Abstract
The aim of this study was to determine whether pregabalin affects nociceptive behavior and central sensitization in a trigeminal neuropathic pain model. A partial infraorbital nerve transection (p-IONX) or sham operation was performed in adult male rats. Nociceptive withdrawal thresholds were tested with von Frey filaments applied to the bilateral vibrissal pads pre-operatively and post-operatively. On post-operative day 7, the behavioral assessment was conducted before and at 30 min, 60 min, 120 min, 180 min, and 24hr after pregabalin (0.1, 1, 10, 100 mg/kg, i.p.) or saline injection. The effects of pregabalin or saline were also examined on the mechanoreceptive field and response properties of nociceptive neurons recorded in the medullary dorsal horn at post-operative day 7–10. Reduced withdrawal thresholds reflecting bilateral mechanical allodynia were observed in p-IONX rats until post-operative day 28, but not in sham-operated rats. At post-operative day 7, pregabalin significantly and dose-dependently reversed the reduced mechanical withdrawal thresholds in p-IONX rats. Pregabalin also attenuated central sensitization of the neurons, as reflected in reversal of their reduced activation threshold, increased responses to pinch/pressure, and enhanced stimulus-response function. This study provides the first documentation that pregabalin attenuates the mechanical allodynia and central sensitization that characterize this trigeminal neuropathic pain model, and supports its clinical use for treating craniofacial neuropathic pain.
Keywords: pregabalin, allodynia, central sensitization, trigeminal neuropathic pain
Introduction
Neuropathic pain is defined as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system 47. Neuropathic pain patients may suffer from spontaneous or evoked pain, and treatment of the pain has often been inadequate 32,54. The gabapentinoid pregabalin has recently been shown to be efficacious in many neuropathic pain states. Pregabalin is a ligand to the a2δ subunit of the voltage-gated calcium channel, and binding at this site attenuates calcium influx at nerve terminals that reduces the release of several neurotransmitters involved in nociceptive transmission, such as glutamate and substance P 12,18,27,30,33. Recent animal studies suggest that pregabalin may impair the development or maintenance of spinal central sensitization underlying the hyperalgesic state 3,53. Pregabalin is effective not only in several types of neuropathic pain in humans 14,19,32,41,45 but also in rat models of neuropathic pain 5,17,27,34 and in other pain models21,35,57. A related gabapentinoid, gabapentin, has also been shown to be effective clinically and in animal pain models 2,10,15,32. Other than reports on its clinical effectiveness specific for trigeminal neuralgia,35,38 there have been no detailed clinical investigations of the effectiveness of pregabalin in human orofacial neuropathic pain states.
Such findings bearing on the clinical efficacy of gabapentin and pregabalin and their possible underlying mechanisms in animal pain models have come almost exclusively from studies of spinally related pain. There is very limited evidence of their actions and mechanisms in craniofacial pain states, even though chronic as well as acute craniofacial pain conditions are very common 28,29. Nociceptive behavior accompanied by central sensitization of nociceptive neurons in the medullary dorsal horn (also termed the trigeminal subnucleus caudalis)13,43 can be produced in animal models of craniofacial neuropathic pain 23,24,36. Although gabapentin has been shown effective in rat behavioral models of craniofacial inflammatory and neuropathic pain 9,20, pregabalin has not been tested on both nociceptive behaviour and central sensitization in animal models of craniofacial neuropathic pain. Therefore, the aim of the present study was to use a craniofacial neuropathic pain model involving partial transection of the infraorbital nerve 31 to determine whether pregabalin affects craniofacial nociceptive behavior and central sensitization in functionally identified nociceptive neurons in the rat’s medullary dorsal horn. Data have been partly reported in abstract form4.
Methods
Animals
Experiments were performed on adult male Sprague-Dawley rats weighing 280–400g (n=84, Charles River, Canada). The animals were housed in a temperature and humidity controlled environment on a 12 h light/dark cycle. Food and water were freely available. All surgeries and procedures were approved by the University of Toronto Animal Care Committee in accordance with the regulations of the Ontario Animal Research Act (Canada).
Infraorbital nerve transection
Under isoflurane anesthesia (5% induction, 2~2.5% maintenance), an incision was made in the right maxillary gingivo-buccal groove of the rats. The infraorbital nerve was exposed and dissected free from the surrounding tissue. As previously described by Miyamoto et al.31 and Xu et al.56, the lateral 1/2 of the nerve was lifted from the maxillary bone and cut with scissors in some rats to produce a partial transection of the nerve (p-IONX); care was taken not to damage facial nerve branches. In other rats, a sham operation was performed but without any nerve injury. After the surgery, the wounds were closed by sutures. The animals were returned to their home cages and fed with mash and chow.
Drug treatments
Pregabalin was dissolved in sterilized saline and administered i.p. at 2 ml/kg in the behavioral testing experiments (see below). P-IONX rats displaying mechanical allodynia (see below) following baseline behavioral testing were randomly placed into 5 different groups (n=6/group) and received a single administration of pregabalin (0.1, 1, 10, 100 mg/kg) or vehicle (saline) at post-operative day 7. This dose range of pregabalin was guided by our recent study showing that pregabalin at doses between 1–100mg/kg were effective in dose-dependently attenuating orofacial EMG activity and medullary release of glutamate evoked by noxious orofacial stimulation 33. The group of sham-operated rats (n=6) received a single administration of 10 mg/kg pregabalin at post-operative day 7. Since 1 mg/kg was the minimum effective dose in the behavioral testing (see Results), during the neuronal recording experiments, pregabalin at a dose of 3 mg/kg (or saline) was selected to be delivered after the baseline properties of each neuron were obtained (see below). The neuronal properties were also assessed at 30 and 60 min after drug administration, based on the time course reported in previous electrophysiological studies using systematically administered pregabalin 5,53.
Behavioral testing
The testing was similar to that used in previous studies 48,49. Briefly, 36 rats were first trained daily prior to surgery to remain in a plastic container and place their noses through a hole in the container. Mechanical withdrawal thresholds were determined at five locations bilaterally on the vibrissal pad by using von Frey filaments (North Coast Medical, Inc. Morgan Hill, USA). The lowest stimulus intensity, in ascending order, to evoke an escape response (i.e. head aversion and scratching) was taken as the withdrawal threshold. Filaments exerting forces greater than 15 g could not be used because their application moved the head before the hair bent, so 15 g was used as the cut-off value. In the group of p-IONX rats treated with saline (n=6) and the sham-operated group treated with pregabalin (n=6), behavioral tests were conducted at pre-operative days 1, 2, and 3 and at post-operative days 1, 3, 5, 7, 10, 14, 21, 28, and 35. In the groups of p-IONX rats treated with different doses of pregabalin (n=24), behavioral tests were conducted at pre-operative days 1, 2, and 3 and at post-operative days 1, 3, 5, 7. On post-operative day 7, the time at which p-IONX rats displayed significant mechanical allodynia (see Results), the behavioral assessments were conducted in all the rats (n=36) from the p-IONX and sham-operated groups before and at 30 min, 60 min, 120 min, 180 min, and 24 hr after injection of pregabalin or saline.
Neuron Recording & Stimulation Procedures
The methods used for animal preparation, stimulation, neuronal recording and classification were similar to those described previously in detail 6,7,55 and so will only be briefly outlined here. As well as the rats used in the behavioral testing experiments outlined above, additional rats were used to provide a total of 48 rats (24 p-IONX rats, 24 sham-operated rats) that were used for the neuronal recording experiments at post-operative days 7–10 (shown in the behavioral experiments to be around the time of peak mechanical allodynia); behavioral testing was also carried out in the 24 p-IONX rats before the neuronal recording experiments to confirm the existence of mechanical allodynia (withdrawal threshold <2g). Each rat was anesthetized by a single injection i.p. of a mixture of α-chloralose (50 mg/kg) and urethane (1 g/kg). Then the left external jugular vein was cannulated, and a tracheal cannula was inserted. The caudal medulla was surgically exposed after the rat was placed in a stereotaxic apparatus, and the dura and subarachnoid membrane were removed. Just before the start of the neuronal recording session, a supplemental dose of urethane (200–300 mg/kg, i.v.) was administered. Then the rat was paralyzed with intravenous pancuronium bromide injection [initial dose, 0.3– 0.4 ml of 1 mg/ml solution, followed by a continuous intravenous infusion of a mixture of 70% urethane solution (0.2 g/ml) and 30% pancuronium solution (1 mg/ml) at a rate of 0.3–0.4ml/h] and artificially ventilated throughout the whole experimental period. A deep level of anesthesia was judged periodically by the lack of spontaneous movements and responses to paw pinching when pancuronium-induced muscle paralysis was allowed to wear off. Heart rate, percentage expired CO2, and rectal temperature were constantly monitored and maintained at physiological levels of 333–430 beats/min, 3.5– 4.5%, and 37–37.5°C, respectively.
Single neuronal activity was recorded extracellularly by means of an epoxy resin-coated tungsten microelectrode. As the microelectrode was advanced with a rostral inclination of 23° into the right caudal medulla, 1.4 –1.8 mm lateral to the midline and 1.5–2.0 mm behind the obex, stimuli (see below) were applied to the craniofacial tissues to search for nociceptive neurons in the medullary dorsal horn that received a craniofacial sensory input. Neuronal activity was amplified, displayed on oscilloscopes, and led to a window discriminator and an analog-to-digital converter (CED 1401 plus; Cambridge Electronic Design, Cambridge, UK) connected to a personal computer running Spike2 software (Cambridge Electronic Design), which digitized and stored the triggered action potentials. Data were analyzed off-line with this same software.
A wide range of mechanical (brush, pressure, and pinch), and noxious thermal (radiant heat; 51–53°C) stimuli were applied to the craniofacial region to classify each neuron as low-threshold mechanoreceptive (LTM), wide dynamic range (WDR), or nociceptive specific (NS) 6,7,55. Only WDR and NS neurons were further tested in this study since LTM neurons do not show central sensitization 6,43. The average spontaneous activity (in Hz) of a nociceptive neuron was determined over the initial 3–5 min recording period. As outlined in our previous studies 6,7,55, the cutaneous tactile mechanoreceptive field (RF) of WDR neurons in the rat medullary dorsal horn is characteristically large (>10mm2) and with a clear boundary and long-lasting evoked busting discharges, and was determined by brushing the skin with a force < 2 g. The cutaneous pinch/pressure RF of each WDR (and NS) neuron was determined through the use of a blunt probe and a pair of non-serrated forceps. A burst response consisting of at least two spikes during each stimulus trial was accepted as the criterion for the RF boundary of the neuron tested. Noxious cutaneous stimulation was used sparingly so as to avoid damage to the skin and the production of peripheral sensitization. The pinch RF of NS neurons is typically more localized than that of WDR neurons but NS neurons can also manifest a very localized tactile RF (<2mm2) following injury or inflammation of orofacial tissues 6,7,49. The activation threshold to a mechanical stimulus applied to the craniofacial RF was also assessed for NS neurons, by using a pair of force-monitoring forceps; in addition, as the mechanical force was gradually increased, the responses of the tested neuron were monitored and recorded simultaneously by the use of a Spike2 program (CED 1401 plus). The neuronal responses to a mechanical pinch stimulus (100g for NS, 40g for WDR) were determined with the force-monitoring forceps applied to the neuronal craniofacial RF. Graded mechanical stimuli were delivered by means of von Frey filament applications at non-noxious force levels (0.4g, 1g, 2g) and at noxious levels (6g, 15g, 26g, 60g, 100g), in ascending order, each for 2 s at an interval of >45 s applied to the RF so as to determine the stimulus-response function 6. The responses were quantified as the mean frequency during the 2s of stimulation.
Statistical analyses
Data were tested for normality (Kolmogorov–Smirnov test) and equal variance. Data are reported as mean±SEM. For the behavioral tests, differences in threshold between the baseline (average of the three pre-operative day values) and each post-operative day were treated by one-way ANOVA followed by the Bonferroni post-hoc test. The effects of pregabalin at different doses compared with saline (as vehicle control) were tested by univariate analysis followed by the Bonferroni post-hoc test. For neuronal recordings, differences in spontaneous activity frequency, RF area, mechanical activation threshold and pinch/pressure-evoked responses between the baseline values (values before pregabalin or saline administration at post-operative day 7–10) of the p-IONX/pregabalin group, the sham-operated group and the p-IONX/saline group were treated by one-way ANOVA followed by the Bonferroni post-hoc test. Differences in spontaneous activity frequency, RF area, mechanical activation threshold and pinch/pressure-evoked responses between the baseline value and values at different post-delivery time points in each group were treated by one-way ANOVA followed by Bonferroni or Dunnett’s post-hoc tests. Differences in stimulus-response functions were treated by univariate analysis followed by Bonferroni or Dunnett’s post-hoc tests. Calculations were carried out by means of SPSS12.0. P <0.05 were considered to reflect statistical significance.
Results
IONX induced behavioral changes and central sensitization
Behavioral features
The withdrawal thresholds to mechanical stimulation of the left and right vibrissal pads were examined after the p-IONX or sham operation. Compared to baseline values, p-IONX rats showed significant decreases of mechanical withdrawal thresholds on both the ipsilateral (one-way ANOVA: F=17.67, p<0.001) and contralateral (one-way ANOVA: F=9.27, p<0.001) sides, while the thresholds of sham-operated rats did not significantly change (one-way ANOVA, ipsilateral: F=1.05, p=0.42; contralateral: F=1.40, p=0.21). The decreases of withdrawal thresholds in the p-IONX group were considered to reflect mechanical allodynia and were significant at post-operative day 1, peaked at post-operative days 5–10 and were maintained to post-operative day 28 before returning to baseline levels at post-operative day 35 (Fig. 1).
Fig. 1.
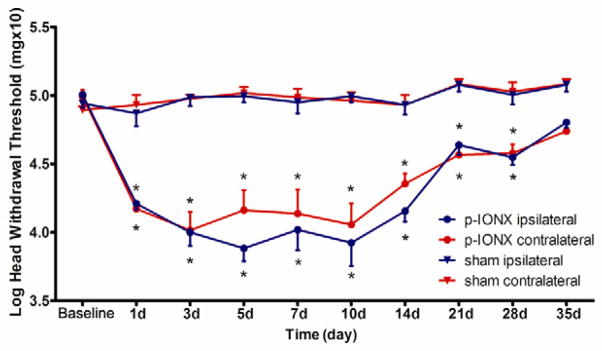
Time course of the mechanical head withdrawal thresholds after p-IONX in bilateral vibrissal pads. In the sham group (n=6), the threshold did not differ from pre-surgery values in bilateral sides. In the p-IONX group (n=6), bilateral mechanical allodynia was established at the day 1 after surgery. The mechanical allodynia persisted until postoperative days 28 and returned to the level of sham group at 35 days following p-IONX. * p < 0.05 for comparison between the baseline value and values at the different time points after p-IONX (one-way ANOVA). All values shown as mean±SEM.
Neuronal properties
Forty-eight functionally identified NS (n=24) and WDR (n=24) neurons responding to ipsilateral craniofacial noxious stimulation were studied at post-operative day 7–10. All of these neurons in the 3 study groups were located in the deep laminae of the medullary dorsal horn (Fig 2), at an average depth of 919±24μm (min 598μm, max 1180μm). Sixteen neurons classified as NS (n=8) or WDR (n=8) neurons were recorded in p-IONX rats and during their recording were treated with pregabalin (p-IONX/pregabalin group); another 16 neurons (eight NS, eight WDR) were from sham-operated rats that were also treated with pregabalin (sham-operated/pregabalin group); the remaining 16 neurons (eight NS, eight WDR) were from p-IONX rats and were treated with the vehicle saline (p-IONX/saline group). All 24 NS neurons (eight from each group) had at baseline an ipsilateral pinch/pressure RF that for most was located in the maxillary division of the trigeminal nerve, involving the vibrissal pad, periorbital region, or nose (Table. 1, Fig. 3). All 24 WDR neurons (eight from each group) had at baseline an extensive ipsilateral craniofacial pinch/pressure RF involving the periorbital and perioral regions and a tactile RF located within the boundary of the pinch/pressure RF. All eight WDR neurons in the sham-operated/pregabalin group had a baseline spontaneous activity, but no spontaneous activity was detected in NS neurons of the sham-operated/pregabalin group.
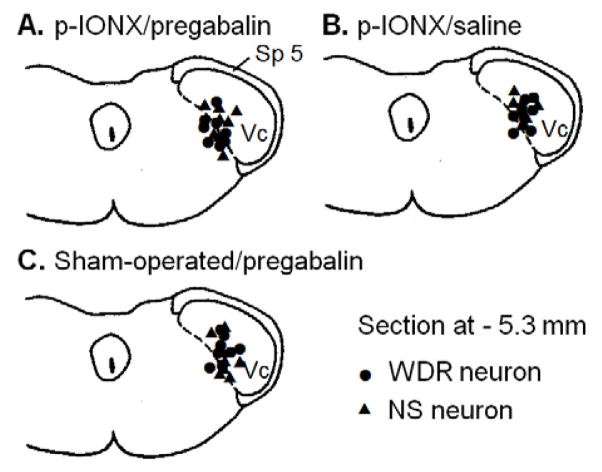
Fig. 2.
Histologically confirmed neuronal recording sites in the p-IONX/pregabalin group (A), the p-IONX/saline group (B), and the sham-operated/pregabalin group (C). The sites were plotted onto a section of the caudal medulla (−5.3 mm behind interaural line). Abbreviations: Sp 5: Trigeminal spinal tract; Vc: trigeminal subnucleus caudalis (MDH: medullary dorsal horn); Dot: WDR neuron; Triangle: NS neuron.
Table 1.
Effects of pregabalin on features of p-IONX induced central sensitization in MDH nociceptive neurons
| Group | Spontaneous activity
|
Tactile RF (cm2) | Pinch RF (cm2) | Mechanical activation threshold (g) | Responses to graded stimuli (sum of spikes) | |
|---|---|---|---|---|---|---|
| Frequency (Hz) | Incidence | |||||
| Sham/Pregabalin | ||||||
| NS neuron (n=8) | ||||||
| baseline | 0 | 0/8 | 1.5±0.3 | 52.6±6.8 | 75.1±16.2 | |
| 30 min | 0 | 0/8 | 1.5±0.3 | 50.4±6.3 | 74.8±22.9 | |
| 60 min | 0 | 0/8 | 1.5±0.3 | 54.3±5.8 | 51.9±6.3 | |
| Drug effect | F=0. 01, p=0.99 | F=0.10, p=0.91 | F=0.65, p=0.54 | |||
| WDR neuron (n=8) | ||||||
| baseline | 0.4±0.2 | 8/8 | 0.75±0.18 | 3.7±0.4 | 62.3±13.9 | |
| 30 min | 0.3±0.2 | 8/8 | 0.75±0.18 | 3.6±0.4 | 47.0±13.7 | |
| 60 min | 0.3±0.1 | 8/8 | 0.75±0.18 | 3.5±0.5 | 35.0±10.6 | |
| Drug effect | F=0.07, p=0.93 | F=0.05, p=0.95 | F=0. 05, p=0.95 | F=1.04, p=0.37 | ||
| IONX/Pregabalin | ||||||
| NS neuron (n=8) | ||||||
| baseline | 0.06±0.05 | 3/8 | 3.2±0.8 | 22.8±1.7 ## | 150.5±16.3 # | |
| 30 min | 0.06±0.05 | 3/8 | 2.7±0.8 | 51.3±7.6 | 69.3±16.0 ** | |
| 60 min | 0.03±0.05 | 3/8 | 2.0±0.5 | 64.8±12.5 ** | 39.1±8.5 ** | |
| Drug effect | F=0.16, p=0.85 | F=2.97, p=0.48 | F=6.37, p=0.007 | F=16.75, p=0.001 | ||
| WDR neuron (n=8) | ||||||
| baseline | 2.0±1.1 | 8/8 | 1.01±0.25 | 5.5±1.1 | 127.4±19.6 # | |
| 30 min | 0.6±0.4 | 8/8 | 0.91±0.35 | 4.2±1.1 | 55.5±14.1** | |
| 60 min | 0.6±0.4 | 8/8 | 0.71±0.24 | 3.9±1.1 | 44.6±9.4** | |
| Drug effect | F=1.27, p=0.30 | F=0.32, p=0.73 | F=0. 61, p=0.56 | F=9.05, p=0.001 | ||
| IONX/Saline | ||||||
| NS neuron (n=8) | ||||||
| baseline | 0.01±0.01 | 3/8 | 2.9±0.3 | 28.4±3.0 # | 150.3±16.5 # | |
| 30 min | 0.13±0.09 | 3/8 | 2.8±0.3 | 29.8±3.0 | 145.6±16.1 | |
| 60 min | 0.07±0.05 | 3/8 | 2.8±0.3 | 25.0±3.1 | 159.5±38.6 | |
| Drug effect | F=0.89, p=0.43 | F=0. 02, p=0.98 | F=0.64, p=0.54 | F=0.21, p=0.81 | ||
| WDR neuron (n=8) | ||||||
| baseline | 1.4±0.9 | 8/8 | 0.86±0.23 | 5.8±0.9 | 126.6±19.7 # | |
| 30 min | 0.4±0.2 | 8/8 | 0.92±0.29 | 5.8±1.0 | 119.6±19.1 | |
| 60 min | 1.0±0.6 | 8/8 | 0.95±0.27 | 6.0±1.0 | 135.6±18.2 | |
| Drug effect | F=0.67, p=0.52 | F=0.03, p=0.97 | F=0.08, p=0.99 | F=0.17, p=0.84 | ||
| Statistical between baseline of 3 groups | ||||||
| NS neuron | F=1.17, p=0.33 | F=3.35, p=0.06 | F=12.89, p=0.001 | F=4.58, p=0.02 | ||
| WDR neuron | F=0.90, p=0.42 | F=0.34, p=0.71 | F=1.68, p=0.21 | F=7.07, p=0.004 | ||
All values shown as mean±SEM.
Drug effect - comparison between the baseline value and values at the different times after Pregabalin or saline in each group,
p<0.05,
p<0.01 (One-way ANOVA followed by the Bonferroni post-hoc test).
p<0.05,
p<0.01 for comparison between the baseline values (values before pregabalin or saline administration at post-operative day 7–14) of the Sham/Pregabalin group and those of the p-IONX/Pregabalin group or the p-IONX/Saline group (One-way ANOVA followed by the Bonferroni post-hoc test).
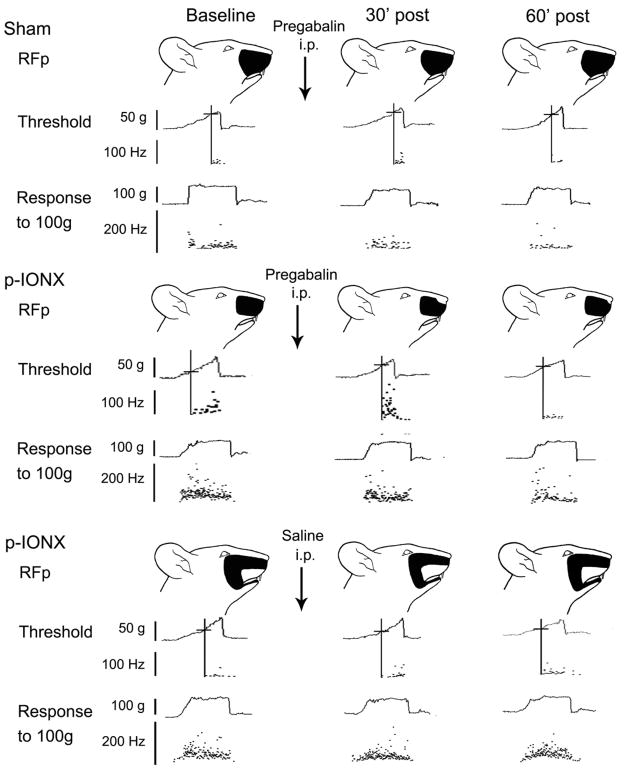
Fig. 3.
Examples showing change of central sensitization in NS neurons after Pregabalin (3mg/kg) i.p. to p-IONX group and sham group or saline to p-IONX group. For each example, the pinch RF (top), activation threshold (middle), and responses to pinch/pressure stimuli (bottom) are shown. Data at baseline, 30 min and 60 min after pregabalin or saline injection of each NS neuron are arranged in columns from left to right. Note that the NS neurons illustrated from both the p-IONX/pregabalin and p-IONX/saline groups showed neuroplastic changes at baseline, comparing to sham-operated/pregabalin group. The cutoff (vertical and horizontal) line shows the activation threshold of a given neuron, and the neuronal discharges are displayed in an interspike instantaneous frequency distribution. The unit responses to pinch/pressure stimuli (100g) are also shown in the instantaneous frequency distribution during the 5 s stimulation period. The black area indicates the pinch/pressure RF.
The NS neurons showed evidence of central sensitization following p-IONX. Comparison between the baseline values of the p-IONX/pregabalin group and the sham-operated/pregabalin group at post-operative day 7–10 revealed that the mechanical activation threshold of NS neurons in the p-IONX rats had decreased significantly (p=0.007) (Table. 1, Fig. 4A); the pinch/pressure-evoked responses increased significantly (p=0.001) (Table. 1, Fig. 4B), and the stimulus-response function was also increased significantly (p-IONX/pregabalin group vs. sham-operated/pregabalin: p=0.001) (Fig. 5A, B). The baseline values of the threshold, pinch/pressure-evoked responses, and stimulus-response function of NS neurons in the p-IONX/saline group had similar features as the p-IONX/pregabalin group (Table.1, Fig. 4, 5A, C). In the two p-IONX groups, the incidence of neurons responding to stimuli below 2g was 100%, while the incidence was 0% in the sham-operated/pregabalin group (Fig. 5A, B, C). Five of eight NS neurons in the p-IONX/pregabalin group and four of eight NS neurons in the p-IONX/saline group had a very localized tactile RF (<2mm2) at baseline, but this was not a characteristic of the sham-operated/pregabalin group. While no NS neurons in the sham-operated/pregabalin group showed spontaneous activity at baseline, three of eight NS neurons from each of the two p-IONX groups showed such spontaneous activity (Table.1).
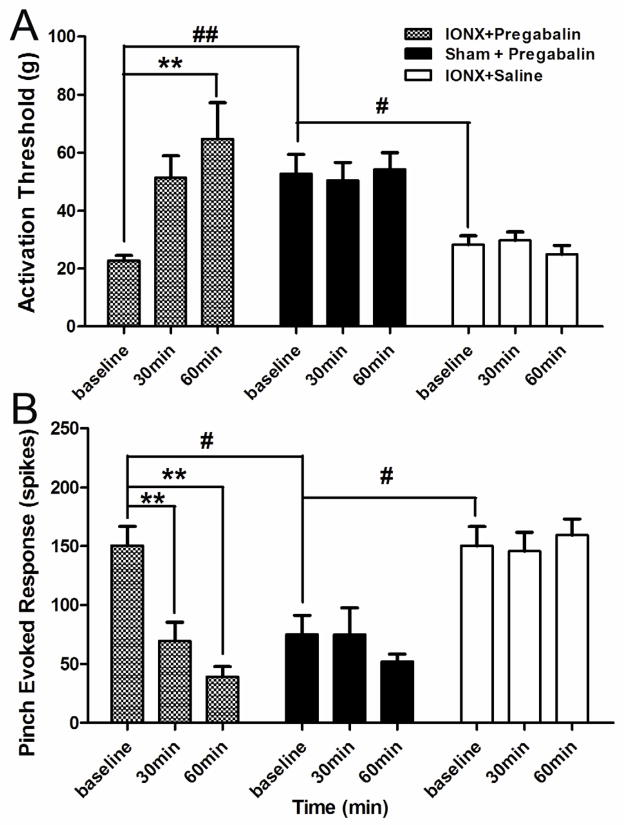
Fig. 4. Changes in mechanical activation threshold and responses to graded mechanical stimuli of NS neurons from p-IONX/pregabalin group, sham-operated/pregabalin group and p-IONX/saline group. Each group consists of eight NS neurons.
A. The baseline thresholds of p-IONX/pregabalin group and p-IONX/saline group showed significantly decreases in comparison to sham/pregabalin group. (one-way ANOVA followed by the Bonferroni post-hoc test, p-IONX/pregabalin group vs. sham-operated/pregabalin: p=0.01; p-IONX/saline group vs. sham-operated/pregabalin: p=0.01). In the p-IONX/pregabalin group, pregabalin produced significant increases in threshold during the 60 min observation period (one-way ANOVA followed by Bonferroni post hoc test, baseline vs. 30 min: p=0. 081; baseline vs. 60 min: p=0.007); in the sham/pregabalin group, pregabalin no longer produced increases in thresholds (one-way ANOVA: F=0.10, p=0.91); in the p-IONX/saline group, saline had no effect on mechanical activation threshold of NS neurons (one-way ANOVA:, F=0.64, p=0.54).
B. In the p-IONX/pregabalin group, pregabalin significantly decreased the response to pinch at 60 min (one-way ANOVA followed by Bonferroni post hoc test, baseline vs. 30 min: p=0. 002; baseline vs. 60 min: p<0.001); in the sham-operated/pregabalin group, pregabalin produced decrease in pinch response but the decrease was not significant. (one-way ANOVA: F=0.65, p=0.54); saline had no effect on pinch response of NS neurons after p-IONX (one-way ANOVA: F=0.21, p=0.81). All values are shown as mean±SEM.
Fig. 5.
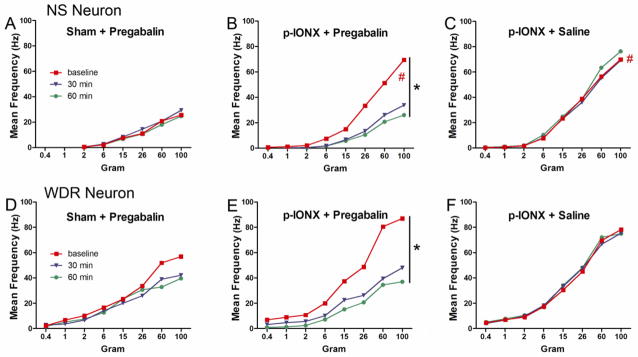
Each figure showed the stimulus-responses functions of NS and WDR neurons from 3 groups. Each stimulus-responses function was determined from the neuronal responses to graded mechanical stimuli applied to the neuronal RF. The functions increased significantly after p-IONX. (#: Compared between baselines of p-IONX/pregabalin group or p-IONX/saline group and sham/pregabalin group. Univariate analysis, p-IONX/pregabalin group vs. sham-operated/pregabalin group: p<0.001; p-IONX/saline group vs. sham-operated/pregabalin group: p<0.001). B. In the p-IONX/pregabalin group, the curves of NS neuron stimulus-response function after pregabalin administration shifted downward significantly (Univariate analysis, baseline vs. 30 min: p=0. 003; baseline vs. 60 min: p<0.001), suggesting that pregabalin depressed the response of NS neurons to the stimuli. Note the curves also shifted to the right, which implies an increase of the response threshold. E. The downward shift of the curves in WDR neurons was also significantly (Univariate analysis, baseline vs. 30 min: p=0.002; baseline vs. 60 min: p<0.001). Pregabalin had no significant effect on stimulus-responses function of either NS or WDR neurons in the sham-operated/pregabalin group (A and D). Saline also did not affect the stimulus-responses function of either NS or WDR neurons in the p-IONX/saline group (C and F)
The WDR neurons also showed evidence of central sensitization after p-IONX. The baseline values of the pinch/pressure-evoked responses of the p-IONX/pregabalin group and p-IONX/saline group increased significantly compared to the sham-operated/pregabalin group (p-IONX/pregabalin group: p=0.001; p-IONX/saline group: p=0.004) (Table. 1, Fig. 6). The stimulus-response function increased in both p-IONX/pregabalin group and p-IONX/saline group, although this change was not significant [p-IONX/pregabalin group vs. sham-operated/pregabalin group: p=0.06; p-IONX/saline group vs. sham-operated/pregabalin group: p=0.37) (Fig. 5D, E, F)]. No significant changes in WDR neuronal spontaneous activity frequency, tactile RF size or pinch RF size were observed in the two p-IONX groups compared to the sham-operated/pregabalin group.
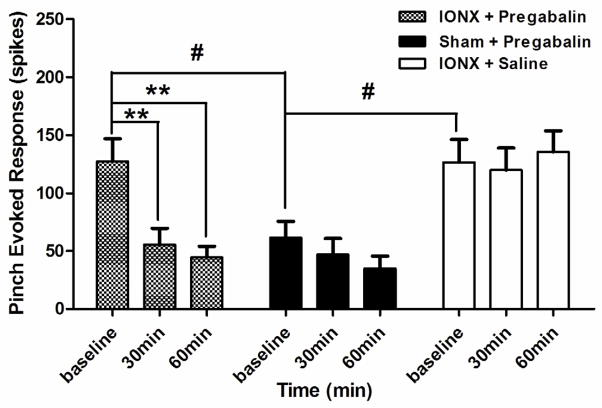
Fig. 6.
Changes in responses to graded mechanical stimuli of WDR neurons from p-IONX/pregabalin group, sham/pregabalin group and p-IONX/saline group. Each group consists of eight WDR neurons. In the p-IONX/pregabalin group, pregabalin significantly decreased the response to pinch at 60 min (one-way ANOVA followed by Bonferroni post hoc test, baseline vs. 30 min: p=0.008; baseline vs. 60 min: p=0.002); in the sham-operated/pregabalin group, pregabalin produced decrease in pinch response but the decrease was not significant (one-way ANOVA: F=1.04, p=0.37); saline had no effect on pinch response of WDR neurons after p-IONX (one-way ANOVA: F=0.17, p=0.84). All values are shown as mean±SEM.
Pregabalin effects on maintenance of mechanical allodynia and central sensitization
Behavioral test
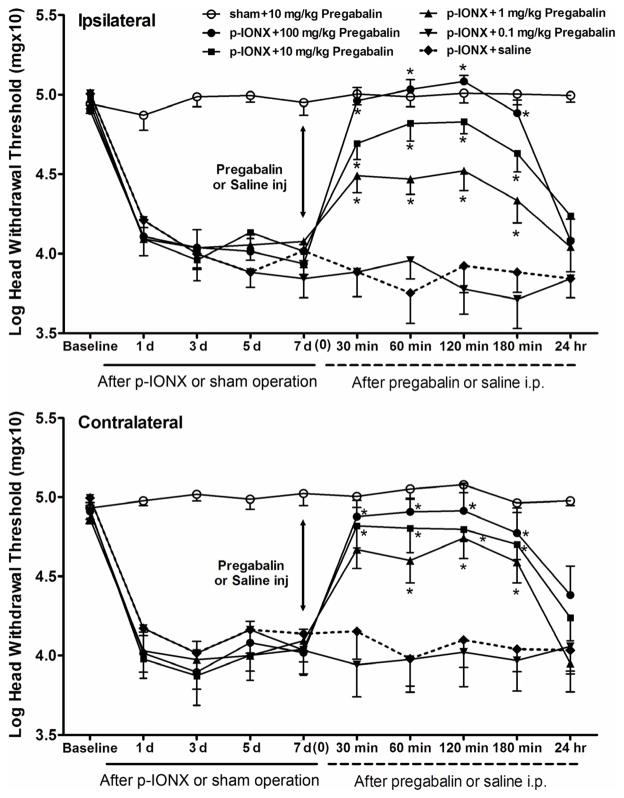
To investigate the effects on the mechanical withdrawal threshold of pregabalin in p-IONX and sham rats, pregabalin (0.1, 1, 10, or 100 mg/kg i.p.) or saline (as vehicle control) was administrated at post-operative day 7. Saline did not affect the mechanical threshold in the p-IONX/saline group (comparison between before and after saline administration, ipsilateral: F=0.94, p=0.50; contralateral: F=0.23, p=0.98) (Fig. 7). However, 1, 10, and 100 mg/kg pregabalin significantly reversed the reduced mechanical threshold bilaterally when compared with saline, while 0.1mg/kg pregabalin had no effect (ipsilateral: F=73.1, p<0.001; contralateral: F=38.6, p<0.001. post-hoc: 1, 10, 100 mg/kg vs saline, p<0.001, 0.1 mg/kg vs saline, p>0.999) (Fig. 7). The pregabalin effect on withdrawal threshold was dose-dependent and was maintained for up to 3 hours after pregabalin delivery but had dissipated within 24 hours. We also observed that pregabalin caused slight lethargy and decreased motor activity tested only at the highest dose used in the behavioral test (100 mg/kg i.p.). Pregabalin (10 mg/kg) had no effect on withdrawal threshold in the sham-operated group (ipsilateral: F=0.45, p=0.90; contralateral: F=0.56, p=0.83) (Fig. 7).
Fig. 7.
The effects of pregabalin on p-IONX induced mechanical allodynia in bilateral vibrissal pads. On post-operative day 7, the behavioral assessment was conducted before and at 30 min, 60 min, 120 min, 180 min, and 24 hr after injection of pregabalin (0.1, 1, 10, 100 mg/kg i.p., n=6 for each group) or isotonic saline. Pregabalin (1 to 100 mg/kg i.p.) significantly increased the mechanical withdrawal thresholds when compared with saline, while 0.1mg/kg pregabalin had no effect. (Univariate analysis followed by the Bonferroni post-hoc test, ipsilateral: F=73.1, p<0.001; contralateral: F=38.6, p<0.001. post hoc: 1, 10, 100 mg/kg vs saline, p<0.001, 0.1 mg/kg vs saline, p>0.999). All values are shown as mean±SEM.
Neuronal properties
In the NS neurons of the p-IONX/pregabalin group, compared to baseline values (values before pregabalin administration), pregabalin delivered at post-operative day 7–10 significantly reversed the lowered mechanical activation threshold typical of this group (see above) at 60 min (baseline vs. 30 min: p=0. 08; baseline vs. 60 min: p=0.007) (Table. 1, Fig. 3, 4A). Pregabalin also significantly reduced the elevated pinch/pressure-evoked responses in this group at 30 and 60 min (baseline vs. 30 min: p=0.002; baseline vs. 60 min: p=0.001) (Table. 1, Fig. 3, 4B). In addition, the elevated stimulus-response function of the p-IONX/pregabalin group shifted downward significantly after pregabalin administration (baseline vs. 30 min: p=0. 003; baseline vs. 60 min: p=0.001) (Fig. 5B). The curve also shifted to the right, consistent with the increase of the response threshold (Fig. 5B). No significant changes occurred in both the spontaneous activity incidence and the discharge frequency. Although the tactile RF seen in some NS neurons in the pIONX/pregabalin group at baseline disappeared after administration of pregabalin, no significant change was observed in pinch/pressure RF size. Pregabalin also significantly reduced the elevated pinch/pressure-evoked responses of WDR neurons in the p-IONX/pregabalin group at 30 and 60 min (baseline vs. 30 min: p=0. 008; baseline vs. 60 min: p=0.002) (Table. 1, Fig. 6), and attenuated the tactile-evoked responses (Fig 5E); in addition, the stimulus-response function was shifted downward significantly (baseline vs. 30 min: p=0.002; baseline vs. 60 min: p=0.001) (Fig. 5E), whereas pregabalin did not affect the stimulus-response function in WDR neurons in sham-operated/pregabalin groups. Pregabalin had no significant effect on WDR neuronal RF size and spontaneous activity (Table 1).
In the p-IONX/saline group, saline had no significant effect on mechanical activation threshold (NS: p=0.54) (Table. 1, Fig. 3, 4A), responses to pinch/pressure stimuli (NS: p=0.81; WDR: p=0.84) (Table. 1, Fig. 3, 4B, 6) and the stimulus-response function (baseline vs. 30 min: p>0.99; baseline vs. 60 min: p=0.99) (Fig. 7C, F) of both the NS and WDR neurons. Saline also had no significant effect on spontaneous activity and size of RF in NS and WDR neurons (Table. 1, Fig 3). In the sham-operated/pregabalin group, the activation threshold (NS: p=0.91) (Table. 1, Fig. 3, 4A), responses to pinch/pressure stimuli (NS: p=0.54; WDR: p=0.37) (Table. 1, Fig. 4B,6), stimulus-response function (NS: p=0.65; WDR: p=0.19) (Fig. 5A, D), tactile RF (WDR: p=0.99) and pinch/pressure RF (NS: p=0.99; WDR: p=0.95) (Table. 1, Fig. 3) were not significantly altered after pregabalin application.
Discussion
This is the first study to document that partial damage of the rat ION induces bilateral facial mechanical hypersensitivity indicative of mechanical allodynia that is accompanied by a central sensitization in functionally identified NS and WDR nociceptive neurons in the medullary dorsal horn. It also demonstrates for the first time that pregabalin is effective in attenuating mechanical allodynia and the trigeminal central sensitization in functionally identified nociceptive neurons of the medullary dorsal horn in this rat model of trigeminal neuropathic pain. Pregabalin did not affect baseline behavioral mechanical sensitivity and the baseline RF and response properties of the nociceptive neurons.
Bilateral sustained mechanical allodynia can be induced by p-IONX
Several trigeminal neuropathic pain models have been developed to study the neural mechanisms underlying craniofacial neuropathic pain conditions 1,22–24,31,39,44,48,52,56. Following a chronic constriction injury of the infraorbital nerve (CCI-ION), rats and mice exhibit a prolonged heat or mechanical hyperalgesia and mechanical allodynia in the vibrissal pad 22,52,56. Iwata and colleagues have reported that the mechanical thresholds in the ipsilateral vibrissal pad area are significantly lower than those of sham-operated rats for 4 weeks following inferior alveolar nerve transection, and that the contralateral side also shows transient decreases in mechanical thresholds 23,24,36. In a mouse p-IONX model, mechanical allodynia is also observed bilaterally 31. The p-IONX rats in the present study also showed mechanical allodynia reflected on both sides of the face as a lowered mechanical withdrawal threshold that began at post-operative day 1, peaked at post-operative day 5–10, which was maintained to day 28, and returned to baseline levels by postoperative day 35. The extent and time course of the behavioral changes induced by p-IONX in our study, including the extra-territorial spread to the contralateral side, are consistent with those reported in these neuropathic pain models. Recent studies reported that minocycline, an inhibitor of microglia, attenuates the development of extra-territorial pain hypersensitivity in a trigeminal neuropathic pain model39 and that administration of the astrocyte inhibitor fluoroacetate also could attenuate extra-territorial allodynia following inferior alveolar nerve transection36. Thus, the extra-territorial pain reported in the trigeminal neuropathic pain models might involve glial cell activation, as suggested in a recent review 8.
Central sensitization can be induced by p-IONX
Relatively little is known about the peripheral and central neuronal changes that occur after trigeminal nerve injury. The allodynia and hyperalgesia that has been reported in trigeminal neuropathic pain models is accompanied by significant increases in WDR spontaneous activity, mechanically evoked responses, and RF size, and are suggestive of neuroplastic changes reflecting central sensitization in the medullary dorsal horn 23,24,36,42,44. Furthermore, a study utilizing the inferior alveolar nerve transection model showed that increases in excitability of WDR neurons receiving afferent inputs from the reinnervated inferior alveolar nerve were long-lasting and were associated with changes in nocifensive behavior 42. Consistent with these results, the WDR neurons in the present study exhibited central sensitization as reflected in significantly increased spontaneous activity and responses to mechanical non-noxious and noxious stimuli. Previous studies of our laboratory have also documented the occurrence of central sensitization of NS neurons in an acute craniofacial pain model6,7, and similar changes in spinal dorsal horn NS neurons associated with central sensitization in different pain models have also been documented 25,40,50,51. In the present study, NS neurons in the medullary dorsal horn showed central sensitization following p-IONX, with heightened excitability reflected especially in reduced mechanical activation threshold and enhanced responses to graded noxious stimuli applied to the neuronal RF. Spontaneous activity of NS neurons was rarely seen in naïve rats in our previous studies, and similar features were noted in the sham-operated group in the present study. However, there was an increased incidence of spontaneous activity in NS neurons in the p-IONX group, although the sample size was small.
Pregabalin can attenuate the nociceptive behavior and central sensitization of nociceptive neurons induced by p-IONX
Several studies in the spinal nociceptive system have shown that pregabalin reduces nociceptive responses in rat pain models at varying doses: neuropathic pain, 10 to 30 mg/kg, i.v.5, 3–30mg/kg, p.o.17, 80mg/kg i.p.34; visceral allodynia, 79 mg/kg s.c. or 63 mg/kg p.o.11; muscle hyperalgesia, 10 to 30 mg/kg i.p.57; acute inflammation, 6 mg/kg p.o.21. In addition, we have recently shown that pregabalin at doses between 1–100mg/kg is effective in dose-dependently attenuating orofacial EMG activity and medullary release of glutamate evoked by noxious orofacial stimulation33. Our study similarly showed that pregabalin at 1, 10 and 100 mg/kg (i.p.) dose-dependently reversed at post-operative day 7 the p-IONX-induced lowered mechanical threshold reflecting a facial mechanical allodynia. At the highest dose used in our study (100 mg/kg pregabalin), we observed slight lethargy and decreased motor activity in some rats, which is consistent with the findings of Yokoyama et al (2007)57. Although pregabalin has been shown to be effective in several types of spinal neuropathic pain states14,19,32,41,45 and in rat models5,17,27,34 our results provide the first documentation that pregabalin attenuates the mechanical hypersensitivity manifested in a rat trigeminal neuropathic pain model.
Pregabalin has been shown to attenuate spinal dorsal horn neuronal nociceptive responses in rat neuropathic pain models3,53. In their study of spinal dorsal horn neuronal hyperexcitability, Wallin et al. (2002) found that pregabalin or gabapentin enhanced the spinal cord stimulation-induced suppression of the hyperexcitability of WDR neurons in the spinal dorsal horn 53, and Bannister et al. (2011) found that pregabalin could reduce responses of WDR neurons in morphine-treated rats to noxious mechanical and thermal stimuli3. Our study in the medullary dorsal horn tested the effects of pregabalin on NS neurons as well as on WDR neurons in the p-IONX rats and sham-operated rats. In p-IONX rats, but not in sham-operated rats, pregabalin (at a dose of 3mg/kg) attenuated the enhanced neuronal spontaneous activity and evoked responses in sensitized NS neurons. It also depressed some of these changes indicative of central sensitization in WDR neurons, i.e., their responses to tactile (2g and less) as well as noxious mechanical stimuli (6g and higher), consistent with our behavioral data of pregabalin’s reversal of the p-IONX-induced mechanical allodynia. We did not specifically test if p-IONX induces behavioral changes reflecting mechanical hyperalgesia, but our findings that pregabalin reduced the responses of sensitized NS neurons to noxious mechanical stimuli raises the possibility that orofacial mechanical hyperalgesia may also be subject to reduction by pregabalin; this needs to be tested in future studies. Also noteworthy are our findings that pregabalin had no effect on normal nociceptive behavior (10mg/kg) or the baseline neuronal properties of nociceptive neurons (3mg/kg) in sham-operated animals, which suggest that pregabalin can block central sensitization in medullary dorsal horn nociceptive neurons without affecting normal nociceptive processing.
Pregabalin and the related compound gabapentin are ligands at the a2δ subunit of the voltage-gated calcium channel, and binding at this site reduces calcium influx at nerve terminals and consequently reduces the stimulated release of synaptic neurotransmitters 12,18,27,30. Enhanced excitability and sustained membrane depolarization are features of central sensitization of spinal and medullary dorsal horn neurons. While the determination of the site(s) of action of pregabalin was not an aim of the present study that utilized systemic administration of the drug, several studies have identified that the spinal cord dorsal horn is an important site of action for gabapentin and pregabalin16,21,26,37,46. However, systemic delivery of pregabalin can also significantly inhibited ectopic discharges from injured afferent neurons in a dose-dependent manner, which suggests that the analgesic effect of pregabalin on neuropathic pain may be mediated at least in part by a peripheral inhibitory action on the generation of ectopic discharges caused by nerve injury 5. Thus it is possible that the effects of pregabalin observed in the present study were partly mediated by a reduction of ectopic afferent activity, thus directly reducing or eliminating the nociceptive afferent input to the medullary dorsal horn, and preventing/reducing the sensitization of the medullary dorsal horn neurons.
Conclusions
The present results indicate that pregabalin effectively attenuates mechanical hypersensitivity and nociceptive neuronal hyperexcitability reflecting central sensitization in a rat model of craniofacial neuropathic pain, and supports its clinical use in the treatment of craniofacial neuropathic pain conditions.
Perspective.
Trigeminal nerve injury in rats produced facial mechanical hypersensitivity and trigeminal central sensitization of medullary dorsal horn neurons that were markedly attenuated by systemically administered pregabalin, suggesting its potential clinical utility for orofacial neuropathic pain.
Footnotes
Disclosures
This work was supported by grants from the Pfizer Canada, as well as Canadian Institutes of Health Research MOP grant 82831 and US National Institutes of Health grant DE04786. We thank Pfizer Canada for providing pregabalin. BJS is the holder of a Canada Research Chair. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain. 2009;13:568–575. doi: 10.1016/j.ejpain.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, Jr, Welge JA, Bishop F, Stanford KE, Hess EV, Hudson JI. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–1344. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 3.Bannister K, Sikandar S, Bauer CS, Dolphin AC, Porreca F, Dickenson AH. Pregabalin suppresses spinal neuronal hyperexcitability and visceral hypersensitivity in the absence of peripheral pathophysiology. Anesthesiology. 2011;115:144–152. doi: 10.1097/ALN.0b013e31821f6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Wang H, Chiang CY, Dostrovsky JO, Sessle BJ. Effects of pregabalin on nociceptive behaviour and central sensitization in a trigeminal neuropathic pain model. Society for Neuroscience Meeting; 2011. p. Abstract No 72.27. [Google Scholar]
- 5.Chen SR, Xu Z, Pan HL. Stereospecific effect of pregabalin on ectopic afferent discharges and neuropathic pain induced by sciatic nerve ligation in rats. Anesthesiology. 2001;95:1473–1479. doi: 10.1097/00000542-200112000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998;80:2621–2631. doi: 10.1152/jn.1998.80.5.2621. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang CY, Dostrovsky JO, Iwata K, Sessle BJ. Role of glia in orofacial pain. Neuroscientist. 2011;17:303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- 9.Christensen D, Gautron M, Guilbaud G, Kayser V. Effect of gabapentin and lamotrigine on mechanical allodynia-like behaviour in a rat model of trigeminal neuropathic pain. Pain. 2001;93:147–153. doi: 10.1016/S0304-3959(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 10.Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J Neurochem. 2007;100:1289–1299. doi: 10.1111/j.1471-4159.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- 11.Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther. 2002;302:1013–1022. doi: 10.1124/jpet.302.3.1013. [DOI] [PubMed] [Google Scholar]
- 12.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Corbin AE, Young JP, Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Cui M, Willis WD. Gabapentin markedly reduces acetic acid-induced visceral nociception. Anesthesiology. 2003;98:729–733. doi: 10.1097/00000542-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther. 1997;282:1242–1246. [PubMed] [Google Scholar]
- 17.Field MJ, Bramwell S, Hughes J, Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: are they signalled by distinct primary sensory neurones? Pain. 1999;83:303–311. doi: 10.1016/s0304-3959(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 18.Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 19.Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20:456–472. doi: 10.1097/ACO.0b013e3282effaa7. [DOI] [PubMed] [Google Scholar]
- 20.Grabow TS, Dougherty PM. Gabapentin produces dose-dependent antinociception in the orofacial formalin test in the rat. Reg Anesth Pain Med. 2002;27:277–283. doi: 10.1053/rapm.2002.30740. [DOI] [PubMed] [Google Scholar]
- 21.Hurley RW, Chatterjea D, Rose Feng M, Taylor CP, Hammond DL. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97:1263–1273. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- 23.Iwata K, Imai T, Tsuboi Y, Tashiro A, Ogawa A, Morimoto T, Masuda Y, Tachibana Y, Hu J. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J Neurophysiol. 2001;86:2868–2877. doi: 10.1152/jn.2001.86.6.2868. [DOI] [PubMed] [Google Scholar]
- 24.Iwata K, Tsuboi Y, Shima A, Harada T, Ren K, Kanda K, Kitagawa J. Central neuronal changes after nerve injury: neuroplastic influences of injury and aging. J Orofac Pain. 2004;18:293–298. [PubMed] [Google Scholar]
- 25.Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko M, Mestre C, Sanchez EH, Hammond DL. Intrathecally administered gabapentin inhibits formalin-evoked nociception and the expression of Fos-like immunoreactivity in the spinal cord of the rat. J Pharmacol Exp Ther. 2000;292:743–751. [PubMed] [Google Scholar]
- 27.Kumar N, Laferriere A, Yu JSC, Leavitt A, Coderre TJ. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2010;113:552–561. doi: 10.1111/j.1471-4159.2010.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeResche L, Drangsholt M. Orofacial pain: from basic science to clinical management. 2. Vol. 13. Chicago: Quintessence; 2008. Epidemiology of orofacial pain: prevalence, incidence and risk factors; p. C18. [Google Scholar]
- 29.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 30.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto M, Tsuboi Y, Takamiya K, Huganir RL, Kondo M, Shinoda M, Oi Y, Iwata K. Involvement of GluR2 and GluR3 subunit C-termini in the trigeminal spinal subnucleus caudalis and C1-C2 neurons in trigeminal neuropathic pain. Neurosci Lett. 2011;491:8–12. doi: 10.1016/j.neulet.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Taenzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narita N, Kumar N, Cherkas PS, Chiang CY, Dostrovsky JO, Coderre TJ, Sessle BJ. Systemic Pregabalin Attenuates Sensorimotor Responses and Medullary Glutamate Release in Inflammatory Tooth Pain Model. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozaki-Taguchi N, Chaplan SR, Higuera ES, Ajakwe RC, Yaksh TL. Vincristine-induced allodynia in the rat. Pain. 2001;93:69–76. doi: 10.1016/S0304-3959(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 35.Obermann M, Yoon MS, Sensen K, Maschke M, Diener HC, Katsarava Z. Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia. 2008;28:174–181. doi: 10.1111/j.1468-2982.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 36.Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge BJ, Chaplan SR, Sakamoto E, Yaksh TL. Characterization of the effects of gabapentin and 3-isobutyl-gamma-aminobutyric acid on substance P-induced thermal hyperalgesia. Anesthesiology. 1998;88:196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Perez C, Navarro A, Saldana MT, Martinez S, Rejas J. Patient-reported outcomes in subjects with painful trigeminal neuralgia receiving pregabalin: evidence from medical practice in primary care settings. Cephalalgia. 2009;29:781–790. doi: 10.1111/j.1468-2982.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 39.Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain. 2006;121:219–231. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher MH, Cervero F. Role of the NKCC1 co-transporter in sensitization of spinal nociceptive neurons. Pain. 2010;151:756–762. doi: 10.1016/j.pain.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, Kondo M, Sessle BJ, Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol. 2008;99:2251–2263. doi: 10.1152/jn.00794.2007. [DOI] [PubMed] [Google Scholar]
- 43.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 44.Shibuta K, Suzuki I, Shinoda M, Tsuboi Y, Honda K, Shimizu N, Sessle BJ, Iwata K. Organization of hyperactive microglial cells in trigeminal spinal subnucleus caudalis and upper cervical spinal cord associated with orofacial neuropathic pain. Brain Res. 2012;1451:74–86. doi: 10.1016/j.brainres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–1800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
- 46.Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–590. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- 47.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 48.Tsuboi Y, Takeda M, Tanimoto T, Ikeda M, Matsumoto S, Kitagawa J, Teramoto K, Simizu K, Yamazaki Y, Shima A, Ren K, Iwata K. Alteration of the second branch of the trigeminal nerve activity following inferior alveolar nerve transection in rats. Pain. 2004;111:323–334. doi: 10.1016/j.pain.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Tsuboi Y, Iwata K, Dostrovsky JO, Chiang CY, Sessle BJ, Hu JW. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci. 2011;34:292–302. doi: 10.1111/j.1460-9568.2011.07747.x. [DOI] [PubMed] [Google Scholar]
- 50.Turnbach ME, Spraggins DS, Randich A. Spinal administration of prostaglandin E(2) or prostaglandin F(2alpha) primarily produces mechanical hyperalgesia that is mediated by nociceptive specific spinal dorsal horn neurons. Pain. 2002;97:33–45. doi: 10.1016/s0304-3959(01)00487-0. [DOI] [PubMed] [Google Scholar]
- 51.Urch CE, Donovan-Rodriguez T, Dickenson AH. Alterations in dorsal horn neurones in a rat model of cancer-induced bone pain. Pain. 2003;106:347–356. doi: 10.1016/j.pain.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallin J, Cui JG, Yakhnitsa V, Schechtmann G, Meyerson BA, Linderoth B. Gabapentin and pregabalin suppress tactile allodynia and potentiate spinal cord stimulation in a model of neuropathy. Eur J Pain. 2002;6:261–272. doi: 10.1053/eujp.2002.0329. [DOI] [PubMed] [Google Scholar]
- 54.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 55.Xie YF, Zhang S, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) Brain Behav Immun. 2007;21:634–641. doi: 10.1016/j.bbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Xu M, Aita M, Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. J Pain. 2008;9:1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]