | 74.4 (4.8) |
| Sex, n (%) | ||||
| Male | 10 (63) | 8 (50) | 9 (60) | 8 (53) |
| Female | 6 (38) | 8 (50) | 6 (40) | 7 (47) |
| MMSE, mean (SE) | 17.1 (4.1) | 16.1 (5.4) | 11.8 (3.1) | 11.1 (3.9) |
| Disease duration (months), mean (SD) | 26.3 (14.1) | 28.2 (18.7) | 41.2 (19.4) | 45.3 (17.6) |
Abbreviations: MMSE, Mini Mental Status Examination; SD, standard deviation; SE, standard error of the mean.
Figure 2.
Mean changes from baseline for patients in the Galantamine Switch study using the MMSE, DAD, MENFIS, CMAI, and NPI.Notes: *P < 0.05 for the 24 mg group versus baseline; #P < 0.05 for the 16 mg group versus baseline (Mann-Whitney U-test). Error bars indicate the SE.Abbreviations: CMAI, Cohen-Mansfield Agitation Inventory; DAD, Disability Assessment for Dementia; MENFIS, Mental Function Impairment Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; SE, standard error of the mean.
Donepezil increase versus additional memantine study
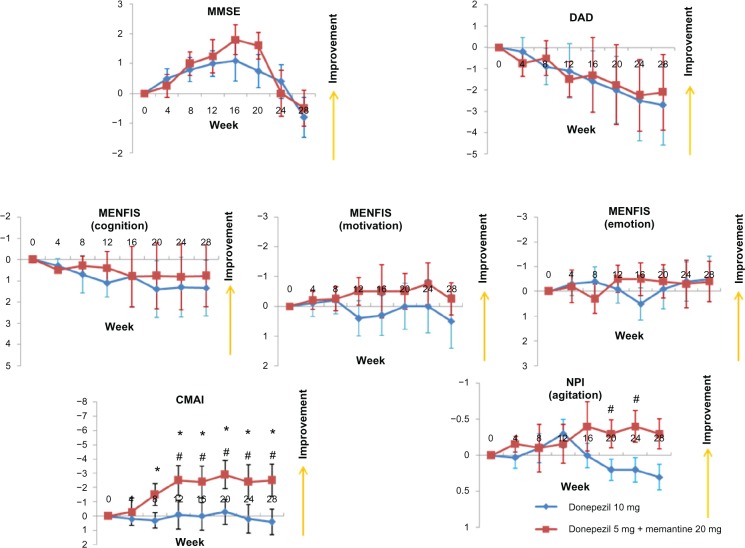
A total of 30 patients (donepezil increase group, n = 15; additional memantine group, n = 15) completed the study. Their demographic data are shown in Table 1. Similar to the Galantamine Switch study, there were no improvements from baseline in MMSE, DAD, or MENFIS scores, and there were no differences in these measures between the donepezil increase and the additional memantine groups (Figure 3). However, for the CMAI, the additional memantine group showed improvement after 8 weeks compared with baseline, and after 12 weeks, a significant difference was observed for the CMAI between the additional memantine and donepezil increase groups. Finally, agitation, as assessed using the NPI, improved significantly in the additional memantine group compared with the donepezil increase group at both 20 and 24 weeks.
Figure 3.
Mean changes from baseline for patients enrolled in the donepezil increase versus additional memantine study using the MMSE, DAD, MENFIS, CMAI, and NPI.Notes: *P < 0.05 for the additional memantine group versus baseline; #P < 0.05 for the additional memantine group versus the donepezil increase group (Mann-Whitney U-test). Error bars indicate the standard error of the mean.Abbreviations: CMAI, Cohen-Mansfield Agitation Inventory; DAD, Disability Assessment for Dementia; MENFIS, Mental Function Impairment Scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
Discussion
All patients included in this trial were on the acetylcholinesterase inhibitor, donepezil, and previous reports indicate that acetylcholinesterase inhibitors reduce behavioral abnormalities in patients with AD.20,21 Some patients with AD may not experience sustained clinical benefit from acetylcholinesterase inhibitor treatment owing to “lack-of-benefit” or “loss-of-response” after long-term treatment or tolerance issues. Therefore, changing acetylcholinesterase inhibitor therapy may benefit patients with AD who initially respond to acetylcholinesterase inhibitor treatment but then experience a decline in cognition, behavior, or activities of daily living, or who experience persistent adverse events.22 No adverse effects of the study drugs, such as nausea and vomiting, were reported in the Galantamine Switch study. In the DIAM study, only one patient (6.3%) in the donepezil increase group and two patients (11.8%) in the additional memantine group discontinued the study due to adverse drug effects. The incidence of adverse effects reported was less frequent than previously reported in patients receiving memantine alone.6
Donepezil and galantamine are widely used to slow the rate of cognitive and behavioral decline in patients with AD. These agents have the same mechanism of action, but galantamine also has allosteric-modulating activity at nicotinic receptors.5 Galantamine is effective and safe in patients with AD, regardless of previous exposure to acetylcholinesterase inhibitors. In a previous study, patients who were taking galantamine and had been previously exposed to an acetylcholinesterase inhibitor achieved significant improvements in cognition compared with those who received placebo.23 The sample size of the current study was limited, so no differences were seen for cognitive improvements from baseline. However, this study did demonstrate a reduction in agitation on the CMAI in the group that switched from donepezil to galantamine. The CMAI consists of 29 agitation behavior items with a total score in the range of 0–203; therefore, this scale is well suited for discriminating small changes in agitation behavior. The NPI also includes an agitation domain, but the scoring range is small (severity 0–3, frequency 0–4) and did not show any differences compared with baseline and when the two groups were compared. Similarly, Howard et al24 used the CMAI in patients with AD and reported that donepezil treatment for 12 weeks was not more effective than placebo for the treatment of agitation. Therefore, the results of this study suggest that switching from donepezil to galantamine is beneficial for improving agitation in patients with AD.
Galantamine is a rather weak acetylcholinesterase inhibitor, but has additional allosteric potentiating effects at nicotinic receptors. This allosteric potentiation contributes not only to neuronal protection against several neurotoxic stimuli but also to enhanced release of neurotransmitters, including dopamine, noradrenaline, glutamate, and γ-aminobutyric acid. In patients with AD, a positive correlation has been seen between aggressive behavior and magnitude of cell loss in the rostral locus coeruleus.25 The locus coeruleus is the major nucleus of origin of noradrenergic fibers in the mammalian brain. Therefore, galantamine-induced allosteric potentiation at nicotinic receptors may result in suppression of agitation.
Previous studies26,27 have reported that for patients with AD, addition of memantine to an established donepezil regimen resulted in better outcomes for cognition and activities of daily living, compared with placebo. Further, addition of memantine reduced agitation, irritability, and appetite/eating disturbances, as measured by the NPI. Memantine reduced agitation in patients who were agitated at baseline and delayed the emergence of agitation in those who were free of agitation at baseline. Data from the current study demonstrated a reduction in agitation in the additional memantine group compared with the donepezil increase group, using both the CMAI and NPI scales. The mechanism by which memantine produces psychotropic benefits is not clear. It is hypothesized that memantine blocks the excitotoxicity associated with chronic low-level glutamate stimulation.28 Glutamate excitotoxicity has been associated with the tau hyperphosphorylation required in the production of neurofibrillary tangles and is thought to be a significant executioner pathway. Neurofibrillary tangles have been linked to frontal lobe dysfunction and agitation,29,30 so it is likely that, through a decrease in glutamate toxicity and tau deposition, memantine would have an effect on frontally mediated behaviors.
The results of this study must be viewed in light of its limitations. This study was limited by small sample sizes. Previous reports have demonstrated the cognitive benefits of switching to galantamine from donepezil and the addition of memantine to donepezil.9,23,26,27 The current results demonstrated an improvement in suppression of agitation in patients in both the Galantamine Switch and DIAM studies, but improvements in cognition compared with baseline were not demonstrated.
Behavior symptoms in patients with AD increase the direct costs of care. For example, a one-point increase in the NPI score was calculated to be equivalent to an increased cost of $247–$409 in total annual direct costs.31 Therefore, the addition of memantine to established therapy is an effective way to suppress agitation compared with an increase in the donepezil dose. In summary, switching to galantamine from donepezil and addition of memantine in patients with AD receiving donepezil were both safe and meaningful treatment options, and particularly efficacious for suppression of agitation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44(9):1078–1081. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 2.Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15(3):346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 3.Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B. Maintaining functional and behavioral abilities in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15( Suppl 1):S34–S40. doi: 10.1097/00002093-200108001-00006. [DOI] [PubMed] [Google Scholar]
- 5.Samochocki M, Hoffe A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305(3):1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- 6.Reisberg B, Doody R, Stoffer A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 7.Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 9.Edwards K, Therriault O’Connor J, Gorman C. Switching from donepezil or rivastigmine to galantamine in clinical practice. J Am Geriatr Soc. 2004;52(11):1965. doi: 10.1111/j.1532-5415.2004.52529_3.x. [DOI] [PubMed] [Google Scholar]
- 10.Engedal K, Davis B, Richarz U, Han J, Schauble B, Andreasen N. Two galantamine titration regimens in patients switched from donepezil. Acta Neurol Scand. 2012;126(1):37–44. doi: 10.1111/j.1600-0404.2011.01594.x. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurolog y. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53(5):471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 14.Homma A, Niina R, Ishii T, Hasegawa K. Development of a new rating scale for dementia in the elderly: Mental function impairment scale (MENFIS) Japanese Journal of Geriatric Psychiatry. 1991;2(10):1217–1222. [Google Scholar]
- 15.Cohen-Mansfield J, Libin A. Assessment of agitation in elderly patients with dementia: correlations between informant rating and direct observation. Int J Geriatr Psychiatry. 2004;19(9):881–891. doi: 10.1002/gps.1171. [DOI] [PubMed] [Google Scholar]
- 16.Homma A, Usui M. Cohen-Mansfield Agitation Inventory (CMAI) Nihon Rinsho. 2004;62(Suppl 4):39–41. Japanese. [PubMed] [Google Scholar]
- 17.Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 18.Hirono N, Mori E, Ikejiri Y, et al. Japanese version of the Neu-ropsychiatric Inventory – a scoring system for neuropsychiatric disturbance in dementia patients. No to Shinkei. 1997;49(3):266–271. Japanese. [PubMed] [Google Scholar]
- 19.Schneider LS, Olin JT. Clinical global impressions in Alzheimer’s clinical trials. Int Psychogeriatr. 1996;8(2):277–288. doi: 10.1017/s1041610296002645. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry. 2000;157(1):4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(3):532–538. doi: 10.1176/appi.ajp.161.3.532. [DOI] [PubMed] [Google Scholar]
- 22.Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer’s disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003;19(8):707–714. doi: 10.1185/030079903125002450. [DOI] [PubMed] [Google Scholar]
- 23.Mintzer JE, Kershaw P. The efficacy of galantamine in the treatment of Alzheimer’s disease: comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. Int J Geriatr Psychiatry. 2003;18(4):292–297. doi: 10.1002/gps.826. [DOI] [PubMed] [Google Scholar]
- 24.Howard RJ, Juszczak E, Ballard CG, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med. 2007;357(14):1382–1392. doi: 10.1056/NEJMoa066583. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KL, Chen C P, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51(5):407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- 26.Cummings JL, Schneider E, Tariot PN, Graham SM. Memantine MEMMDSG. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67(1):57–63. doi: 10.1212/01.wnl.0000223333.42368.f1. [DOI] [PubMed] [Google Scholar]
- 27.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 28.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. Neuro Rx. 2004;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senanarong V, Cummings JL, Fairbanks L, et al. Agitation in Alzheimer’s disease is a manifestation of frontal lobe dysfunction. Dement Geriatr Cogn Disord. 2004;17(1–2):14–20. doi: 10.1159/000074080. [DOI] [PubMed] [Google Scholar]
- 30.Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49(3):355–361. [PubMed] [Google Scholar]
- 31.Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59(11):1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]